Abstract
Prenatal stress and prenatal nutrition each have demonstrable impact on fetal development, with implications for child neurodevelopment and behavior. However, few studies have examined their joint influences despite evidence of potential interactive effects. We examined associations among prenatal stress, prenatal antioxidant intakes, and child temperament in a sociodemographically diverse pregnancy cohort (N=137 mother-child dyads). In mid-pregnancy, mothers completed an assessment of recent negative life events as a measure of prenatal stress and an assessment of prenatal diet. When the children were 30 months of age, mothers completed the Early Childhood Behavior Questionnaire-Very Short form, which provides scores on child Negative Affectivity, Effortful Control, and Surgency/Extraversion. Linear regressions tested associations between maternal prenatal negative life events and child temperament and effect modification by maternal prenatal antioxidant intakes (vitamins A, C, and E, magnesium, zinc, selenium, beta-carotene). Analyses revealed that increased maternal prenatal negative life events were associated with higher child Negative Affectivity (β=0.08, P=0.009) but not with child Effortful Control (β= −0.03, P=0.39) or Surgency/Extraversion (β=0.04, P=0.14). Prenatal intakes of zinc and selenium modified this effect: Maternal exposure to prenatal negative life events was associated with higher child Negative Affectivity in the presence of lower intakes of zinc and selenium. Modification effects approached significance for vitamins A and C. The results suggest that the combination of elevated stress exposures and lower antioxidant intakes in pregnancy increases the likelihood of heightened child temperamental negative affectivity. Increased antioxidant intakes during pregnancy may protect against influences of prenatal stress on child temperament.
Keywords: prenatal programming, prenatal stress, diet, nutrition, temperament Associations among Prenatal Stress, Maternal Antioxidant Intakes in Pregnancy, Child Temperament at Age 30 Months
Introduction
Research indicates that childhood temperament has significant long-term consequences for development, including influencing later personality and social development as well as risk for psychopathology.1 Thus, explicating modifiable factors that shape child temperament may contribute to the development of intervention strategies that have long-term impact. An extensive literature indicates that the in utero environment can dramatically influence fetal development, with implications for neurodevelopment across the lifespan.2 Maternal psychosocial stress in pregnancy has been identified as a robust programmer of offspring neurobehavioral outcomes, including temperament.3–7
Studies suggest three overarching temperament domains, including negative affectivity (e.g., fear, frustration, sadness); regulation (“orienting/regulation” in infancy, e.g., attentional persistence, soothability; “effortful control” in childhood, e.g., attentional and inhibitory control); and surgency/extraversion (e.g., high activity, impulsivity, positive affectivity).1 Prenatal stress has most frequently been associated with child negative affectivity, including increased distress in response to novelty, poor recovery from distress, fearfulness, and difficult temperament.8–11 Studies have also linked prenatal stress and anxiety to poorer behavioral and attentional regulation in infants and toddlers.8,12–14 Relatedly, there is evidence that prenatal stress negatively impacts child cognitive development,12,14–16 which has implications for the temperament domains of orienting/regulation and effortful control, given their dependence on attentional capacities. Very limited data suggest that prenatal stress may be associated with increased surgency/extraversion.11 Notably, the majority of studies examining links between prenatal stress exposures and offspring temperament have focused on infant outcomes; more research is needed to explore potential effects on temperament later in development.
The mechanisms via which prenatal stress influences child temperament are as yet not well explicated and likely complex and varied. One potential mechanism that has received little attention is oxidative stress. Oxidative stress results from increased production of reactive oxygen species, decreased antioxidant defense, and/or failure to repair oxidative damage, potentially leading to cell damage or death.17,18 Animal studies link prenatal stress to oxidative damage and decreased activity of antioxidant enzymes.19,20 In humans, psychological stress is associated with increased oxidative stress and reduced antioxidant activity in plasma.21 The fetus may be particularly vulnerable to oxidative stress due to immature antioxidant defenses and differing detoxification capacity at the level of the placenta that results in increased oxidative stress in fetal tissues.22,23 Oxidative stress, in turn, has been found to damage neural precursors, impair neurogenesis, and contribute to neuronal degeneration in the central nervous system17,19,24,25 and has been implicated in the etiology of numerous brain conditions (e.g., mild cognitive impairment, psychiatric disorders).17,19,24–28 Notably, research suggests that dietary intakes of micronutrients that have antioxidant properties may reduce environmentally induced oxidative stress/inflammation in the placenta and developing fetal brain.29–31 Together, these data suggest that maternal antioxidant intakes in pregnancy may mitigate the effects of psychosocial stress on neurodevelopment.
There are additional reasons for considering the joint effects of prenatal stress and prenatal nutrition on child neurodevelopment. Increased exposure to psychosocial stress and inadequate nutrient intakes have been found to co-occur in women of childbearing age and during pregnancy.15,32,33 For example, in a study of pregnant women, participants who reported higher levels of anxiety and stress had decreased folate intake despite overall increased caloric intake compared to those with lower levels of stress.34 A population-based study of pregnant women found high levels of both stress and food insecurity (defined as having to cut or skip a meal, eat less than considered adequate, or go hungry) in women of low socioeconomic status.35
Although prenatal stress and poor nutrition correlate and have each been linked to neurodevelopmental outcomes, to date they have largely been examined separately.15 Our group was the first to provide evidence that maternal nutrition during pregnancy may modify prenatal stress effects on infant temperament.7 Specifically, within the same cohort described in the current study, we found that prenatal dietary intakes of polyunsaturated fatty acids partially attenuated the association between increased maternal prenatal stress and poorer orienting/regulation in 6-month-olds.7 Demonstrating that such associations persist into later childhood will strengthen evidence of joint prenatal stress and nutrition effects on temperament. Moreover, because maternal prenatal stress and nutrition are both potentially modifiable risk factors, an understanding of their independent and synergistic effects will inform intervention development. Such findings may be particularly instructive for developing interventions to reduce health disparities, given that lower-income populations are more likely to experience stress and suboptimal dietary intakes in pregnancy.36–38 Notably, earlier work from our group demonstrated that women with low education and self-reported economic-related food insecurity were at increased risk for multiple antioxidant deficiencies.36
The objectives of this study were to examine associations among maternal prenatal stress, maternal antioxidant intakes during pregnancy, and child temperament. We leveraged a sociodemographically diverse longitudinal pregnancy cohort with data on maternal exposures to negative life events during pregnancy, prenatal micronutrient intakes, and child temperament assessed at age 30 months. Based on the extant literature, we hypothesized that increased prenatal stress and lower prenatal antioxidant intakes (vitamins A, C, and E, magnesium, zinc, selenium, beta-carotene) would each be associated with greater child negative affectivity and surgency/extraversion and diminished child effortful control. We further hypothesized that greater prenatal antioxidant intakes would attenuate associations between maternal prenatal stress and child temperament. We specifically hypothesized that zinc, selenium, and vitamin A would have particular impact, given that these antioxidants have been identified as influencing neuronal cell growth and development during the late fetal and neonatal time period.39
Method
Participants
Participants were mothers and their 30-month-old infants enrolled in the PRogramming of Intergenerational Stress Mechanisms (PRISM) study, a prospective pregnancy cohort designed to examine the potential roles of maternal and child stress exposures on child development. Between March 2011 and December 2013, pregnant women were recruited from prenatal clinics in a Boston area hospital and a community health center. Recruitment sites were chosen given desired heterogeneity in sociodemographic characteristics. Eligibility criteria included: (a) English- or Spanish-speaking; (b) age ≥ 18 years at enrollment; and (c) single gestation pregnancy. Exclusion criteria included (a) maternal endorsement of drinking ≥ 7 alcoholic drinks/week at any point during pregnancy, as usage above these thresholds has been associated with increased risk for numerous health and developmental problems40,41 and (b) maternal or child chronic health conditions that would impede study participation. Approximately 70% of eligible women approached agreed to participate and continued follow-up after delivery (N = 311). Based on screening data, there were no significant differences in race/ethnicity, education, or income between women who enrolled and those who declined.
The current study included PRISM mother-child dyads who provided data on maternal prenatal stress exposures and child temperament when the child was approximately 30 months of age. Due to truncation of funding supporting neurodevelopmental assessments, a subsample of the PRISM cohort (N = 137 dyads) provided data for the current analyses. Dyads who provided data for the current analyses did not differ from those who did not on prenatal stress exposures, smoking during pregnancy, or child sex, Ps > 0.15. Mothers who provided data for the current analyses were older, P = 0.02, more likely to be White, P < 0.001, and more likely to have greater than a high school education, P < 0.001. Compared to mothers who did not provide data for the current study, mothers who provided data had higher intakes of vitamin E, magnesium, selenium, and beta-carotene, Ps < .05, but did not differ on intakes of vitamins A or C or zinc, Ps > .05.
Measures
Prenatal stress.
Maternal prenatal stress during pregnancy was assessed using the Crisis in Family Systems-Revised (CRISYS-R) survey, which inquires about recent exposure to negative life events.42 The CRISYS-R is suitable for sociodemographically diverse populations, has good test/retest reliability, has been validated in English and Spanish in samples of parents, and has been utilized in several studies as a measure of prenatal stress.43–48 The survey encompasses 11 domains (financial, legal, career, stability in relationships, medical issues pertaining to self, medical issues pertaining to others, safety in the community, safety in the home, housing problems, difficulty with authority, discrimination), with multiple items assessing each domain. Participants indicated whether each event had occurred in the previous 6 months and rated endorsed items as positive, negative, or neutral. Research suggests increased vulnerability when exposed to negative events across multiple domains.49 Thus, the number of domains with one or more negative events endorsed was summed to create a Negative Life Events domain score (NLE; possible range 0–11), as done in prior research.45,50 Higher scores indicate greater stress exposure during pregnancy. Because participants completed the CRISYS-R, on average, during the beginning of the 3rd trimester (see Procedure, below), the measure assessed stress exposures from the beginning of pregnancy.
Antioxidant intakes.
Maternal dietary and supplemental intakes were assessed using the modified Block98 Food Frequency Questionnaire (FFQ).51,52 The FFQ food list was based on the National Health and Nutrition Examination Survey III (NHANES) dietary recall data and was modified for the current study to include a more extensive list of fish and seafood items.53 The measure has been validated in multicultural populations, including women, and can be administered in English or Spanish. 52,54 For each food/beverage item, participants were asked how often (daily, weekly, monthly, or rarely/never) and how much (small, medium, or large serving, with portion size pictures provided) they consumed. The FFQ also inquired about the type and frequency of vitamins, minerals, and other dietary supplements used in the prenatal period.
Details of the dietary assessment and processing of the FFQ data in this cohort have been previously described.36 Briefly, FFQ data were processed through the Block Dietary Data System to calculate daily energy, macronutrient, and micronutrient intakes using the NHANES and the United States Department of Agriculture (USDA) Nutrient Database for Standard Reference.51,55,56 Micronutrient data included estimated antioxidant intakes, which were validated within a subset (n = 42) of the cohort using 24-hour dietary recalls,57 considered the gold standard for validation studies.58,59 The specific antioxidant intakes estimated included vitamins A, C, and E and magnesium, zinc, selenium, and beta-carotene.57 Dietary antioxidant intake values were energy-adjusted using the residual method, as previously described.60 Supplemental intakes were then added to the energy-adjusted dietary intakes to provide total energy-adjusted intakes of each micronutrient, which were used in the analyses.
Child temperament.
Child temperament was assessed using the Early Childhood Behavior Questionnaire-Very Short form (ECBQ-VS).61,62 The 36-item ECBQ-VS has demonstrated reliability and validity62 and is available in English and Spanish. The ECBQ-VS was administered to mothers as an interview. Mothers rated the frequency that their child engaged in specific day-to-day behaviors in the prior two weeks using a 7-point scale, with responses ranging from 1 (never) to 7 (always). Scores were summed across items according to ECBQ-VS scoring criteria to create three scales assessing different behavioral domains: Negative Affectivity, Effortful Control, and Surgency/Extraversion. Negative Affectivity assesses the tendency to express negative affect, including fear, sadness, irritability, and anger. Effortful Control assesses attentional abilities (attentional flexibility and control), inhibitory control, and engagement in positive low-intensity activities (e.g., cuddling). Surgency/Extraversion assesses tendencies toward high activity level, impulsivity, excitement/pleasure, and engagement with others.61 These three scales conform to theory regarding the structure of temperament in younger and older children, adolescents, and adults63 and are consistent with the factor structure found in prior analyses of the standard 201-item ECBQ.62,64 For each scale, higher scores indicate greater levels of that temperamental trait.
Sociodemographic covariates.
Mothers self-reported their age, race/ethnicity, and highest level of education. Race/ethnicity was categorized into White, Black/Haitian, Hispanic, and other; the majority categorized as “other” self-identified as Asian or multi-racial. Education was dichotomized into “completion of high school education or less” or “greater than high school education.” Child sex and child age at the ECBQ-VS assessment were also considered in analyses.
Procedure
Maternal sociodemographics were assessed shortly following recruitment (M = 26.9 weeks gestation, SD = 8.1 weeks gestation). Within two weeks of enrollment, trained research assistants administered the CRISYS-R, and mothers completed the FFQ. When the children were approximately 30 months of age (M = 31.8 months, SD = 1.8 months), mothers completed the ECBQ-VS. Study procedures were approved by the relevant institutions’ human studies ethics committees. Mothers provided written informed consent and were administered questionnaires in their preferred language.
Data Analytic Plan
Data analyses proceeded in several steps. First, descriptive statistics were calculated. Next, linear regression models tested the association between prenatal stress exposures (i.e., continuous NLE score from CRISYS-R) and the child ECBQ-VS scores (Negative Affectivity, Effortful Control, Surgency/Extraversion). Each ECBQ-VS score was modeled separately. Adjusted models controlled for child sex and age at the time of the ECBQ-VS administration as well as maternal race/ethnicity, education, and age, as these sociodemographic factors have been linked to maternal stress and child behavioral outcomes.54,65 Next, linear regression models tested the association between each prenatal antioxidant intake and child ECBQ-VS scores. Because the distributions of intakes were skewed for some of the individual antioxidants, the individual antioxidant intake scores were dichotomized into high versus low intake, based on the median split for each antioxidant. The interactions between prenatal stress exposures and antioxidant intake levels as predictors of ECBQ-VS scores were then examined by formal tests of interaction by adding the product term prenatal stress (continuous NLE score) x antioxidant intake level (dichotomized) to the models. The interactions were modeled separately for each antioxidant (vitamins A, C, E and magnesium, zinc, selenium, beta-carotene). The interactions were tested only for temperament outcomes that were significantly associated with prenatal stress exposures in the linear regression models.
Results
Descriptive Data
Table 1 details sample characteristics and descriptive statistics for the main study variables. The sample was racially/ethnically diverse, with 47% of mothers self-reporting their race/ethnicity as White, 28% as Black/Haitian, 15% as Hispanic, and 10% as other. The rates of child prematurity (< 37 weeks = 5.1%) and low birthweight (< 2500 grams = 3.6%) were very low.
Table 1.
Sample characteristics and distributions of main study variables (N = 137)
| n | % | M/MDa | SD/IQRb | |
|---|---|---|---|---|
| Maternal education: ≤ High school diploma | 16 | 12 | ||
| Maternal race/ethnicity | ||||
| White | 65 | 47 | ||
| Black/Haitian | 38 | 28 | ||
| Hispanic | 21 | 15 | ||
| Otherc | 13 | 10 | ||
| Maternal age at pregnancy (years) | 32.10 | 4.95 | ||
| Prenatal smoking | 25 | 18 | ||
| Child sex (male) | 78 | 57 | ||
| Child age at assessment (months) | 31.84 | 1.77 | ||
| Child birthweight (grams) | 3362 | 500 | ||
| Child gestational age (weeks) | 39.29 | 1.43 | ||
| Prenatal stress exposures: CRISYS-R: NLEd | 2.15 | 1.97 | ||
| Prenatal antioxidant intakese | ||||
| Vitamin A (μg/day) | 1759.65 | 1551 | ||
| Vitamin C (mg/day) | 194.58 | 107.74 | ||
| Vitamin E (mg/day) | 13.53 | 8.08 | ||
| Magnesium (mg/day) | 350.73 | 129.31 | ||
| Zing (mg/day) | 16.39 | 11.16 | ||
| Selenium (μg/day) | 120.52 | 59.10 | ||
| Beta-carotene (μg/day) | 5286.92 | 4142 | ||
| Child temperament scales (ECBQ-VS)f | ||||
| Negative Affectivity | 3.00 | 0.71 | ||
| Effortful Control | 4.95 | 0.69 | ||
| Surgency/Extraversion | 5.71 | 0.62 |
Mean (M) data presented for maternal and child age, child birthweight and gestational age, prenatal stress exposures, and child temperament scale scores. Median (MD) data presented for prenatal antioxidant intakes due to non-normality of distribution of data.
Standard deviation (SD) data presented for maternal and child age, child birthweight and gestational age, prenatal stress exposures, and child temperament scale scores. Interquartile range (IQR) presented for prenatal antioxidant intakes due to non-normality of distribution of data.
The majority of mothers categorized as “other” race/ethnicity self-identified as Asian or multi-racial.
Prenatal stress exposures assessed via the Negative Life Events domain score (NLE) from the Crisis in Family Systems-Revised (CRISYS-R) survey.
Antioxidant intake scores available for 125 participants.
Child temperament scores derived from the Early Childhood Behavior Questionnaire-Very Short form (ECBQ-VS).
As in other studies,64 the Pearson correlation coefficients among the ECBQ-VS scale scores were small and in different directions, supporting the decision to analyze them separately: Negative Affectivity and Effortful Control r = −0.26, P = 0.002; Negative Affectivity and Surgency/Extraversion r = 0.13, P = 0.13; Surgency/Extraversion and Effortful Control r = 0.25, P = 0.003. The Spearman bivariate correlation coefficients among the individual antioxidants (continuous measures) were predominantly in the moderate range, average rs = 0.59. Prenatal stress was not significantly correlated with any of the individual antioxidant intakes, Ps ≥ 0.10.
Associations between Prenatal Stress and Child Temperament
Table 2 displays the results from the linear regression models examining associations between prenatal stress and child Negative Affectivity, Effortful Control, and Surgency/Extraversion. In an unadjusted model, higher prenatal stress was associated with increased child Negative Affectivity (β = 0.08, P = 0.009). In a model adjusted for maternal race/ethnicity, education, and age and child sex and age, the association between prenatal stress and child Negative Affectivity remained significant (β = 0.06, P = 0.05). In this model, none of the covariates reached significance. In unadjusted and adjusted models, prenatal stress was not significantly associated with Effortful Control (β = −0.03, P = 0.39 and β = −0.01, P = 0.68, respectively) or Surgency/Extraversion (β = 0.04, P = 0.14 and β = 0.02, P = 0.40, respectively).
Table 2.
Results of linear regression models testing associations between maternal prenatal stress exposures and child temperament domains
| Variable | Beta Estimate | CI Lower | CI Upper | P-value |
|---|---|---|---|---|
| Child Temperament Scalesa | ||||
| Negative Affectivity | 0.08 | 0.02 | 0.14 | 0.009 |
| Effortful Control | −0.03 | −0.09 | 0.03 | 0.39 |
| Surgency/Extraversion | 0.04 | −0.01 | 0.09 | 0.14 |
| Child Temperament Scalesb | ||||
| Negative Affectivity | 0.06 | −0.001 | 0.12 | 0.05 |
| Effortful Control | −0.01 | −0.08 | 0.05 | 0.68 |
| Surgency/Extraversion | 0.02 | −0.03 | 0.08 | 0.40 |
Prenatal stress assessed via the Negative Life Events domain score (NLE) from the Crisis in Family Systems-Revised (CRISYS-R) survey. Child temperament scale scores derived from the Early Childhood Behavior Questionnaire-Very Short form (ECBQ-VS).
Unadjusted analyses, N = 137.
Analyses adjusted for child sex and age at ECBQ-VS assessment and for maternal race/ethnicity, education, and age at study enrollment, N = 135.
Associations between Prenatal Antioxidant Intakes and Child Temperament
Table 3 displays the results from the linear regression models examining associations between the individual prenatal antioxidant intakes (high versus low) and child Negative Affectivity, Effortful Control, and Surgency/Extraversion. In unadjusted models, greater child Negative Affectivity was associated with lower prenatal intakes of vitamin A (β = −0.25, P = 0.047) and beta-carotene (β = −0.27, P = 0.03), and greater child Effortful Control was associated with higher prenatal intakes of vitamin C (β = 0.25, P = 0.03). In models adjusted for maternal race/ethnicity, education, and age and child sex and age, greater child Effortful Control continued to be associated with higher prenatal intakes of vitamin C (β = 0.26, P = 0.03), and greater child Surgency/Extraversion was associated with higher intakes of vitamin A (β = 0.23, P = 0.047), vitamin C (β = 0.22, P = 0.045), and selenium (β = 0.27, P = 0.02).
Table 3.
Results of linear regression models testing associations between maternal prenatal antioxidant intakes and child temperament domains (N = 125)
| Antioxidant Variable | Beta Estimate | CI Lower | CI Upper | P-value |
|---|---|---|---|---|
| Child Negative Affectivitya | ||||
| Vitamin A | −0.25 | −0.49 | −0.01 | 0.047 |
| Vitamin C | −0.009 | −0.25 | 0.24 | 0.94 |
| Vitamin E | −0.01 | −0.26 | 0.23 | 0.91 |
| Magnesium | 0.002 | −0.24 | 0.25 | 0.99 |
| Zinc | −0.18 | −0.42 | 0.07 | 0.15 |
| Selenium | −0.23 | −0.48 | 0.009 | 0.06 |
| Beta-carotene | −0.27 | −0.51 | −0.03 | 0.03 |
| Child Effortful Controla | ||||
| Vitamin A | 0.069 | −0.16 | 0.29 | 0.55 |
| Vitamin C | 0.25 | 0.03 | 0.47 | 0.03 |
| Vitamin E | −0.02 | −0.25 | 0.20 | 0.83 |
| Magnesium | 0.11 | −0.11 | 0.34 | 0.32 |
| Zinc | 0.01 | −0.21 | 0.24 | 0.92 |
| Selenium | 0.05 | −0.18 | 0.27 | 0.68 |
| Beta-carotene | −0.02 | −0.24 | 0.21 | 0.88 |
| Child Surgency/Extraversiona | ||||
| Vitamin A | 0.11 | −0.11 | 0.33 | 0.33 |
| Vitamin C | 0.19 | −0.03 | 0.40 | 0.09 |
| Vitamin E | −0.09 | −0.31 | 0.13 | 0.42 |
| Magnesium | 0.01 | −0.20 | 0.23 | 0.90 |
| Zinc | 0.05 | −0.16 | 0.27 | 0.63 |
| Selenium | 0.13 | −0.08 | 0.35 | 0.23 |
| Beta-carotene | −0.007 | −0.22 | 0.21 | 0.95 |
| Child Negative Affectivityb | ||||
| Vitamin A | −0.13 | −0.40 | 0.14 | 0.34 |
| Vitamin C | 0.05 | −0.19 | 0.30 | 0.66 |
| Vitamin E | 0.11 | −0.15 | 0.37 | 0.39 |
| Magnesium | 0.09 | −0.16 | 0.34 | 0.50 |
| Zinc | −0.07 | −0.34 | 0.19 | 0.58 |
| Selenium | −0.12 | −0.39 | 0.14 | 0.36 |
| Beta-carotene | −0.17 | −0.43 | 0.09 | 0.21 |
| Child Effortful Controlb | ||||
| Vitamin A | 0.06 | −0.19 | 0.31 | 0.65 |
| Vitamin C | 0.26 | 0.03 | 0.48 | 0.03 |
| Vitamin E | −0.01 | −0.25 | 0.23 | 0.93 |
| Magnesium | 0.12 | −0.12 | 0.35 | 0.33 |
| Zinc | 0.01 | −0.24 | 0.25 | 0.96 |
| Selenium | 0.06 | −0.19 | 0.31 | 0.63 |
| Beta-carotene | −0.01 | −0.25 | 0.24 | 0.96 |
| Child Surgency/Extraversionb | ||||
| Vitamin A | 0.23 | 0.01 | 0.47 | 0.047 |
| Vitamin C | 0.22 | 0.01 | 0.42 | 0.045 |
| Vitamin E | −0.04 | −0.27 | 0.18 | 0.72 |
| Magnesium | 0.06 | −0.16 | 0.28 | 0.59 |
| Zinc | 0.14 | −0.09 | 0.37 | 0.23 |
| Selenium | 0.27 | 0.04 | 0.50 | 0.02 |
| Beta-carotene | 0.10 | −0.13 | 0.33 | 0.39 |
Child temperament scale scores derived from the Early Childhood Behavior Questionnaire-Very Short form (ECBQ-VS).
Unadjusted analyses.
Analyses adjusted for child sex and age at ECBQ-VS assessment and for maternal race/ethnicity, education, and age at study enrollment.
Modification of Prenatal Stress Effects on Child Temperament by Prenatal Antioxidant Intakes
In linear regression models adjusted for maternal race/ethnicity, education, and age and child sex and age, the product term prenatal stress (continuous NLE score) x individual antioxidant intakes (high versus low) were significant in predicting child Negative Affectivity for zinc, P = 0.047, and selenium, P = 0.024, and approached significance for vitamin A, P = 0.075, and vitamin C, P = 0.059. In each instance, the association between higher prenatal stress and increased child Negative Affectivity was stronger among children of mothers with lower prenatal antioxidant intakes (Fig 1). The interaction terms were not significant for vitamin E, P = 0.85, magnesium, P = 0.69, or beta-carotene, P = 0.86, in predicting child Negative Affectivity. Effect modification was not tested for child Effortful Control or Surgency/Extraversion given the lack of main effects of prenatal stress on these temperament domains.
Figure 1.
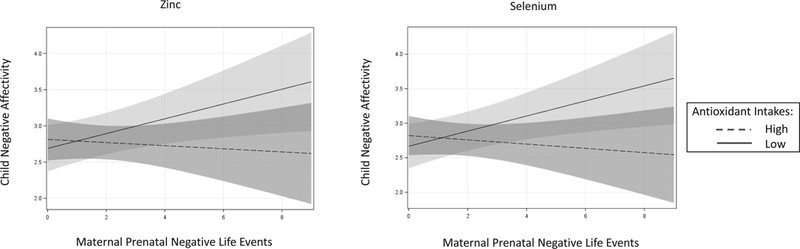
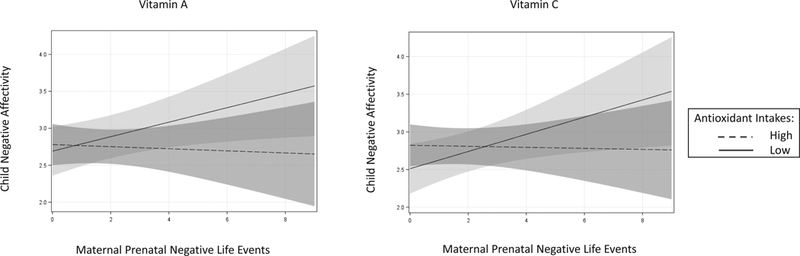
Associations between prenatal stress and child negative affectivity: Effect modification by maternal prenatal antioxidant intakes (N = 122). Prenatal stress assessed via the Crisis in Family Systems-Revised (CRISYS-R) survey. Child negative affectivity assessed via the Early Childhood Behavior Questionnaire-Very Short form (ECBQ-VS). Individual antioxidant intakes assessed via the Block98 Food Frequency Questionnaire (FFQ), modified. Figure depicts the change in child ECBQ-VS Negative Affectivity scores associated with each unit increase in prenatal Negative Life Events domain score exposures (NLE) assessed via the CRISYS-R, grouped by maternal prenatal intakes of zinc, selenium, and vitamins A and C. High and low antioxidant intake groups were determined by median split (see Table 1 for median split values for each antioxidant). Analyses adjusted for maternal race/ethnicity, education, and age and child sex and age. The formal interaction term (continuous NLE score x dichotomized antioxidant intake score) was significant for zinc, P = 0.047, and selenium, P = 0.024, and approached significance for vitamin A, P = 0.075, and vitamin C, P = 0.059. In each panel, the light shaded region represents 95% confidence intervals for predicated average child Negative Affectivity among mothers with low antioxidant intakes (solid line); the medium shaded region represents 95% confidence intervals for predicated average child Negative Affectivity among mothers with high antioxidant intakes (dashed line); and the dark shaded region represents the overlap between 95% confidence intervals. The interaction term was not significant for vitamin E, magnesium, or beta-carotene, Ps > 0.60 (not shown).
Discussion
The overall goal of this study was to examine associations among prenatal maternal stress, antioxidant intakes during pregnancy, and child temperament at age 30 months in a sociodemographically diverse longitudinal pregnancy cohort. Animal and human studies strongly suggest that prenatal stress and maternal nutrition during pregnancy influence fetal brain development and, consequently, child neurocognitive and neurobehavioral outcomes. However, little research has examined their joint effects, particularly in relation to child temperament. Data from various lines of research suggest that maternal antioxidant intakes during pregnancy may mitigate the effects of prenatal stress on child neurodevelopment.15,29–31,39 This is the first longitudinal population-based study to examine potential modification of prenatal stress effects on child temperament by maternal prenatal antioxidant intakes.
Analyses revealed that maternal exposure to negative life events during pregnancy (i.e., prenatal stress) was associated with increased child negative affectivity but not with child effortful control or surgency/extraversion. These results are consistent with the many studies that have linked maternal prenatal stress to various indictors of infant negative affectivity.8–11 Fewer studies have examined associations between prenatal stress and child effortful control, although some have documented negative associations with child attentional and behavioral regulation. The absence of an association between prenatal stress and child effortful control in our study may be attributable to differences across studies in how prenatal stress is operationalized. In the current study, prenatal stress was quantified as the number of domains (e.g., financial, relationship) in which the mother experienced a negative event during pregnancy. A wide variety of conceptualizations of prenatal stress have been used across studies, including exposure to stressful events, stress reactions (e.g., feelings of stress, anxiety, depression), and pregnancy-specific concerns. Different associations with measures of child attentional and behavioral regulation as well as neurocognitive outcomes emerge for different conceptualizations of prenatal stress.8,12,13 Studies testing associations between prenatal stress and child surgency/extraversion are lacking. Because surgency/extraversion incorporates characteristics that may be perceived as positive traits (e.g., engagement with others), as well as potential risk factors (e.g., impulsivity), high or low levels of surgency/extraversion do not necessarily indicate behavioral or mental health risk.66
More research is needed to specify the different types of stress exposures and stress reactions that influence different domains of temperament and to explicate the underlying mechanisms that contribute to these patterns of association. Data suggest that multiple pathways are involved in prenatal stress effects on fetal development (e.g., oxidative stress, programming of the fetal hypothalamic-pituitary-adrenal axis [HPAA] and autonomic nervous system, shaping of the structure and functioning of the amygdala and prefrontal cortex).12,13 Different patterns of involvement of these various neural and stress regulatory systems are hypothesized to contribute to the various temperament domains.45,64 Negative affectivity may show the most consistent associations with prenatal stress because the hypothesized underlying associated systems (e.g., HPAA, amygdala) are particularly susceptible to prenatal stress exposure effects.45
There was modest evidence that prenatal antioxidant intakes were associated with child temperament. Specifically, lower intakes of vitamin A and beta-carotene were associated with increased child negative affectivity, and higher intakes of vitamin C with greater effortful control. However, in analyses adjusted for sociodemographic factors, the associations between prenatal antioxidant intakes and child negative affectivity were no longer significant, and associations between greater Surgency/Extraversion and higher intakes of vitamin A, vitamin C, and selenium emerged. More research is needed to determine the nature of associations between prenatal dietary intakes and child temperament. Although inadequate prenatal nutrition has been identified as a risk factor for poor child neurodevelopmental outcomes,15 links specifically to temperament are limited.7 For temperament outcomes, antioxidant intakes may not have general effects but rather exert influence under specific conditions (e.g., high oxidative stress) and for temperament domains that are most susceptible to prenatal stress effects (e.g., negative affectivity).
The current study findings, in fact, suggest that intakes of certain antioxidants during pregnancy may modify the impact of prenatal stress exposures on child negative affectivity. Specifically, the association between higher prenatal stress exposures and increased child negative affectivity was stronger among children of mothers with lower prenatal intakes of zinc and selenium and tended to be stronger among children of mothers with lower prenatal intakes of vitamins A and C. No modification of prenatal stress effects on child negative affectivity were found for vitamin E, magnesium, or beta-carotene. These results are consistent with the literature indicating that certain antioxidants exert greater influence on neurodevelopment than others (Georgieff, 2007). Notably, zinc, selenium, and vitamin A have been specifically identified as nutrients with particular impact on neuronal cell growth and development during the late fetal and neonatal time period.39 An examination of the pattern of results suggests that higher intakes of these antioxidants may provide protection against the effects of prenatal stress on child negative affectivity, possibly by reducing the impact of oxidative stress on developing fetal stress regulatory systems. Further study is needed to determine if the results were driven by insufficient antioxidant intakes in the low antioxidant intake groups or by enhanced intakes among those in the high intake groups.
This study has several strengths. It is the first prospective population-based study to report associations among maternal prenatal stress, maternal micronutrient antioxidant intakes, and child temperament. Notably, the sample comprised predominantly normal birthweight children. This study is timely given that, in high-income countries such as the United States and the United Kingdom, micronutrient inadequacies appear to be emerging as dietary patterns of high fat and sugar and low nutrient density are increasingly reported.67 The diversity of the sample allows for increased generalizability of the findings, although care should be taken in applying the results to clinically at-risk populations (e.g., extreme temperament), for whom developmental processes may differ.68 The dietary exposure measure used has been validated in multicultural and periconceptional populations51,52 and has been widely used to estimate dietary intakes over long periods of time (e.g., months), including during pregnancy.69 Moreover, the measure was validated within a subset of the current cohort using an extensive four-step automated dietary recall process.57 Also, this study considered associations among prenatal stress and nutrition and temperament in childhood; the majority of studies to date in this area have focused on infancy and often on specific temperament domains (e.g., only negative affectivity). By extending our earlier findings showing that prenatal diet may mitigate prenatal stress effects on temperament from infants to toddlers, this study provides further support for the hypothesis that prenatal dietary interventions may provide some long-term protection against prenatal stress exposure effects.
This study also has limitations. The prenatal stress measure may not have captured all events that occurred during the entire pregnancy and may have covered varying periods of gestational age across participants. Current data indicate that timing of stress exposures may influence the nature of neurobehavioral effects, including temperament;10,14 our measures did not allow us to quantify stress exposure by gestational age. This measurement error would be expected to be similar in women regardless of child temperament characteristics (i.e., non-differential misclassification), resulting in an underestimation of the association between prenatal stress and child temperament. Future studies should attempt to determine if timing of stress exposures and dietary intakes during pregnancy influences the various domains of child temperament; possibly different temperamental domains are more vulnerable to diet and stress exposure effects during different stages of pregnancy.
Maternal report of child temperament offers the strength of taking advantage of the mother’s ability to observe her child’s behavior over a range of contexts; however, reliance on maternal report increases risk for diminished validity due to the potential impact of maternal psychopathology or personality on the accurate perception and reporting of child behaviors.10 The ECBQ-VS was designed to reduce the influence of such biases by inquiring about concrete child behaviors rather than asking for abstract judgments, and the measure has shown strong psychometric properties.62,64 Future studies should consider incorporating independent assessments of child temperament to more fully explicate associations among prenatal stress, nutrition, and child temperament. Future studies may also consider measuring serum micronutrient concentrations to ascertain potential differences in bioavailability due to expansion in plasma volume during gestation and other adaptations to the pregnant state. Finally, this study did not consider parental (maternal or paternal) or child postnatal stress exposures or child nutritional status, which may have influenced child temperament. Notably, data suggest that prenatal exposures exert effects on child emotional functioning independent of postnatal factors.10,70–72 Nevertheless, studies should consider whether the associations assessed here may be influenced by postnatal factors.
We controlled for a number of possible confounders, including maternal age, race/ethnicity, and education and child sex and age. A number of other potential risk factors were minimally present in this sample (e.g., prematurity, low birthweight, prenatal alcohol and drug use). Such risk factors may contribute to associations between prenatal stress and child temperament. For example, the effects of maternal prenatal stress on child temperament may be mediated, in part, by premature birth. Future research with larger samples and greater variability in perinatal health factors should explore the role of perinatal health in associations among prenatal stress and nutrition and child behavioral outcomes. Other possible confounders were not considered, including variables that may covary with maternal stress and result in increased oxidative stress to the fetus (e.g., prenatal smoking, other nutritional intakes). Including such variables may have resulted in overcorrection of the tested associations, particularly in a sample of this size. Notably, another study examining links between maternal prenatal stress and infant temperament found significant associations even after excluding or controlling for multiple potential confounders, including maternal age, income, and education, medical risk during pregnancy, mode of delivery, adverse birth outcomes, birthweight, gestational age, breastfeeding status, and child sex.14 Moreover, prenatal antioxidant intakes may reduce oxidative stress effects on infant temperament regardless of the specific source(s) of oxidative stress. Studies that include direct assessment of oxidative stress levels before and after dietary intervention may help address this question. Such studies should also consider whether the source of antioxidant intakes (food, supplements) influences these processes. Finally, mothers with relatively higher and lower prenatal antioxidant intakes may have differed in unmeasured ways that influenced the tested associations. For example, mothers with higher intakes may have had better overall nutrition, greater access to compensatory instrumental and social resources, and/or exposure to different sources of stress that differentially impacted fetal development compared to mothers with lower intakes. These hypotheses need to be tested in future studies.
In summary, the current study’s findings are in line with previous reports linking maternal prenatal stress exposures to child negative affectivity and suggest that maternal intake of select antioxidants during pregnancy may mitigate these effects. Research indicates that negative affectivity may have particular import for developmental outcomes. Measures of infant and child negative affectivity show similarity to the adult personality factor of neuroticism,1 suggesting that negative affectivity may be relatively stable across the life course. Moreover, elevated negative affectivity in early childhood appears to heighten sensitivity to environmental influences, increasing the likelihood of negative outcomes (e.g., psychopathology) under poor environmental conditions, including high stress.73–79 Thus, explicating factors that influence negative affectivity may inform intervention efforts to optimize child neurobehavioral and mental health outcomes, particularly in stress-exposed populations. The current findings support ongoing efforts to determine if optimizing maternal nutrition during pregnancy mitigates the effects of prenatal stress exposures on child developmental outcomes.
Acknowledgements
The research was supported by a grant from the National Heart, Lung & Blood Institute (R01HL095606; RJW, MBE, multi-PIs), the National Institute of Environmental Health Sciences R21ES021318 (RJW, PI), and the National Center for Advancing Translational Sciences TL1TR001434 (LL). During preparation of this manuscript, the authors were supported by TL1TR001434 (LL), K99ES024116 (KJB), R01HL095606 (RJW, MBE), R21ES021318 (SK, RJW), and the Program for Behavioral Science in the Department of Psychiatry at Boston Children’s Hospital (MBE). None of the funding agencies had any role in the study design, the collection, analysis or interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not represent the official views of any granting agency.
The authors thank Thalita Morin and Fernanda Borges de Figueiredo for their diligent assistance in processing the dietary data. The authors also thank the families whose generous donation of time made this project possible.
Footnotes
Statement of Interest
None.
References
- 1.Gartstein MA, Rothbart MK. Studying infant temperament via a revision of the Infant Behavior Questionnaire. Infant Behav Dev. 2003; 7, 517–522. [Google Scholar]
- 2.Bale TL, Baram TZ, Brown AS, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010; 68, 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knudsen EI, Heckman JJ, Cameron JL, et al. Economic, neurobiological, and behavioral perspectives on building America’s future workforce. Proc Natl Acad Sci U S A. 2006; 103, 10155–10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012; 129, e232–246. [DOI] [PubMed] [Google Scholar]
- 5.Charil A, Laplante DP, Vaillancourt C, et al. Prenatal stress and brain development. Brain Res Rev. 2010; 65, 56–79. [DOI] [PubMed] [Google Scholar]
- 6.Weiss B, Bellinger DC. Social ecology of children’s vulnerability to environmental pollutants. Environ Health Perspect. 2006; 114, 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunst K, Enlow M, Kannan S, et al. Effects of prenatal social stress and maternal dietary fatty acid ratio on infant temperament: Does race matter? Epidemiology: Open Access. 2014; 4, 1000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huizink AC, de Medina PG, Mulder EJ, et al. Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry. 2002; 41, 1078–1085. [DOI] [PubMed] [Google Scholar]
- 9.Brand SR, Engel SM, Canfield RL, et al. The effect of maternal PTSD following in utero trauma exposure on behavior and temperament in the 9-month-old infant. Ann N Y Acad Sci. 2006; 1071, 454–458. [DOI] [PubMed] [Google Scholar]
- 10.Davis EP, Glynn LM, Schetter CD, et al. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007; 46, 737–746. [DOI] [PubMed] [Google Scholar]
- 11.Lin B, Crnic KA, Luecken LJ, et al. Maternal prenatal stress and infant regulatory capacity in Mexican Americans. Infant Behav Dev. 2014; 37, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buitelaar JK, Huizink AC, Mulder EJ, et al. Prenatal stress and cognitive development and temperament in infants. Neurobiol Aging. 2003; 24 Suppl 1, S53–60; discussion S67–58. [DOI] [PubMed] [Google Scholar]
- 13.Gutteling BM, de Weerth C, Willemsen-Swinkels SH, et al. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. Eur Child Adolesc Psychiatry. 2005; 14, 41–51. [DOI] [PubMed] [Google Scholar]
- 14.Zhu P, Sun MS, Hao JH, et al. Does prenatal maternal stress impair cognitive development and alter temperament characteristics in toddlers with healthy birth outcomes? Dev Med Child Neurol. 2014; 56, 283–289. [DOI] [PubMed] [Google Scholar]
- 15.Monk C, Georgieff MK, Osterholm EA. Research review: maternal prenatal distress and poor nutrition - mutually influencing risk factors affecting infant neurocognitive development. J Child Psychol Psychiatry. 2013; 54, 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004; 75, 1085–1097. [DOI] [PubMed] [Google Scholar]
- 17.Michel TM, Frangou S, Thiemeyer D, et al. Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder—a postmortem study. Psychiatry Research. 2007; 151, 145–150. [DOI] [PubMed] [Google Scholar]
- 18.Moylan S, Jacka FN, Pasco JA, et al. How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: a critical review of biological pathways. Brain and Behavior. 2013; 3, 302–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arcego DM, Krolow R, Lampert C, et al. Stress during the pre-pubertal period leads to long-term diet-dependent changes in anxiety-like behavior and in oxidative stress parameters in male adult rats. Neurochemical Research. 2013; 38, 1791–1800. [DOI] [PubMed] [Google Scholar]
- 20.Feng Z, Zou X, Jia H, et al. Maternal docosahexaenoic acid feeding protects against impairment of learning and memory and oxidative stress in prenatally stressed rats: Possible role of neuronal mitochondria metabolism. Antioxidants & Redox Signaling. 2012; 16, 275–289. [DOI] [PubMed] [Google Scholar]
- 21.Sivonova M, Zitnanova I, Hlincikova L, et al. Oxidative stress in university students during examinations. Stress. 2004; 7, 183–188. [DOI] [PubMed] [Google Scholar]
- 22.Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med. 2010; 15, 191–195. [DOI] [PubMed] [Google Scholar]
- 23.Myllynen P, Pasanen M, Vahakangas K. The fate and effects of xenobiotics in human placenta. Expert Opin Drug Metab Toxicol. 2007; 3, 331–346. [DOI] [PubMed] [Google Scholar]
- 24.Wolkowitz OM, Epel ES, Reus VI, et al. Depression gets old fast: do stress and depression accelerate cell aging? Depression and Anxiety. 2010; 27, 327–338. [DOI] [PubMed] [Google Scholar]
- 25.McKinney BC, Oh H, Sibille E. Age-by-disease biological interactions: implications for late-life depression. Frontiers in Genetics. 2012; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kananen L, Surakka I, Pirkola S, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010; 5, e10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson AW, Jaaro-Peled H, Shahani N, et al. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc Natl Acad Sci U S A. 2013; 110, 12462–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teyssier JR, Ragot S, Chauvet-Gelinier JC, et al. Expression of oxidative stress-response genes is not activated in the prefrontal cortex of patients with depressive disorder. Psychiatry Res. 2011; 186, 244–247. [DOI] [PubMed] [Google Scholar]
- 29.Gallo C, Renzi P, Loizzo S, et al. Potential therapeutic effects of vtiamin E and C on placental oxidative stress induced by nicotine: An in vitro evidence. The Open Biochemistry Journal. 2010; 4, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelvin EA, Edwards S, Jedrychowski W, et al. Modulation of the effect of prenatal PAH exposure on PAH-DNA adducts in cord blood by plasma antioxidants. Cancer Epidemiol Biomarkers Prev. 2009; 18, 2262–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hossain M, Mazzone P, Tierney W, et al. In vitro assessment of tobacco smoke toxicity at the BBB: do antioxidant supplements have a protective role? BMC Neurosci. 2011; 12, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stringhini S, Sabia S, Shipley M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA: the journal of the American Medical Association. 2010; 303, 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouchacca J, Abbott GR, Ball K. Associations between psychological stress, eating, physical activity, sedentary behaviours and body weight among women: a longitudinal study. BMC Public Health. 2013; 13, 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurley KM, Caulfield LE, Sacco LM, et al. Psychosocial influences in dietary patterns during pregnancy. J Am Diet Assoc. 2005; 105, 963–966. [DOI] [PubMed] [Google Scholar]
- 35.Braveman P, Marchi K, Egerter S, et al. Poverty, near-poverty, and hardship around the time of pregnancy. Matern Child Health J. 2010; 14, 20–35. [DOI] [PubMed] [Google Scholar]
- 36.Brunst KJ, Wright RO, DiGioia K, et al. Racial/ethnic and sociodemographic factors associated with micronutrient intakes and inadequacies among pregnant women in an urban US population. Public Health Nutr. 2014; 17, 1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee S, Coxe S, Fennie K, et al. Stressful life event experiences of pregnant women in the United States: A latent class analysis. Womens Health Issues. 2017; 27, 83–92. [DOI] [PubMed] [Google Scholar]
- 38.Burns ER, Farr SL, Howards PP. Stressful life events experienced by women in the year before their infants’ births--United States, 2000–2010. MMWR Morb Mortal Wkly Rep. 2015; 64, 247–251. [PMC free article] [PubMed] [Google Scholar]
- 39.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007; 85, 614S–620S. [DOI] [PubMed] [Google Scholar]
- 40.Patra J, Bakker R, Irving H, et al. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG. 2011; 118, 1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Testa M, Quigley BM, Eiden RD. The effects of prenatal alcohol exposure on infant mental development: A meta-analytical review. Alcohol Alcohol. 2003; 38, 295–304. [DOI] [PubMed] [Google Scholar]
- 42.Berry C, Shalowitz M, Quinn K, et al. Validation of the Crisis in Family Systems-Revised, a contemporary measure of life stressors. Psychol Rep. 2001; 88, 713–724. [DOI] [PubMed] [Google Scholar]
- 43.Shalowitz MU, Berry CA, Rasinski KA, et al. A new measure of contemporary life stress: Development, validation, and reliability of the CRISYS. Health Serv Res. 1998; 33, 1381–1402. [PMC free article] [PubMed] [Google Scholar]
- 44.Berry CA, Quinn KA, Portillo N, et al. Reliability and validity of the Spanish Version of the Crisis in Family Systems-Revised. Psychol Rep. 2006; 98, 123–132. [DOI] [PubMed] [Google Scholar]
- 45.Bosquet Enlow M, Devick KL, Brunst KJ, et al. Maternal lifetime trauma exposure, prenatal cortisol, and infant negative affectivity. Infancy. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cowell WJ, Bellinger DC, Coull BA, et al. Associations between prenatal exposure to black carbon and memory domains in urban children: Modification by sex and prenatal stress. PLoS One. 2015; 10, e0142492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suglia SF, Staudenmayer J, Cohen S, et al. Cumulative stress and cortisol disruption among Black and Hispanic pregnant women in an urban cohort. Psychol Trauma. 2010; 2, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tse AC, Rich-Edwards JW, Koenen K, et al. Cumulative stress and maternal prenatal corticotropin-releasing hormone in an urban U.S. cohort. Psychoneuroendocrinology. 2012; 37, 970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers HF. Ethnicity- and socio-economic status-related stresses in context: an integrative review and conceptual model. J Behav Med. 2009; 32, 9–19. [DOI] [PubMed] [Google Scholar]
- 50.DiPietro JA. Maternal stress in pregnancy: considerations for fetal development. J Adolesc Health. 2012; 51, S3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Block G, Hartman AM, Dresser CM, et al. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986; 124, 453–469. [DOI] [PubMed] [Google Scholar]
- 52.Snook Parrott M, Bodnar LM, Simhan HN, et al. Maternal cereal consumption and adequacy of micronutrient intake in the periconceptional period. Public Health Nutr. 2009; 12, 1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siega-Riz AM, Bodnar LM, Savitz DA. What are pregnant women eating? Nutrient and food group differences by race. Am J Obstet Gynecol. 2002; 186, 480–486. [DOI] [PubMed] [Google Scholar]
- 54.Palmer FB, Anand KJ, Graff JC, et al. Early adversity, socioemotional development, and stress in urban 1-year-old children. J Pediatr. 2013; 163, 1733–1739 e1731. [DOI] [PubMed] [Google Scholar]
- 55.Block G Invited commentary: another perspective on food frequency questionnaires. Am J Epidemiol. 2001; 154, 1103–1104; discussion 1105–1106. [DOI] [PubMed] [Google Scholar]
- 56.Department of Agriculture. USDA National Nutrient Database for Standard Reference Beltsville, MD: Nutrient Data Laboratory; 1998. [Google Scholar]
- 57.Brunst KJ, Kannan S, Ni YM, et al. Validation of a food frequency questionnaire for estimating micronutrient intakes in an urban US sample of multi-ethnic pregnant women. Matern Child Health J. 2016; 20, 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dwyer J, Picciano MF, Raiten DJ. Future directions for the integrated CSFII-NHANES: What We Eat in America-NHANES. J Nutr. 2003; 133, 576S–581S. [DOI] [PubMed] [Google Scholar]
- 59.Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008; 88, 324–332. [DOI] [PubMed] [Google Scholar]
- 60.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997; 65, 1220S–1228S; discussion 1229S-1231S. [DOI] [PubMed] [Google Scholar]
- 61.Putnam SP, Rothbart MK. Development of Short and Very Short forms of the Children’s Behavior Questionnaire. J Pers Assess. 2006; 87, 103–113. [DOI] [PubMed] [Google Scholar]
- 62.Putnam SP, Jacobs J, Gartstein MA, et al. Development and assessment of short and very short forms of the Early Childhood Behavior Questionnaire. Paper presented at the International Conference on Infant Studies, 2010; Baltimore, MD. [Google Scholar]
- 63.Putnam S, Ellis LK, Rothbart MK. The structure of temperament from infancy through adolescence In Advances/Proceedings in Research on Temperament (eds. Eliasz A, Angleitner A), 2001; pp. 165–182. Germany: Pabst Scientist. [Google Scholar]
- 64.Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: The Early Childhood Behavior Questionnaire. Infant Behav Dev. 2006; 29, 386–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nomaguchi K, House AN. Racial-ethnic disparities in maternal parenting stress: the role of structural disadvantages and parenting values. Journal of health and social behavior. 2013; 54, 386–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hovens JG, Giltay EJ, van Hemert AM, et al. Childhood maltreatment and the course of depressive and anxiety disorders: The contribution of personality characteristics. Depress Anxiety. 2016; 33, 27–34. [DOI] [PubMed] [Google Scholar]
- 67.Parisi F, Laoreti A, Cetin I. Multiple micronutrient needs in pregnancy in industrialized countries. Ann Nutr Metab. 2014; 65, 13–21. [DOI] [PubMed] [Google Scholar]
- 68.Perez-Edgar K, Schmidt LA, Henderson HA, et al. Salivary cortisol levels and infant temperament shape developmental trajectories in boys at risk for behavioral maladjustment. Psychoneuroendocrinology. 2008; 33, 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blumfield ML, Hure AJ, Macdonald-Wicks L, et al. A systematic review and meta-analysis of micronutrient intakes during pregnancy in developed countries. Nutr Rev. 2013; 71, 118–132. [DOI] [PubMed] [Google Scholar]
- 70.Blair MM, Glynn LM, Sandman CA, et al. Prenatal maternal anxiety and early childhood temperament. Stress. 2011; 14, 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davis EP. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004; 6, 319–331. [Google Scholar]
- 72.Davis EP, Glynn LM, Dunkel Schetter C, et al. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci. 2005; 27, 299–305. [DOI] [PubMed] [Google Scholar]
- 73.Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009; 135, 885–908. [DOI] [PubMed] [Google Scholar]
- 74.Belsky J, Hsieh KH, Crnic K. Mothering, fathering, and infant negativity as antecedents of boys’ externalizing problems and inhibition at age 3 years: differential susceptibility to rearing experience? Dev Psychopathol. 1998; 10, 301–319. [DOI] [PubMed] [Google Scholar]
- 75.Feldman R, Greenbaum CW, Yirmiya N. Mother-infant affect synchrony as an antecedent of the emergence of self-control. Dev Psychol. 1999; 35, 223–231. [DOI] [PubMed] [Google Scholar]
- 76.Kochanska G Toward a synthesis of parental socialization and child temperament in early development of conscience. Child Development. 1993; 64, 325–347. [Google Scholar]
- 77.Kochanska G, Aksan N, Joy ME. Children’s fearfulness as a moderator of parenting in early socialization: Two longitudinal studies. Dev Psychol. 2007; 43, 222–237. [DOI] [PubMed] [Google Scholar]
- 78.Pluess M, Belsky J. Differential susceptibility to rearing experience: the case of childcare. J Child Psychol Psychiatry. 2009; 50, 396–404. [DOI] [PubMed] [Google Scholar]
- 79.van Aken C, Junger M, Verhoeven M, et al. The interactive effects of temperament and maternal parenting on toddlers’ externalizing behaviours. Infant and Child Development. 2007; 16, 553–572. [Google Scholar]


