Abstract
Background:
Raltegravir in combination therapy has demonstrated potent suppression of HIV-1 with a favorable safety profile. This report provides 96-week efficacy and safety data from Protocol 005, a Phase II study.
Methods:
HIV-infected patients with very limited treatment options and failing antiretroviral therapy were randomized to raltegravir 200, 400, or 600 mg or placebo b.i.d., plus optimized background therapy for ≥24 weeks; all patients were then offered open-label raltegravir 400 mg b.i.d. Efficacy measurements included changes in viral load and CD4 count from baseline and percent of patients with HIV-1 RNA <400 and <50 copies/mL.
Results:
One hundred and thirty-three patients received raltegravir and 45 received placebo. No dose-dependent differentiation in the safety or antiviral activity of raltegravir was observed during the double-blind phase. For the combined raltegravir groups, mean change in viral load from baseline was −1.60 log10 copies/mL at week 48 and −1.38 log10 copies/mL at week 96 (observed failure approach). At week 48, HIV-1 RNA levels were <400 copies/mL in 68% of raltegravir recipients and <50 copies/mL in 55%; these levels were maintained in 55% and 48% of raltegravir recipients, respectively, at week 96 (noncompleter = failure). There were few discontinuations of raltegravir (4%) due to adverse events.
Conclusions:
In patients with limited treatment options, raltegravir with OBT had a potent and sustained antiretroviral effect and was generally well tolerated through 96 weeks.
Keywords: HIV, AIDS, antiretroviral therapy, integrase inhibitor, resistance, raltegravir
INTRODUCTION
Raltegravir (also known as MK-0518) is an HIV-1 integrase strand transfer inhibitor that has been shown to be active against multidrug-resistant HIV-1 and both CCR5-tropic and CXCR4-tropic HIV-1 in vitro.1,2 Raltegravir is additive or synergistic in vitro with currently available antiretroviral drugs. Strains of HIV resistant to raltegravir remain susceptible to other agents.2 In patients with no prior antiretroviral therapy (ART), raltegravir with tenofovir and lamivudine was generally well tolerated and had potent and durable antiretroviral activity, which was similar to that of efavirenz with tenofovir and lamivudine after 96 weeks of therapy (83% of patients in the raltegravir group and 84% in the efavirenz group had HIV-1 RNA <50 copies/mL).3 In treatment-experienced patients infected with HIV-1 resistant to nucleoside (NRTI) and nonnucleoside reverse transcriptase inhibitors (NNRTI) and protease inhibitors (PIs), raltegravir 200, 400, and 600 mg b.i.d. in combination with optimized background therapy (OBT) was compared with placebo plus OBT (Protocol 005). Antiretroviral effects were observed as early as week 4 and were sustained through week 24 across all raltegravir groups.4 This report presents the long-term (week 96) safety and efficacy of raltegravir plus OBT in patients who completed the double-blind, dose-ranging period of the study, and then continued to receive raltegravir (400 mg b.i.d.) plus OBT in an open-label extension.
METHODS
The first part of the study was a double-blind (with inhouse blinding), randomized, dose-ranging period to evaluate the safety, tolerability, and efficacy of raltegravir (200 mg, 400 mg, or 600 mg b.i.d.) plus OBT compared with placebo plus OBT in HIV-infected, treatment-experienced patients with multidrug resistant virus. When raltegravir 400 mg b.i.d. was selected as the dose for phase III studies, this study (Protocol 005) was amended to allow patients who had completed at least 24 weeks in the double-blind period to receive raltegravir 400 mg b.i.d. in an open-label extension. This study is currently in progress at a total of 31 sites, 15 in the United States, and 16 in Europe, Latin America, and Asia, and is registered at Clinicaltrials.gov (NCT # 00105157). The study protocol was approved by the Ethical Review Committee at each site, and written consent was obtained from each patient before study entry.
Patients
HIV-seropositive patients ≥ 18 years of age were eligible if they had plasma HIV-1 RNA levels >5000 copies/mL and CD4+ T-cell (CD4) counts >50 cells/mm3 on stable ART for more than 3 months and were infected with HIV-1 with documented genotypic and phenotypic resistance to at least 1 NNRTI, 1 NRTI, and 1 PI. Additional eligibility criteria have been described previously.4
Procedures
Before randomization, the investigators selected each patient’s OBT based on the patient’s prior antiretroviral treatment history, results from all available genotypic and phenotypic resistance tests, previous or current laboratory abnormalities, and intolerance to prior ARTs. Neither tipranavir nor darunavir was permitted as part of the OBT because they were investigational at the time this study was initiated and potential drug interactions with raltegravir were unknown. Subsequent changes to OBT were permitted only for toxicity management or when patients switched to the virologic failure arm (see below). In addition to the OBT, raltegravir tablets or matching placebo were taken twice daily (about 10–14 hours apart) without regard to food intake. Details of treatment allocation and blinding have been previously described.4 The original treatment group assignment remained blinded when patients entered the open-label extension. Physical examinations, blood samples for the measurement of plasma HIV-1 RNA and CD4 cell count, and blood and urine samples for laboratory tests were collected at screening; randomization (day 1); weeks 2, 4, 8, 12, 16, and every 8 weeks thereafter; and 14 days after the last dose of study therapy in patients who discontinued the trial.
Patients with documented virologic failure after week 16 could switch to the open-label postvirologic failure (OLPVF) arm to receive raltegravir 400 mg b.i.d. and to reoptimize their other ART. Virologic failure was defined as either lack of efficacy (ie, confirmed decrease in plasma viral load from baseline of less than 1.0 log10 copies/mL and viral load >400 copies/mL) or virologic relapse (ie, viral load >400 copies/mL, on 2 consecutive measurements at least 1 week apart, after initial response with viral load <400 copies/mL; or an increase >1.0 log10 copies/mL above nadir level, on 2 consecutive measurements at least 1 week apart). These patients were counted as virologic failures in the efficacy analysis. Resistance testing (PhenoSense GT, Monogram Biosciences, San Francisco, CA) was performed at baseline and at the time of virologic failure. In patients with confirmed virologic failure, the emergence of resistance to raltegravir was investigated by isolating viral RNA and determining the amino acid sequences of the integrase gene as compared with the baseline sequence of integrase in each subject.
Statistical Analysis
The objective of the week 96 analysis was to evaluate the long-term safety and efficacy of treatment with raltegravir plus OBT. All randomized patients who received at least one dose of study medication were included in the analyses. The primary endpoints for this study were safety, as measured by the occurrence of adverse events and laboratory abnormalities, and antiretroviral activity, as measured by the change from baseline in HIV-1 RNA (log10 copies/mL) at week 24.4 Secondary endpoints included the proportions of patients with viral load <400 and <50 copies/mL and the change from baseline in CD4 cell count. Because over 85% of patients had transitioned to the open-label extension and were receiving raltegravir 400 mg b.i.d. by week 48, efficacy data were analyzed for the combined raltegravir group and for the original raltegravir dose groups after this time point. Data for the placebo group are not displayed after week 24 because only 6 patients in the placebo group completed more than 24 weeks in the double-blind phase; however, the virologic responses of these 6 patients are described in the results.
For the calculation of the change from baseline in viral load and in CD4 cell count, patients who discontinued early due to virologic failure (lack of efficacy) were deemed to be failures [Observed Failure (OF) approach] and in the analysis were assumed to have returned to their baseline level at subsequent visits (ie, baseline value carried forward); patients who discontinued for other reasons were censored at the time of discontinuation. For the proportion of patients achieving HIV-1 RNA levels <400 and <50 copies/mL, all patients who discontinued, irrespective of reasons, were deemed to be failures at subsequent visits [noncompleter = failure (NC = F) approach]; intermittent missing values were counted as failures unless they were preceded and succeeded by successes, in which case no result was imputed. Summary statistics and the corresponding 95% CIs are presented; no hypothesis testing was done.
Logistic regression models were utilized to examine the potential effects of prognostic factors for viral response of HIV-1 RNA <50 copies/mL at week 96 for patients randomized to raltegravir; a separate univariate model was fit for each covariate examined [enfuvirtide use in OBT in enfuvirtide naïve patients vs. no use or continued use, baseline HIV-1 RNA ≤50,000 vs. >50,000 and ≤100,000 vs. >100,000 copies/mL, baseline genotypic sensitivity score (GSS) >0 vs. 0, and baseline phenotypic sensitivity score (PSS) >0 vs. 0]. Prognostic factor and original treatment group were included in the models initially; however, because treatment group was not significant in any model, nominal P-values are reported from univariate models for each covariate. The OF approach was used for these analyses.
Although the efficacy analyses provide data through week 96 for all patients, the safety analysis included all data from the double-blind phase and the open-label extension that were available through the visit cut-off date of December 10, 2007, including data beyond week 96 for 87 patients. The severity of laboratory abnormalities was graded according to the 1992 Division of AIDS toxicity guidelines for adults (http://rcc.tech-res-intl.com/tox_tables.htm). Adverse events were counted as drug related if they were judged by the investigator as definitely, probably, or possibly related to any component of the antiretroviral regimen (including drugs used in the OBT). Safety data are displayed for the double-blind period alone and for the double-blind period plus the open-label extension (ie, the entire study period). Only 6 patients from the placebo group received raltegravir in the open-label extension; data for these patients are displayed with the placebo group in the safety tables.
RESULTS
Patient Characteristics
A total of 178 patients received at least one dose of study drug; 133 were randomized to raltegravir and 45 to placebo. The demographics and baseline characteristics of randomized patients were comparable among the 4 originally assigned treatment groups.4 Overall, patient demographics were indicative of patients with AIDS with extensive prior antiretroviral treatment (Table 1). The number of antiretroviral drugs in the OBT ranged from 2 to 7 (median 4). Enfuvirtide was included in the OBT of 64 patients (36%), including 43 patients (24%) who were naïve to this drug. Counting enfuvirtide use in enfuvirtide-naive patients as one active drug, there were no effective antiretrovirals in the OBT of 99 patients (56%) and 63 patients (35%) on the basis of genotypic and phenotypic resistance tests, respectively (Table 1).
TABLE 1.
Patient Baseline and OBT Characteristics
| Raltegravir + OBT |
|||||
|---|---|---|---|---|---|
| 200 mg b.i.d. (N = 43) |
400 mg b.i.d. (N = 43) |
600 mg b.i.d. (N = 45) |
Placebo + OBT (N = 45) |
Total (N = 178) |
|
| Age (yrs), median (range) | 43 (18–57) | 43 (32–69) | 44 (25–63) | 43 (29–59) | 43 (18–69) |
| Male, n (%) | 36 (84) | 40 (89) | 41 (91) | 40 (89) | 157 (88) |
| HIV RNA (log10 copies/mL), mean ± SD | 4.6 ± 0.6 | 4.8 ± 0.5 | 4.7 ± 0.5 | 4.7 ± 0.6 | 4.7 ± 0.5 |
| CD4 T-cell count (cells/mm3), mean ± SD | 245 ± 186 | 221 ± 115 | 220 ± 161 | 274 ± 188 | 240 ± 165 |
| History of AIDS, n (%) | 34 (79) | 39 (87) | 38 (84) | 36 (80) | 147 (83) |
| Years of prior antiretroviral treatment, median (range) | 10 (0–16) | 11 (2–14) | 9 (3–16) | 10 (2–17) | 10 (0–17) |
| Number of prior antiretroviral therapies, median (range) | 12 (3–18) | 13 (8–17) | 12 (3–21) | 12 (5–17) | 12 (3–21) |
| OBT: Number of antiretrovirals, median (range) | 4(2–6) | 4 (3–7) | 4 (2–7) | 4 (3–7) | 4 (2–7) |
| GSS*: 0 to all antiretrovirals, n (%) | 18 (42) | 32 (71) | 25 (56) | 24 (53) | 99 (56) |
| PSS*: 0 to all antiretrovirals, n (%) | 14 (33) | 22 (49) | 13 (29) | 14 (31) | 63 (35) |
| PSS*: 0 to PIs, n (%) | 42 (98) | 42 (93) | 40 (89) | 38 (84) | 162 (91) |
| Enfuvirtide use in OBT, n (%) Enfuvirtide-naive patients |
13 (30) | 8 (18) | 12 (27) | 10 (22) | 43 (24) |
| Enfuvirtide-experienced patients | 1 (2) | 10 (22) | 4 (9) | 6 (13) | 21 (12) |
GSS/PSS score determined by PhenoSense GT (Monogram Biosciences). GSS was defined as the total number of drugs in the OBT to which a patient’s viral isolate showed genotypic sensitivity. PSS was defined as the total number of drugs in the OBT to which a patient’s viral isolate showed phenotypic sensitivity.
Enfuvirtide use in OBT in enfuvirtide-naive patients was counted as one active drug in OBT and added to the GSS and PSS.
Transition to open-label raltegravir occurred at various time points after week 24. The median (range) duration of the double-blind phase was approximately 40 (3–59) weeks for the combined raltegravir groups and 24 (12–50) weeks for the placebo group. For the original raltegravir treatment groups, the median (range) duration of the double-blind phase was 40 (3–59) weeks, 40 (20–55) weeks, and 40 (19–55) weeks for patients receiving raltegravir 200, 400, and 600 mg, respectively.
Of the 133 patients randomized to all raltegravir groups, 94 (71%) entered the open-label phase, and 86 (65%) completed at least 96 weeks of follow-up (Table 2). Of the 45 patients randomized to placebo, 6 (13%) received raltegravir 400 mg b.i.d. in the open-label extension and completed at least 96 weeks of follow-up. The median duration of follow-up for the entire study was 115 weeks (range, 3–138 weeks) for the combined raltegravir groups and 25 weeks (range, 12–131 weeks) for the placebo group.
Table 2.
Patient Disposition
| Raltegravir* + OBT N = 133 n (%) |
Placebo + OBT N = 45 n (%) |
Total N = 178 n (%) |
||
|---|---|---|---|---|
| Double-blind (DB) phase | ||||
| Treated | 133 | 45 | 178 | |
| Discontinued† DB | 39 (29) | 39 (87) | 78 (44) | |
| Lack of efficacy | 36 (27) | 38 (84) | 74 (42) | |
| Clinical adverse event |
2 (2) | 1 (2) | 3 (2) | |
| Laboratory adverse event |
1 (1) | 0 | 1 (1) | |
| Open-label (OL) extension | ||||
| Total entered from DB | 94 (71) | 6 (13) | 100 (56) | |
| Discontinued†OL | 8 (6) | 0 | 8 (4) | |
| Lack of efficacy | 3 (2) | 0 | 3 (2) | |
| Clinical adverse event | 2 (2) | 0 | 2 (1) | |
| Consent withdrawn | 1 (1) | 0 | 1 (1) | |
| Other | 2 (2) | 0 | 2 (1) | |
All original raltegravir dosage groups combined.
Reasons for discontinuation were investigator determined.
Efficacy
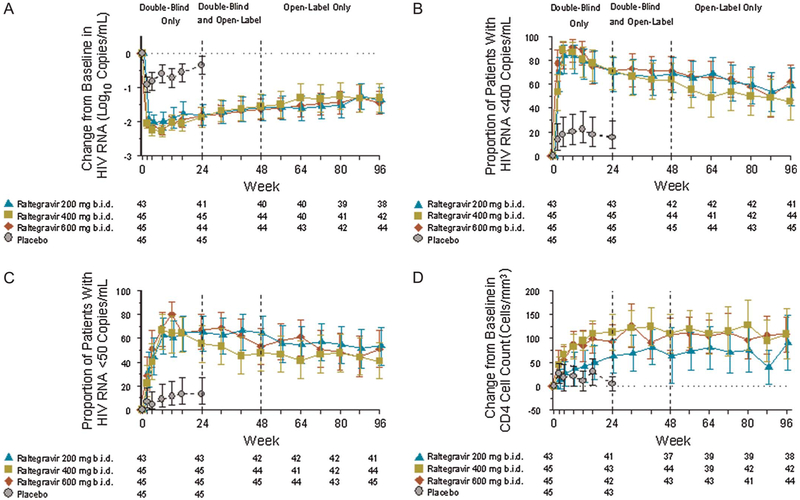
In the combined raltegravir groups, the mean change from baseline in HIV-1 RNA was −1.60 log10 copies/mL [95% confidence interval (CI), −1.79 to −1.41] at week 48 and − 1.38 log10 copies/mL (95% CI, −1.59 to −1.17) at week 96 (OF analysis). HIV-1 RNA <400 copies/mL was achieved in 68% of the raltegravir group at week 48 and in 55% at week 96, whereas HIV-1 RNA <50 copies/mL was achieved in 55% and 48% of patients at weeks 48 and 96, respectively (NC = F analysis). Increases in CD4 cell counts were observed as early as week 4 and were sustained through week 96; for the combined raltegravir groups, the mean change (95% CI) from baseline was +96 cells/mm3 (71, 121) at week 48 and +104 cells/mm3 (76, 131) at week 96 (OF analysis). For each of these parameters, there were no significant differences between the original raltegravir treatment groups at week 96 (Fig. 1). Among the 6 patients from the placebo group who switched to raltegravir 400 mg b.i.d. (with viral load <400 copies/mL), HIV-1 RNA levels at week 96 remained <400 copies/mL in 5 patients and <50 copies/mL in 4 (data not shown).
FIGURE 1.
Response to raltegravir: entire study period. A, Mean change from baseline in log10 HIV RNA (OF approach); B, proportion of patients achieving HIV RNA <400 copies/mL (NC = F approach);C, proportion of patients achieving HIV RNA <50 copies/mL (NC = F approach); D, mean change from baseline in CD4+ T-cell count (OF approach). Error bars indicate 95% CI.
Patients with first use of enfuvirtide in the OBT were 7.9 times more likely (P < 0.001) to have HIV-1 RNA <50 copies/mL at week 96 than were patients without enfuvirtide in OBT or with nonnaive use of enfuvirtide in the OBT (Table 3). Patients with a baseline viral load ≤100,000 copies/mL were 2 times more likely than those with higher baseline RNA levels to have HIV-1 RNA <50 copies/mL at week 96 (P = 0.067). Although patients with a GSS (PSS) score greater than zero were approximately 1.5 times more likely than patients with a GSS (PSS) score of zero to have viral suppression <50 copies/mL at week 96, the difference was not statistically significant.
TABLE 3.
Predictors of HIV RNA <50 copies/ml at Week 96 for Patients Receiving Raltegravir (OF Approach)*
| Prognostic Factor | Percent of Patients With HIV RNA < 50 copies/mL |
Odds Ratio for Prognostic Factor† |
||
|---|---|---|---|---|
| n/N | % (95% CI) | Odds Ratio (95% CI) | P | |
| Total | 63/124 | 50.8 (41.7, 59.9) | ||
| Enfuvirtide use in OBT | ||||
| No | 32/79 | 40.5 (29.6, 52.1) | ||
| Yes | 31/45 | 68.9 (53.4, 81.8) | ||
| Yes in enfuvirtide naïve patients | 26/31 | 83.9 (66.3, 94.5) | ||
| Yes in enfuvirtide experienced patients | 5/14 | 35.7 (12.8, 64.9) | ||
| Naive enfuvirtide use (yes vs. no) | 7.869 (2.772, 22.334) | <0.001 | ||
| Baseline HIV RNA (copies/mL) | ||||
| ≤50,000 | 38/65 | 58.5 (45.6, 70.6) | ||
| >50,000 | 25/59 | 42.4 (29.6, 55.9) | ||
| ≤50,000 vs. >50,000 | 1.914 (0.937, 3.909) | 0.075 | ||
| ≤100,000 | 47/83 | 56.6 (45.3, 67.5) | ||
| >100,000 | 16/41 | 39.0 (24.2, 55.5) | ||
| ≤100,000 vs. >100,000 | 2.040 (0.951, 4.375) | 0.067 | ||
| Baseline GSS‡ | ||||
| 0 | 31/68 | 45.6 (33.5, 58.1) | ||
| 1–2 | 29/50 | 58.0 (43.2, 71.8) | ||
| 3 or more | 3/6 | 50.0 (11.8, 88.2) | ||
| >0 vs. 0 | 1.591 (0.780, 3.245) | 0.201 | ||
| Baseline PSS‡ | ||||
| 0 | 20/45 | 44.4 (29.6, 60.0) | ||
| 1–2 | 38/70 | 54.3 (41.9, 66.3) | ||
| 3 or more | 5/9 | 55.6 (21.2, 86.3) | ||
| >0 vs. 0 | 1.493 (0.715, 3.117) | 0.286 | ||
Patients who discontinued assigned therapy due to lack of efficacy were considered as failures (OF approach).
An odds ratio of(<1, =1, >1) indicates (decreased, equal, increased) probability to respond at week 96. Odds ratio, CI, and P value were calculated using a logistic regression model adjusted for the particular prognostic factor.
GSS,PSS were defined as the total number of drugs in the OBT to which a patient’s viral isolate showed genotypic sensitivity or phenotypic sensitivity, respectively. Enfuvirtide use in OBT in enfuvirtide-naive patients was counted as one active drug in the OBT and added to the GSS and PSS.
Efficacy data are available through 76 weeks of treatment with open-label raltegravir 400 mg b.i.d. in patients who experienced virologic failure during the double-blind phase and chose to enter OLPVF treatment group. At this time point, HIV-1 RNA levels were <50 copies/mL in 3 (9%) of 35 patients who had received raltegravir in the double-blind phase and in 13 (35%) of 37 who had received placebo in the double-blind phase. Due to limited treatment options, 54% of patients from the raltegravir group and 49% of those from the placebo group stayed on their failing background therapy regimen upon entering the OLPVF treatment group.
Resistance
During the double-blind phase, virologic failure was observed in 38 (29%) of 133 raltegravir recipients; the majority (68%) of patients with virologic failure was receiving OBT with a GSS of 0. Integrase mutations were observed in 35/38 patients, and there was no difference in the number of patients with integrase mutations among the 3 raltegravir dose groups. Mutations at either amino acid 148 (Q changed to H, K, or R) or amino acid 155 (N changed to H) were observed in 33 patients, and 31 of these sequences also contained secondary changes. Mutations at Q148 were always observed in combination with secondary mutations at 138, 140, or both. Viruses with the N155H mutation contained secondary mutations at residues 74, 92, 97, 143, or 163.
During the open-label extension, 9 patients (3 from each of the original raltegravir treatment groups) who had achieved HIV-1 RNA <50 copies/mL by week 48 had virologic rebound with confirmed HIV-1 RNA >50 copies/mL by week 96 (Table 4); 7 of these patients had limited active ARTs in the OBT (GSS of 0). Of the 6 patients whose HIV-1 strains could be genotyped, 5 had acquired signature integrase mutations at either Q148 (n = 4) or N155 (n = 1), in combination with secondary mutations in the HIV integrase gene (Table 4).
TABLE 4.
Characteristics of Patients With Virologic Rebound (>50 copies/mL) During Open-Label Extension
| Patient | Original Treatment Assignment |
Integrase Amino Acid Changes From Baseline |
Baseline HIV RNA (copies/mL) |
Baseline OBT GSS/PSS* |
Enfuvirtide Use in OBT |
|---|---|---|---|---|---|
| 1 | 600 mg | Q148H, G140S, Q7K/Q, S81R/S, S255N/S/K/R, V280G/V | 136,000 | 0/3 | Yes (used before) |
| 2 | 400 mg | Q148H, G140S | 29,800 | 0/0 | Yes (first-time user) |
| 3 | 200 mg | D279D/N | 11,700 | 0/0 | Yes (used before) |
| 4 | 600 mg | Q148R, E138K | 10,900 | 0/2 | No |
| 5 | 200 mg | T66A, V79V/A, Y143C, E232Q, N155H–(present on different PCR products) | 276,000 | 1/1 | No |
| 6 | 400 mg | Q148R, G140S, E138A | 20,700 | 0/1 | No |
| 7† | 200 mg | 41,400 | 1/1 | No | |
| 8† | 400 mg | 9950 | 0/2 | No | |
| 9‡ | 600 mg | 31,300 | 0/0 | Yes (first-time user) |
GSS/PSS score by PhenoSense GT, enfuvirtide use in OBT is not counted here.
Patient’s viral load never returned to above 400 copies/mL and was not tested.
Multiple PCR tests failed.
Safety
At the end of the double-blind period, the safety profile of raltegravir in combination with OBT was comparable with that of placebo with OBT (Table 5). Among patients randomized to raltegravir from the start of study, drug-related clinical adverse events occurred in 46% of patients during the double-blind period and in 58% after approximately 1 additional year of exposure. The profile of drug-related clinical adverse events observed during the entire study was similar to that observed during the double-blind phase (Table 5). Injection site reaction due to enfuvirtide accounted for most of the drug-related clinical adverse events emerging after week 48.
TABLE 5.
Summary of Adverse Events (AEs)
| Double-Blind Phase |
Entire Study Period |
|||
|---|---|---|---|---|
| Raltegravir (N = 133) | Placebo (N = 45) | Raltegravir (N = 133) | Placebo* (N = 45) | |
| Duration of follow-up (wks), median (range) | 40 (3–59) | 24 (12–50) | 115 (3–138) | 25 (12–131) |
| Clinical adverse events, n (%) | 115 (86) | 37 (82) | 130 (98) | 38 (84) |
| Drug related† (DR) AE | 61 (46) | 24 (53) | 77 (58) | 26 (58) |
| Diarrhea | 2 (2) | 5 (11) | 2 (2) | 5 (11) |
| Nausea | 10 (8) | 5 (11) | 10 (8) | 5 (11) |
| Fatigue | 5 (4) | 1 (2) | 6 (5) | 1 (2) |
| Headache | 6 (5) | 2 (4) | 7 (5) | 2 (4) |
| Injection-site reaction | 0 | 1 (2) | 12 (9) | 3 (7) |
| Serious AE | 14 (11) | 3 (7) | 18 (14) | 3 (7) |
| Serious DR AE | 2 (2) | 2 (4) | 2 (2) | 2 (4) |
| Death | 2 (2) | 0 (0) | 4 (3) | 0 (0) |
| Discontinuations due to AE | 2 (2) | 1 (2) | 4 (3) | 1 (2) |
| Discontinuations due to DR AE | 0 (0) | 1 (2) | 0 (0) | 1 (2) |
| Laboratory adverse events, n (%) | 36 (27) | 11 (24) | 44 (33) | 12 (27) |
| Drug related† (DR) AE | 22 (17) | 8 (18) | 29 (22) | 8 (18) |
| Serious AE | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Serious DR AE | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Death | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Discontinuations due to AE | 1 (1) | 0 (0) | 1 (1) | 0 (0) |
| Discontinuations due to DR AE | 1 (1) | 0 (0) | 1 (1) | 0 (0) |
Raltegravir and placebo were administered with OBT.
N = number of patients randomized and treated at start of study.
Six patients from placebo treatment group entered open-label extension and received raltegravir.
Related to raltegravir or placebo (alone or with OBT); specific events listed occurred in ≥5% of any group.
As previously reported, serious drug-related adverse events occurred during the double-blind phase in 2 patients from the raltegravir group (1 acute pancreatitis; 1 metabolic acidosis with renal insufficiency) and in 2 patients from the placebo group (1 lacunar infarction; 1 worsening lipoatrophy).4 No serious drug-related clinical adverse events were reported during the open-label extension. Four patients died during the study, 2 in the double-blind phase (1 cardiac arrest; 1 suicide)4 and 2 in the open-label extension (1 acute myocardial infarction; 1 lymphadenopathy with splenic abscess); none of the deaths was considered drug-related. One malignancy (anal squamous cell carcinoma in situ) was diagnosed during the study and was also considered not drug related.
Drug-related laboratory adverse events occurred in 17% of the combined raltegravir groups during the double-blind phase and in 22% during the entire study period (Table 5). One patient had a serious laboratory adverse event (blood platelet count decreased) during the open-label extension that was considered possibly related to raltegravir plus OBT; this patient continued in the study and the adverse event resolved. Laboratory abnormalities graded according to the Division of AIDS toxicity guidelines for adults (which included all laboratory abnormalities whether considered by investigators to be adverse events or not) are shown in Table 6. The most common laboratory abnormality was increased bilirubin, which occurred only in patients receiving atazanavir or indinavir in their OBT. Liver function abnormalities were uncommon. In the combined raltegravir groups, grade 3 elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) occurred in 3% and 2% of patients, respectively, during the entire study period. There were no grade 4 abnormalities for AST, ALT, or alkaline phosphatase.
TABLE 6.
Number (%) of Patients With Grade 3 or 4 Laboratory Abnormalities*
| Double-Blind Phase |
Entire Study Period |
||||
|---|---|---|---|---|---|
| Laboratory Test (Unit) | Toxicity Criteria | Raltegravir (N = 133) | Placebo (N = 45) | Raltegravir (N = 133) | Placebo (N = 45) |
| Duration of follow-up (wks), median (range) | 40 (3–59) | 24 (12–50) | 115 (3–138) | 25 (12–131) | |
| Absolute neutrophil count | <750 cells/mm3 | 0 | 1 (2%) | 0 | 1 (2%) |
| Hemoglobin | <7.5 g/dL | 0 | 0 | 0 | 0 |
| Platelet count | <50,000/mm3 | 1 (1%) | 0 | 1 (1%) | 0 |
| Fasting LDL cholesterol‡ | ≥190 mg/dL | 3 (2%) | 2 (6%) | 7 (6%) | 2 (6%) |
| Fasting total cholesterol‡ | >300 mg/dL | 4 (3%) | 1 (3%) | 7 (5%) | 3 (8%) |
| Fasting triglycerides‡ | >750 mg/dL | 7 (5%) | 1 (3%) | 11 (8%) | 1 (3%) |
| Fasting glucose‡ | >250 mg/dL | 0 | 0 | 1 (1%) | 0 |
| Creatinine | >1.8 × ULN | 2 (2%) | 0 | 2 (2%) | 0 |
| Total bilirubin | >2.5 × ULN | 15 (11%) | 3 (7%) | 25 (19%) | 3 (7%) |
| Alkaline phosphatase | >5 × ULN | 0 | 0 | 0 | 0 |
| Pancreatic amylase | > 2 × ULN | 2 (2%) | 0 | 2 (2%) | 1 (2%) |
| Lipase | > 3 × ULN | 2 (2%) | 0 | 4 (3%) | 0 |
| Aspartate aminotransferase | > 5 × ULN | 2 (2%) | 0 | 4 (3%) | 0 |
| Alanine aminotransferase | > 5 × ULN | 1 (1%) | 0 | 2 (2%) | 0 |
| Creatine kinase | >10 × ULN | 5 (4%) | 2 (4%) | 8 (6%) | 2 (4%) |
Raltegravir and placebo were administered with OBT.
For inclusion in this analysis, both a baseline and at least one on-treatment laboratory value had to be present. A patient was included as a Grade X event if his/her highest grade during treatment was X and the laboratory value was worse than baseline.
Fasting laboratory test results were not available for all patients. For the raltegravir group: N = 120 (double-blind) and N = 125 (entire study) for LDL, and N = 130 for total cholesterol, triglycerides, and glucose (both periods); for the placebo group: N = 36 for LDL, N = 39 for total cholesterol and triglycerides, and N = 43 for glucose (both periods).
DISCUSSION
This study in patients with advanced HIV disease, as evidenced by a high percentage with an AIDS diagnosis and with a very limited choice of ARTs, provides a stringent test for the efficacy of raltegravir. Patients were heavily pretreated with ARTs with HIV becoming resistant to at least 3 classes (NRTI, NNRTI, and PI). At the time this study was initiated, newer ARTs such as darunavir and tipranavir were still investigational and therefore not permitted in the OBT; and enfuvirtide, the newest available ART in this period, had already been used in 26% of the enrolled patients.4 Nonetheless, HIV-1 RNA levels <50 copies/mL were maintained through 48 weeks of treatment in 55% of patients receiving raltegravir, and through 96 weeks in 48%, in a rigorous NC = F analysis. In subsequent phase III studies in a similar patient population, BENCHMRK-1 and -2, where investigational ARTs were permitted in the OBT, response rates were improved in both the raltegravir and control groups, with 57% and 26% of patients, respectively, maintaining HIV-1 RNA <50 copies/mL at week 96.5 Immunological benefits, as measured by superior increases in CD4 cell counts, were also maintained through week 48 in the individual raltegravir treatment groups and through week 96 with all patients receiving raltegravir 400 mg b.i.d. In addition, the week 96 response rates on all efficacy measures were not significantly different between the original raltegravir treatment groups, suggesting that the original raltegravir dose level did not affect the long-term response.
In the current study, the potent efficacy of raltegravir was observed even in patients with no active ARTs in their OBT (GSS = 0); in this subgroup, approximately 46% of patients receiving raltegravir had undetectable levels of HIV-1 RNA (<50 copies/mL) at week 96 (Table 3). However, functional monotherapy with raltegravir should be avoided wherever possible,6 given the greater absolute response rates in patients with more active OBT (GSS or PSS > 0) and the lower risk for virologic failure and development of resistance in patients receiving more active OBT.7 As shown by the lower response rates in patients who switched from placebo to raltegravir after virologic failure compared with patients who received raltegravir from the start of the study, raltegravir should be initiated with optimized ART rather than added to a failing regimen. The use of enfuvirtide in the OBT has been shown to augment the antiretroviral effect observed in other studies8–12 with similar patient populations. In the current study, enfuvirtide also improved the efficacy of raltegravir; 84% of patients in the raltegravir group using enfuvirtide for the first time in their OBT had HIV-1 RNA <50 copies/mL at week 96 (Table 3).
Treatment-related mutations in the HIV integrase region were noted in the majority of patients receiving raltegravir who experienced virologic failure. In most of these cases, HIV-1 RNA isolated at virologic failure had a primary mutation in integrase at either amino acid 155 (N155 changed to H) or amino acid 148 (Q148 changed to H, R, or K), all of which confer reduced susceptibility to raltegravir when introduced into HIV-1 in cell culture studies.13 In addition, most viruses with a primary mutation at amino acid position 155 or 148 also had one or more secondary mutations at any of a number of other positions in integrase. In cell culture studies with site-directed HIV-1 mutants, many of these secondary mutations augment the level of resistance to raltegravir and/or improve replication capacity when combined with one of the aforementioned primary mutations.13 Thus, virologic failure in raltegravir-treated patients was associated with the development of resistance to raltegravir and, in most cases, was associated with 2 or more mutations in HIV integrase.
During double-blind treatment, raltegravir in combination with OBT demonstrated a safety profile similar to that of placebo with OBT; this is important in a population in advanced stages of disease, with several concurrent medical conditions, and receiving multiple concomitant medications. The safety profile of raltegravir during the entire study period was similar to that observed in the double-blind phase. Overall, there were few discontinuations due to adverse events and few serious drug-related adverse events. Most clinical adverse events were mild to moderate, and grade 3 and 4 laboratory abnormalities were both uncommon and similar between treatment groups. The observed elevations in serum bilirubin levels occurred in patients who were receiving concomitant medications that have been associated with hyperbilirubinemia.14,15
The results of this long-term study show that in HIV-infected, treatment-experienced patients failing ART with triple-class resistant HIV, the potent efficacy of raltegravir in combination with OBT observed at week 244 and week 48,16 was sustained through 96 weeks of therapy. In addition, raltegravir was generally well tolerated over approximately 2 years, with very few adverse events leading to discontinuation of treatment.
ACKNOWLEDGMENTS
We thank all the patients and their caregivers who participated in this study, and we gratefully recognize the important contributions of the investigators who enrolled their patients in this study.
Disclosures: This study was sponsored and funded by Merck & Co., Inc., which manufactures raltegravir under the brand name ISENTRESS. The study was designed, managed, and analyzed by the sponsor in conjunction with external investigators. Authors had access to all study data upon request. This report was reviewed by the sponsor and was critically reviewed and subsequently approved by each coauthor in its essentially final form.
Presented at the ICAAC 2007 (week 48); HIV DART 2008 (week 96).
Supported by the Merck Research Laboratories.
Footnotes
Potential Conflicts of Interest: R.M.D., H.W., J.Z., A.R.M., C.M.H., K.M.S., R.D.I., and B.-Y.T.N. are current or former employees of Merck & Co. Inc and may own stock and/or stock options in the company. The remaining authors participated as clinical investigators for Protocol 005. In addition, the following potential conflicts of interest have been disclosed by the authors: J.M.G. has received research grants, honoraria, speaker fees, and/or consultancy payments from Merck, Gilead, Pfizer, Bristol-Myers Squibb, Glaxo-SmithKline, and Abbott. C.K. has received honoraria for advisory boards or lectures from Merck, Gilead, Roche, GlaxoSmithKline, Tibotec, Bristol Myers Squibb, and Boehringer Ingelheim. J.J.E. received research grants from Merck, Panacos, Glaxo-SmithKline, and TaiMed; and honoraria, speaker fees, and/or consultancy payments from Merck, Avexa, Panacos, Bristol-Myers Squibb, Glaxo-SmithKline, Tibotec, Virco Laboratories, Pfizer, Tobira, Chimerix, Roche, and Gilead. A.L. has received honoraria for lectures or Advisory Board meetings and/or research support from Merck, Glaxo-SmithKline, Bristol-Myers Squibb, Gilead, Roche, Tibotec, Pfizer, Boehringer-Ingelheim, Abbott, Monogram, and Schering-Plough.
Principal investigators: The MK-0518 Protocol 005 principal investigators by country are: Belgium: N. Clumeck; Brazil: B. Grinsztejn; France: C. Katlama, D. Vittecoq; Germany: J. Rockstroh, S. Staszewski; Italy: G. Carosi, A. Lazzarin, M. Moroni; Malaysia: C. Lee; Mexico: J. Sierra; Spain: J. Blanco, B. Clotet, J. Gatell, V. Soriano; Switzerland: M. Opravil, R. Weber; UK: M. Nelson; USA: J. Aberg, S. Brown, C. Crumpacker, J. Eron, J. Gallant, C. Gonzalez, E. Jones-Lopez, S. Swaminathan, M. Kozal, P. Kumar, D. Kuritzkes, J. Lennox, R. Larsen, R. Liporace, S. Little, D. McMahon, M. Rowley, K. Squires, K. Tashima.
REFERENCES
- 1.Hazuda DJ, Felock P, Witmer M, et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science. 2000;287:646–650. [DOI] [PubMed] [Google Scholar]
- 2.Miller M, Witmer M, Stillmock K, et al. Biochemical and antiviral activity of MK-0518, a potent HIV integrase inhibitor. Presented at: 16th International AIDS Conference; Toronto, Canada; August 2006 [Abstract THAA0302]. [Google Scholar]
- 3.Markowitz M, Nguyen B-Y, Gotuzzo E, et al. Sustained antiretroviral effect of raltegravir after 96 weeks of combination therapy in treatment-naïve patients with HIV-1 infection. J Acquir Immune Defic Syndr. 2009;52:350–356. [DOI] [PubMed] [Google Scholar]
- 4.Grinsztejn B, Nguyen B-Y, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomized controlled trial. Lancet. 2007;369:1261–1269. [DOI] [PubMed] [Google Scholar]
- 5.Steigbigel RT, Cooper DA, Eron JE, et al. 96-Week results from BENCHMRK 1 &2, phase III studies of raltegravir (RAL) in patients (pts) failing antiretroviral therapy (ART) with triple-class resistant HIV. Presented at: 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada; February 2009 [Abstract K-103]. [Google Scholar]
- 6.Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:651–681. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008;359:355–365. [DOI] [PubMed] [Google Scholar]
- 8.Lalezari JP, Henry K, O’Hearn M, et al. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003;348:2175–2185. [DOI] [PubMed] [Google Scholar]
- 9.Lazzarin A, Clotet B, Cooper D, et al. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N Engl J Med. 2003;348:2186–2195. [DOI] [PubMed] [Google Scholar]
- 10.Hicks CB, Cahn P, Cooper DA, et al. Durable efficacy of tipranavir-ritonavir in combination with an optimised background regimen of antiretroviral drugs for treatment-experienced HIV-1-infected patients at 48 weeks in the Randomized Evaluation of Strategic Intervention in multi-drug resistant patients with Tipranavir (RESIST) studies: an analysis of combined data from two randomised open-label trials. Lancet. 2006;368:466–475. [DOI] [PubMed] [Google Scholar]
- 11.Clotet B, Bellos N, Molina J-M, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–1178. [DOI] [PubMed] [Google Scholar]
- 12.Fatkenheuer G, Nelson M, Lazzarin A, et al. Subgroup analyses of maraviroc in previously treated R5 HIV-1 infection. N Engl J Med. 2008;359:1442–1455. [DOI] [PubMed] [Google Scholar]
- 13.Miller MD, Danovich RM, Ke Y, et al. Longitudinal analysis of resistance to the HIV-1 integrase inhibitor raltegravir: results from P005, a phase 2 study in treatment experienced patients [abstract #6]. Presented at: XVII International HIV Drug Resistance Workshop; Sitges, Spain; 10–14 June 2008. [Google Scholar]
- 14.Havlir DV O’Marro SD. Atazanavir: new option for treatment of HIV infection. Clin Infect Dis. 2004;38:1599–1604. [DOI] [PubMed] [Google Scholar]
- 15.Dicenzo R, Luque A, Larppanichpoonphol P, et al. Association of total bilirubin with indinavir and lopinavir plasma concentrations in HIV-infected patients receiving three different double-boosted dosing regimens. J Antimicrob Chemother. 2006;58:393–400. [DOI] [PubMed] [Google Scholar]
- 16.Grinsztejn B, Nguyen B-Y, Katlama C, et al. 48 week efficacy and safety of MK-0518, a novel HIV-1 integrase inhibitor, in patients with triple-class resistant virus. Presented at: 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL; September 2007 [abstract #H-713]. [Google Scholar]