Abstract
Introduction
Prostate Brachytherapy (PB) has well-documented excellent long-term outcomes in all risk groups. There are significant uncertainties regarding the role of Androgen Deprivation Therapy (ADT) with brachytherapy. The purpose of this report is to review systemically the published literature and summarize present knowledge regarding the impact of ADT on Biochemical Progression Free Survival (bPFS), Cause Specific Survival (CSS) and Overall Survival (OS).
Material and Methods
A literature search was conducted in Medline and Embase covering the years 1996-2016. Selected were articles with >100 patients, minimum follow-up 3 years, defined risk stratification and directly examining the role and impact of ADT on bPFS, CSS and OS. The studies were grouped to reflect disease risk stratification. We also reviewed the impact of ADT on OS, cardiovascular morbidity, mortality, and ongoing brachytherapy Randomized Controlled Trials (RCTs).
Results
52 selected studies (43,303 patients) were included in this review; 7 HDR (High Dose Rate), and 45 LDR (Low Dose Rate). Twenty-five studies were multi-institutional and 27 single institution, (retrospective review or prospective data collection) and two were RCTs. The studies were heterogeneous in patient population, risk categories, risk factors, follow-up time, and treatment administered, including ADT administration and duration (median 3-12 months).
Seventy one percent of the studies reported a lack of benefit, while 28% show improvement in bPFS with addition of ADT to PB. The lack of benefit was seen in LR and favourable IR disease, as well as the majority of HDR studies. A bPFS benefit of up to 15% was seen with ADT use in: patients with suboptimal dosimetry, those with multiple adverse risk factors (unfavourable IR) and most HR studies. Four studies reported very small benefit to CSS (2%). None of the studies showed OS advantage, however 3 studies reported an absolute 5-20% OS detriment with ADT. Literature suggests OS detriment is more likely in older patients or those with pre-existing cardiovascular disease (CVD). Four RCTs with an adequate number of patients and well defined risk stratification are in progress. One RTC will answer the question regarding the role of ADT with PB in favourable IR patients, and the other 3 RTCs will focus on optimal duration of ADT in the unfavourable IR and favourable HR population.
Conclusions
Patients treated with brachytherapy have excellent long-term disease outcomes. Existing evidence shows no benefit of adding ADT to PB in LR and favourable IR patients. Unfavourable IR, HR patients and those with suboptimal dosimetry may have up to 15% improvement in bPFS with addition of 3-12 months of ADT, with uncertain impact on CSS and a potential detriment on OS. In order to minimize morbidity one should exercise caution in prescribing ADT together with PB, in particular to older men and those with existing CVD. Due to the retrospective nature of this evidence, significant selection and treatment bias, no definitive conclusions are possible. RCT is urgently needed to define the potential role and optimal duration of ADT in unfavourable IR and favourable HR disease.
Keywords: Prostate Cancer, Brachytherapy, Androgen Deprivation Therapy, Outcomes, bPFS, CSS, OS
Graphical abstract
Introduction
Having emerged in the dawn of the PSA era, Prostate Brachytherapy (PB) has gained worldwide acceptance and is currently considered a standard treatment for organ confined prostate cancer. Excellent long-term results have been published for all risk groups (1). Despite a large body of retrospective and prospective single or multi-institutional data, significant uncertainties remain regarding the role of Androgen Deprivation Therapy (ADT), external beam radiation (EBRT) or both, in patients treated with prostate brachytherapy (PB) both with Low Dose Rate (LDR) and High Dose Rate (HDR), particularly for Intermediate-Risk (IR) and High-Risk (HR) Prostate Cancer (PCa). Data from prospective randomized control trials will not be available for several years.
The purpose of this article is to review the published literature systematically, and to summarize present knowledge regarding the role of ADT with PB. Clinical trials will be reviewed and future directions for research outlined. The mechanism of interaction between ADT and radiation, adverse effects, and impact on cardiovascular morbidity, mortality, and overall survival (OS) will be described. We separately considered the effects of ADT on biochemical Progression Free Survival, (bPFS), Cause Specific Survival (CSS), and Overall Survival (OS) in Low-Risk (LR) intermediate (IR) and high-risk (HR) risk group stratification. We considered both LDR and HDR retrospective institutional and multi-institutional studies; reviewed the limited data on this subject available from randomized controlled trials (RTCs), and reviewed on-going RTCs. We summarize the current available clinical evidence regarding the use of ADT with PB and provided recommendations regarding its use.
Material and Methods
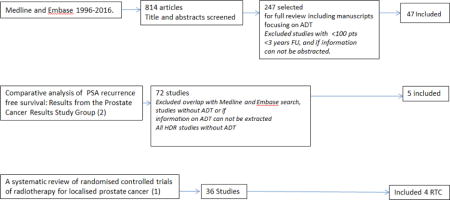
A literature search was conducted in Medline and Embase covering the years 1996-2016. We searched articles on Androgen Deprivation Therapy searching under the subject heading “androgen deprivation therapy” in Embase and searching the titles of articles in Medline for the words “androgen” and “depriv*. 814 articles were identified; those directly focused on toxicity, or the use of ADT and PB were reviewed in great detail (n=247). Outcome articles were cross-referenced with the systematic outcome analysis (1) and the systematic review of randomized trials in prostate cancer (2). Fifty-two were selected for this review, all with >100 patients, with clearly defined risk stratification and directly examining the role and impact of ADT on primarily bPFS, in addition to CSS and OS where available. Excluded were those with follow up of <3 years, those where no ADT was given, or where data required could not be extracted (for example, studies where results between PB and EBRT alone were compared, but effect of ADT on clinical outcomes was assessed together for PB, and non-PB cohorts)(Graph 1). Factors predictive of bPFS, CSS and OS were extracted from multivariable MVA analysis in 50 out of 52 articles, and are included in the tables.
ABS, ACR, ASTRO, ESTRO/EUE/EORTC and NCCN recommendations regarding use of ADT with PB
Most of the above best practice guideline recommendations underline the controversy regarding use of ADT and PB, and do not give firm recommendations apart from recommending ADT for downsizing. For example ABS recommends no ADT in LR, its use in IR is optional and more strongly recommended in HR (3). ABS recommendations for HDR do not refer to use of ADT with HDR, apart from recommending ADT for downsizing (4). ACR similarly states that the use of ADT is “usually not appropriate” for LR disease, “may be appropriate” for IR disease and is “usually appropriate” for HR disease (5). 2016 NCCN guidelines do not recommend ADT for IR treated with PB. For HR disease, ADT “may or may not be used” together with EBRT and PB boost and duration is specified between 0-36 months (6). ESTRO/EUE/EORTC (7), GEC/ESTRO-EUE (8) and ASTRO (9) have no specific recommendation or mention the use of ADT with PB.
Androgen Deprivation Therapy in Prostate Cancer
In 1940, Canadian-born Charles Huggins recognized the androgen dependence of prostate cancer. In 1966, he was awarded the Nobel Prize for medicine for his “discoveries concerning hormonal treatment of prostate cancer”. (http://www.nobelprize.org/nobel_prizes/medicine/laureates/1966/). This discovery revolutionized the treatment of metastatic prostate cancer (10,11). In 1997, Zietman et al. published another landmark observation that revolutionized treatment of localized prostate cancer (12). The combination of radiation with orchiectomy for Shionogi tumours treated in-vivo resulted in a significant increase in control. In addition, orchiectomy 1-12 days before radiation increased radiation effectiveness, suggesting that not only the combination but also the timing was crucial to maximize treatment effect. Two decades later, several large national and international RCTs confirmed and quantified the therapeutic benefit of ADT in combination with EBRT (2).
When combined with EBRT or brachytherapy, ADT improves the geometry of the prostate target by decreasing the volume juxtaposed to adjacent organs at risk. (13). There may also be a synergistic relationship between RT and the concurrent administration of ADT, producing a biological advantage. Several RTCs of ADT and EBRT have reported improvement in not only bPFS and local control, but also in DSS, and OS (2). In order to produce the above-mentioned clinical benefits, ADT must have a biological effect on both local and systemic disease. Clinical evidence supports the hypothesis that ADT can eliminate subclinical micro-metastasis (14).
Interaction between ADT and radiation
Basic clinical research provides evidence of the profound effect of ADT on the local tumour microenvironment. ADT induces apoptosis in normal epithelial cells through p53 expression and inhibition of bcl-2 and inhibition of cell proliferation and repopulation in tumor cells (15). Prostate cancer is often hypoxic and this drives endothelial growth factor (VEGF) expression, which in turn stimulates angiogenesis (16,17). Neo-vasculature is structurally disorganized, highly permeable and leads to interstitial hypertension and insufficient delivery of nutrients and oxygen. ADT inhibits both endothelial growth factor (VEGF) expression and angiogenesis (18). New discovery suggests that androgen receptor(AR) regulates a transcriptional program of DNA repair genes, and with that, AR promotes prostate cancer radio resistance, adding yet another potential mechanism by which ADT increase radio -sensitivity, by deactivating AR and with that DNA repair mechanism, in an experimental setting (19)”.
Therefore, if given prior to EBRT in experimental setting, anti-angiogenesis effect may “normalize” the vasculature and lead to better tissue perfusion, increase in oxygenation, radiation tumour sensitivity, and ultimately increasing local control. Reducing local failure may consequently reduce second wave metastatic spread and thus improve OS (20).
Brachytherapy increases local control by delivering a higher radiation dose. Studies of metabolic activity using MRI and MRSI (magnetic resonance spectroscopic imaging) showed significantly higher complete prostate metabolic atrophy and lower nadir PSA at 48 months after PB vs. EBRT(21). This higher intra-prostatic tumour control is indicative of a positive therapeutic effect of the higher biological dose given with PB vs. EBRT. This observation is supported by clinical results from 3 RCTs of dose escalation using EBRT vs. EBRT and PB (22,23)(24). All 3 RCTs showed significantly higher bPFS with use of PB in addition to EBRT vs. EBRT alone. Therefore the benefits of ADT reported even with dose-escalated EBRT (78-81 Gy) may be due to compensation for suboptimal radiation dose and less effective local therapy. Due to very high intra prostatic dose and excellent disease control, ADT is likely to have less biological effect with PB, except perhaps in cases with very high volume local disease, or through spatial cooperation for suppression of micrometastatic disease (25,26). Addition of ADT to LDR-PB in Intermediate Risk (IR) and High Risk (HR) patients has been shown to significantly decrease 2 year post PB positive biopsy rate from 14% to 3.5% (p=0.002) (27). While it is unclear whether the difference seen would have translated in to difference in PSA outcomes with further follow up (due to testosterone recovery in ADT arm and presence of indeterminate biopsies) the results are intriguing. Taken all together, these somewhat contradictory observations suggest possible benefits of ADT even with high doses of radiation.
EBRT, Dose escalation and ADT
If we disregard normal tissues tolerance for a moment, one can speculate that any truly localized cancer can be cured with radiation alone, given sufficiently high radiation dose and ensuring complete coverage of the tumour target. Therefore, increase in radiation dose should in fact increase the tumour eradication and cure. Five dose escalation RCTs have so far shown improved bPFS of average 15% at 5-10 years with dose increase from 65-78Gy (28). No CSS or OS benefit was observed, in part due to a variety of factors including underpowered studies, the long natural history of prostate cancer, improved treatment of metastatic disease, competing causes of death, and the fact that any effect on OS may be very small or even non-existent (29).
EBRT, ADT and improved OS in IR and HR PCa
With addition of ADT to EBRT, RCTs have shown benefit in improving OS, CSS and bPFS in HR (RTOG 85-31, RTOG 86-10, EORTC 22863, TROG 96-01, RTOG 92-02, RTOG 94-08, Harvard/DFCI, EORTC 22961)(2,29) and IR (RTOG 94-08, Harvard/DFCI 95-096 (2,30) (31)for a duration of 4-36 months, using conventional doses of radiation. A recently published Spanish RCT showed that even in setting the dose escalation to 78 Gy, 24 vs. 4 months of ADT improves bPFS, metastatic free survival (MFS) and OS in patients with intermediate and high risk disease (32). Hence, it is clear that ADT has an additive effect on improving disease outcomes with EBRT even to high doses of 78 (32) and 81 Gy (33). Despite toxicity concerns, patients who get ADT live longer, and therefore should be treated with ADT, with exception of perhaps those with significant cardiac history. The optimal ADT duration with EBRT for each risk category has not been established.
Dose Escalation with Brachytherapy
Brachytherapy for any disease site is considered as the ultimate dose escalation modality, with clearly documented OS benefit in cervical cancer over EBRT alone (34). Randomized trials in prostate cancer comparing EBRT (78Gy) with EBRT and brachytherapy boost in high and high tier-intermediate risk prostate cancer indicate further improvement of PSA RFS (20-30% at 7-10years)(22,23)(24), with no documented CSS or OS benefit. Recent publications using large national databases indicate an increase in CSS (35) and OS (36) in prostate cancer patients treated with any form of brachytherapy. Brachytherapy results in superior disease outcomes, particularly bPFS (24)(22,23,35,36) higher complete prostate metabolic atrophy, and lower nadir PSA(21). For these reasons, addition of ADT to either brachytherapy monotherapy or a boost, may have less impact on outcomes than when ADT is combined with EBRT.
Side Effects of Androgen Deprivation Therapy
The use of even short term ADT has deleterious effects to QOL (37,38) and may increase morbidity and mortality(39) (40). Initially recognized and well-documented side effects of ADT include sexual dysfunction, loss of libido, and hot flashes, fatigue, anemia and decreased muscle mass. Cognitive dysfunction and depression have also been documented (41) where up to 27% of patients on ADT may suffer psychiatric illness during their treatment (42). As experience grew, the more ominous systemic and metabolic effects were documented (43). There is an increased risk of osteoporosis with 23% increase in incidence of fractures. The incidence of metabolic syndrome is 50% for men with ADT vs. 20% in general population, even with one year of ADT. Central and peripheral obesity is common with 9-11% increase in fat mass after 1 year of ADT (44), total cholesterol is elevated by 9%, triglycerides by 27% and HDL decreased by 11% after only 3 months of ADT (40,44–46). In addition, ADT is documented to elevate blood pressure, elevate fasting glucose and fasting insulin by 26%, decrease insulin sensitivity by 13% and increase diabetes by 44%(40,42,47). All of these changes act to increase the risk of cardiovascular events 12 – 60 months after starting ADT (24 vs. 18% P <0.001) (48) and sudden cardiac death, by adjusted HR of 1.16 (p<.004) (40). Several studies have documented a decrease in OS in patients with localized prostate cancer treated with ADT and brachytherapy (39)(49,50). Therefore, even with short duration of only 3 months ADT can negatively impacts on quality of life, and increase morbidity and mortality (48).
ADT, Cardiovascular Morbidity, Mortality and OS
The cardiovascular morbidity and excess mortality (3.5-6%) has been reported in observational studies (40,48,51,52), but not confirmed in RCTs that used ADT (37,53,54). This discrepancy between randomized and non-randomized data may be due to several factors. Older and less healthy men are more likely to be included in observational rather than RCTs studies (40,48,52). In addition, observational data included non-fatal cardiovascular events, which have been considered a more sensitive outcome than fatal cardiovascular events (52).
The primary cause of death in men with PCa treated with brachytherapy is cardiovascular disease (55,56). This is well illustrated in a report from Bittner et al (57). With median follow-up of 5.4 years primary cause of death in 1,354 patients treated with PB + EBRT + ADT is CVD (42% of all deaths) followed by other cancers (30%) and prostate cancer representing only 8.7% of deaths. Even though MVA analysis shows no association between use of ADT and risk of cardiovascular death, CSS or OS, it remains unclear why HR patients had double the risk of dying from CVD when compared to IR and LR patients (19.8% vs. 9.3% vs. 8.7% for HR, IR and LR respectively) (57).
Recent evidence suggests that excess cardiovascular morbidity and mortality is seen predominantly in patients with pre-existing cardiovascular comorbidity. After a median follow-up of 5.1 years, Nanda et al. reported that neoadjuvant ADT use was significantly associated with an increased risk of all-cause mortality only in the subgroup of patients with pre-existing CVD (including heart failure and MI). In their study, mortality had increased from 11% in ADT naïve, to 26% in ADT patients (HR of 1.9, 95%CI 1.04-3.71. p=0.04) (58). Similarly, Nguyen et al. found a significant increase in all-cause mortality (ACM)(adjusted HR 1.76 CI-1.32-2.34 p=0.001) in 1378 men with a history of congestive heart failure or MI treated with PB based radiation with or without median 4 months of ADT (ACM 22.7% vs. 11.6% with and without ADT) (59). Ziehr et al. reported a 5% absolute excess in cardiac specific mortality in men with a history of congestive heart failure (CHF) or myocardial infarction (MI) who received ADT for minimum 4 months (60).
A recent publication from Memorial Sloan-Kettering presented long term follow-up results on 2211 patients treated with EBRT± PB, who received neoadjuvant or adjuvant (45%) or salvage ADT (16%). With median follow-up of 9.3 years, short course of ADT was associated with an increased risk of cardiovascular morbidity (absolute increase 5.3% at 10 years, or, increase from 14.3% to 19.6%). The authors also presented nomograms to quantify the risk of cardiovascular death for patients (61). In addition to pre-existing comorbidity as a predictor of inferior OS, Tiara et al. reported a decrease in OS with ADT in men with low baseline testosterone (62).
Further information regarding impact of pre-existing comorbidity on risk of cardiovascular morbidity and mortality with ADT will be available form an ongoing RCT (RTOG 08-15) which randomizes patients between 0 vs 6 months of ADT and stratifies patients by Adult Comorbidity Evaluation-27 score (ACE-27) (63). Based on a re-analysis of 6 RCTs, Albertsen et al. speculated that the increase in cardiovascular morbidity and cardiovascular mortality might be a LHRH agonist class effect. The authors have reported significantly less CVD events in men treated with LHRH antagonists vs. LHRH agonists (HR 0.44; 95% CI 0.26-0.74; p= 0.002) (64)(65). More information will be available upon completion of the randomized clinical trial (RTC) comparing major cardiovascular events with LHRH agonists vs. antagonists in patients with pre-existing cardiovascular comorbidity (PRONOUNCE NCT02663908).
PCa Risk stratification
The National Comprehensive Cancer Network (NCCN) risk stratification criteria are perhaps the most commonly cited and represent the standard for most modern clinical trials (6). Even though studies included in this report were grouped based on risk stratification, the risk stratification used is not very clear or uniform, apart from a clear definition of LR disease. Evidence suggests that IR and HR PCa are rather heterogeneous disease. Recent publications propose subdividing each risk group (LR, IR and HR) into favourable and unfavourable risk, based on actual patient outcomes. Understanding the new proposed risk stratification and its impact on clinical outcomes is critical when interpreting the literature, formulating treatment decisions and evidence-based recommendations. Hence, this issue has been reviewed here in some detail.
Zumsteg et al (66) supported this concept with their report on 1024 patients treated with high dose EBRT (81Gy) and with median follow-up of 71 months. Unfavorable IR was defined as: primary Gleason pattern of 4, >50% PPC, or multiple intermediate-risk factors (cT2b/c, PSA 10–20, or GS 7). Patients with unfavorable IR (uIR) disease had inferior bPFS (HR: 2.37; p < 0.0001), higher risk of Distant Metastasis (DM) (HR: 4.34; p = 0.0003), and worse Prostate Cancer Specific Mortality (PCSM) (HR: 7.39; p = 0.007) compared with those with favorable IR (fIR) disease, despite being more likely to receive neoadjuvant ADT together with 81Gy EBRT. Nguyen et al reported outcomes on 1063 patients treated with radical prostatectomy, or with EBRT, with or without ADT and stratified by the number of risk features in both IR and HR disease (PSA >10 ng/mL, GS >7, ≥T2b, pre-treatment PSA velocity >2.0 ng/mL/y)(67). The 5-year cumulative incidence of PCSM was 2.4% for one factor, 2.4% for two factors, 7.0% for three factors, and 14.7% for all four factors. Prostate cancer deaths as a proportion of all deaths was 19% for one factor, 33% for two factors, 53% for three factors, and 80% for four factors. Recent data on outcomes on PCSM in HR disease from the SEER database (45,078 patients treated with EBRT with or without PB boost) further outline efforts in redefining risk stratification. HR disease was divided into favorable (T1c, GS4+4, and PSA <10 or T1C, GS6 and PSA >20) and unfavorable HR (all others)(68). Only men with unfavourable HR had a significantly reduced PCSM with EBRT alone vs. EBRT + PB boost (3.9% vs. 5.3% AHR 0.73, 95% CI 0.55-0.59 p=0.022). Unfortunately, with median follow-up of only 3.6 years, conclusions are premature.
The Genito-Urinary Oncologists of Canada (GUROC) have proposed new, refined risk stratification based on recursive partitioning analysis (RPA) analyses of the ProCaRS database (7974 patients from four Canadian Institutions) with long-term follow-up 48-94 months (69). The new risk groups accommodate six separate and statistical unique groups based on differences in long term bPFS. The LR group has been divided into favourable LR and LR based on PSA <6 and PSA >6. IR was sub-classified into favourable and unfavourable IR (PSA ≥10 and, either T2b/c, or T1T2a and GS 7) and the HR group was divided into favourable HR and extreme-risk (ER) group (HR and positive cores >87.5% or PSA >30). Most importantly, unfavourable IR and favourable HR have the same long-term PSA outcomes, when treated with minimum 74Gy EBRT or brachytherapy alone. Furthermore, extreme risk patients had significantly worse long term outcomes when compared to patients with favourable HR disease. Two ongoing RCTs (see below) stratify patients into favourable IR, unfavourable IR and favourable HR groups.
Review of the published literature on ADT and PB
The summary of all studies is given in tables 1–5. For the purpose of this review, studies were grouped based on risk stratification. Out of 52 studies, 36 (68%) included a mixture of risk groups (Tables 1, 3 and 5) and 17(32%) report on single risk group (Tables 2 and 4). Almost half of the studies are multi-institutional (47%). The treatment varied widely between patients, and the majority were treated with LDR-PB monotherapy, or combination LDR-PB with EBRT, all with or without ADT. Only 9 HDR studies are included in this report, as the majority of institutions do not give ADT with HDR. Risk stratification is extracted from the studies where possible and included in the tables. For LR and IR patients, ADT was most often prescribed to downsize the prostate prior to PB (Table 1 and 2). Higher risk patients and patients with multiple risk factors tended to receive ADT more often, and also for a longer duration (Table 4 and 5). Factors predictive of outcomes (bPFS, CSS and OS) were extracted from multivariable (MVA) analysis in all but two studies.
Table 1.
Low Risk (LR) and Intermediate Risk (IR) disease
| LR and IR |
Type of study/institution |
Year of study |
Number of patients |
Median FU |
Risk stratification |
Treatment | % on ADT |
Median ADT duration (range) |
Overall bPFS |
ADT benefit for bPFS |
Overall CSS |
ADT benefit for CSS |
Overall OS |
ADT benefit for OS |
Comments and factors predictive of outcome bPFS, CSS and OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDR | |||||||||||||||
| Ciezki (70) | MultiinstitutionalUS | 1996-2001 | 1668 | 4y | LR:64% IR:36% |
LDR±ADT | 37% | 6 mo | 87.8% | No benefit | NR | NR | NR | NR | NR |
| Potters (71) | New York Institutions US | 1992-2000 | 1449 | 6.8y | NR | LDR±EBRT±ADT | 27% | 5.2 mo (1-24) | 77% | No benefit | 93% | NR | 81% | NR | bPFS (GS, iPSA, D90) |
| Ohashi (72) | Multiinstitutional Japan | 2003-2009 | 663 | 5y | LR: 67% fIR: 33% |
LDR±ADT | 44% | 3 mo | 95.9% | No benefit | 99% | NR | 96% | NR | bPFs (D90, risk group) |
| Morris (73) | British Columbia, Canada | 1998-2003 | 1006 | 7.5y | LR: 58% fIR: 42% |
LDR±ADT | 65% | 6 mo | 95% | No benefit | 99% | NR | 83% | No benefit | bPFS (log iPSA, D90 in ADT naïve) OS (Age, log iPSA) |
| Martin (74) | Quebec City Canada | 1994-2001 | 396 | 5y | LR: 69% fIR: 31% |
LDR±ADT | 65% | 6mo | 88.5% | No benefit | NR | NR | NR | NR | bPFS (GS and stage) |
Table 5.
All risk categories
| All Risk Groups |
Institution/ Type of the study |
Year of the study |
Number of patients |
Median FU |
Risk Stratification |
Treatment | % on ADT |
Median ADT duration |
Overall bPFS |
ADT benefit to bPFS |
Overall CSS |
ADT benefit to CSS |
Overall OS |
ADT benefit to OS |
Comments and factors predictive of outcome bPFS, CSS and OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDR | |||||||||||||||
| Stock (98) | Mount Sinai NY | 1990-2010 | 2427 | 6.5 | LR :44% IR : 34% HR :21% |
LDR±EBRT±ADT | 54% | 6mo (3-36mo) | 85vs 86% for ±ADT | Benefit if BED <150Gy (24% at 10y) | NR | NR | NR | NR | bPFS (ADT, BED) Post PB Biopsy (benefit to ADT with BED < 200Gy) |
| Stone (49) | Mount Sinai NY | 1990-2007 | 1669 | 10 mean | LR :45% IR : 38% HR :16% |
LDR±EBRT±ADT | 54% | 6mo (6–36) | 89%/67% 10 and 15 y | NR | 94.[0-9]% | No benefit | 57%(15y) | OS worse with ADT (5% at 15y) | CSS (stage and GS) OS (age, ADT, smoking, diabetes, emphysema, atrial fib.) |
| Beyer (39) | Arizona Oncology Services | 1998-2001 | 2378 | 4.1 | LR :47% IR : 33% HR :19% |
LDR±EBRT±ADT | 19.5[0-9]% | 3-6 mo (3-12mo) | NR | NR | 88% | No benefit | 43% | OS worse with ADT (20%) | OS (ADT, age, GS) |
| Hinnen (99) | Utrecht Netherlands | 1989-2004 | 921 | 5.7 | LR :25% IR : 40% HR :35% |
LDR±ADT | 18% | 6mo | 79%/57% 5 and 10y | No benefit | 82% | No benefit | 59% | NR | bPFS(year of PB, HR, IR) OS (year of PB, HR) |
| Burri (100) | multiinstitutional US | 1990-2005 | 1665 | 5.6 | LR :60% IR : 27% HR :12% |
LDR±EBRT±ADT | 54% | 3-9mo | 94%/88% 5 and 8 y | No benefit | NR | NR | NR | NR | bPFS (GS, iPSA, BED) |
| Merrick (101) | multiinstitutional US | 1995-2002 | 938 | 5.4 | LR :35% IR : 35% HR :19% |
LDR+EBRT±ADT | 40% | 7-40mo | 96% | Benefit to longer ADT (15%) | 96% | No benefit | 78% | No benefit | bPFS (PPC, longer ADT) OS(age tobacco), |
| Tiara (102) | multiinstitutional US | 1992-1997 | 1656 | 7 | LR :35% IR : 36% HR :28% |
LDR±EBRT±ADT | 37% | <6 and >6 mo | 95.6% | No benefit | 98.[0-9]% | No benefit | 72.6% | No benefit | bPFS (PPC, Risk groups, CAD) CSS (GS, hypertension) OS (age, diabetes, tobacco) |
| Potters (103) | Multiinstitutional-matched pair analysis | 1992-1997 | 263 (612 all pts) | 3.8 | NR | LDR±EBRT,±ADT | 50% | 3.4 mo (3-8mo) | 87%/87% for±ADT | No benefit | NR | NR | NR | NR | bPFS (iPSA, GS, stage) |
| Bittner (57) | Multiinstitutional US | 1995-2004 | 1354 | 5.4 | LR :35% IR : 46% HR :18% |
LDR±EBRT,±ADT | 39% | 6mo (max 36) | NR | NR | 97% | No benefit | 76.7% | No benefit | CSS(GS, Risk Factor) OS(age, smoking) |
| Stone (26) | Mt Sinai NY | 1990-2005 | 584 | 7.1 | LR :44% IR : 24% HR :31% |
LDR±EBRT,±ADT | 48% | 6mo (3-9mo) | 85%/59% for positive vs negative bx | No benefit | 99%/87% for positive vs negative bx | No benefit | NR | NR | bPFS (GS, iPSA BED, bx) CSS(BED, positive Bx) Results: (ADT benefit in IR) |
| Dosoretz (50) | 21st Centurut Oncology | 1991-2005 | 2474 | 4.8 | LR :65% IR : 23% HR :12% |
LDR ±ADT | 69%-83% | 3-3.4mo | NR | NR | NR | NR | NR | OS worse with ADT in men >73y | ACM detriment with ADT (AHR 1.24 CI1.01-1.53 p=0.04) |
| Merrick (104) | Multiinstitutional US | 1995-2001 | 668 | 4.8 | LR :33% IR : 37% HR :28% |
LDR±EBRT±ADT | 58% | 4mo (3–36) | 98%/98%-88% LR/IR/HR | ADT benefit only for HR (9-12%) |
NR | NR | NR | NR | bPFS (HR, ADT, GS, PPC) |
| Kollmeier (105) | Mount Sinai NY | 1990-1996 | 243 | 6.2 | LR :61% IR : 47% HR :1.1% |
LDR±ADT | 60% | 6 mo | NR | No benefit | NR | NR | NR | NR | bPFS (iPSA, GS, BED) |
| Senzaki (106) | Tokushima University Hospital Japan | 2004-2012 | 431 | 5.3 | LR :40% IR : 45% HR : 14% |
LDR±ADT | 63% | 6.5 mo (6–10) | 98, 94 and 89% for LR, IR and HR | ADT benefit | NR | NR | NR | NR | bPFS(ADT and BED <180Gy) |
| Wilson (107) | Sir Charles Gairdner Hospital, Australia | 1994-2007 | 207 | 7.8 | LR :51% IR : 47% HR :1.1% |
LDR±ADT | 58% | 3-6mo | 89% at 10y | No benefit | NR | NR | NR | NR | Only 1% was HR |
| Henry (108) | St. James Hospital Leeds UK | 1995-2004 | 1298 | 4.9y | LR: 44% IR : 33% HR :14% |
LDR±ADT | 44% | 3-4 (all < 6mo) | 79% | Detriment with ADT in IR | 95% | NR | 95% | NR | bPFS (jPSA, GS, worse with ADT, D90 <140Gy, year of PB) |
| LDR and HDR | |||||||||||||||
| Zelefsky (109) | Memorial Sloan- Kettering NY US | 1998-2009 | 1466 | 4y | LR: 57% IR : 38% HR :5% |
LDR/HDR±EBRT±ADT | 31% | 3mo | LR: 98% IR : 95% HR :80% |
No benefit | NR | NR | NR | NR | bPFS(iPSA, GS, D90) |
| HDR | |||||||||||||||
| Tselis (110) | Offenbach Germany | 2004-2008 | 351 | 4.9 | LR: 56% IR : 23% HR :21% |
HDR monotherapy ±ADT | 19% | 9mo | 94% | No benefit | 98% | NR | 98% | NR | bPFS (trend to ADT benefit-5%, p=NS) |
| Demanes (111) | Oakland CA | 1991-1998 | 411 | 6.4 | LR: 27% IR : 45% HR :27% |
HDR+EBRT±ADT | 48% | <6mo | 81% | No benefit | 97% | NR | NR | NR | NR |
| Galalae (112) | Multiinstitutional US and Germany | 1986-2000 | 611 | 5 mean | LR:[0-9]% 1 risk factor 31% ≥2 risk factors 60% | HDR+EBRT±ADT | 28% | 4 mo | 77%/73% 5 and 10y | No benefit | 96/9 2% 5 and 10y | NR | NR | NR | bPFS (Risk group, iPSA, GS, stage) |
| Phan (113) | University Of California-Irvine | 1996-2003 | 309 | 4.9 | LR:21% 1 risk factor 35% ≥2 risk factors 43% | HDR+EBRT±ADT | 36% | 3 mo | 86% | No benefit | 98% | NR | 91% | NR | bPFS (risk group, iPSA) |
| Martinez (114) | Multiinstitutional US | 1986-2000 | 507 | 4.8 | NR | HDR+EBRT±ADT | 35% | 6 mo | 74%/76% for ± ADT | No benefit | 90%/98% for ± ADT | No benefit | 81%/76% for ± ADT | No benefit | bPFS (iPSA, GS) |
Table 3.
Intermediate Risk (IR) and High Risk (HR) disease
| IR and HR |
Type of the study/institution |
Year of study |
Number of patients |
Median FU in years |
Treatment | Risk Stratification |
% ADT |
Median ADT duration |
Overall bPFS |
ADT benefit to bPFS |
Overall CSS |
ADT benefit to CSS |
Overall OS | ADT benefit to OS |
Comments and factors predictive of outcome for bPFS, CSS and OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDR | |||||||||||||||
| Lee (81) | Mount Sinai NY | 1990-1998 | 201 | 3.5y | LDR±ADT | IR: 33% HR: 67% |
66% | 6mo | 68% | Benefit to ADT for low D90 | NR | NR | NR | NR | bPFS(ADT, RS, iPSA, D90 in ADT naïve - 25% bPFS benefit to ADT with low D90) |
| Strom (82) | Tampa FL | 2001-2011 | 120 | 5.2y | LDR+EBRT±ADT | IR: 76% HR: 24% |
45% | IR 4 mo HR 28mo | NR | No benefit | NR | No benefit | NR | No benefit | OS (age, trend for ADT benefit in HR (12% p=NS) |
| Merrick (83) | Multiinstitutional US | 1995-2003 | 530 | 5.7y | LDR+EBRT±ADT | IR: 73% HR: 27% |
33% | 4-7mo (3-36mo) | 95.2% | No benefit | 95.2% | No benefit | 77.3% | No benefit | bPFS (iPSA, CS) CSS(CS) OS (age, diabetes, tobacco) |
| Merrick (84) | Multiinstitutional US RCT - 20 vs 44Gy EBRT + PB | 1999-2004 | 247 | 9y | LDR+EBRT±ADT | PSA>10; 15% GS 8-9: 15% |
32% | 4 and 9 mo | 93.2% | No benefit | 97.7% | No benefit | 80% | No benefit | bPFS (PSA, CS) |
| Dattoli (85) | Multiinstitutional US | 1992-1997 | 321 | 10.5y | LDR+EBRT±ADT | IR: 49% HR: 51% |
44% | 4mo (3–6) | 82% | No benefit | NR | NR | NR | NR | bPFS (GS, PAP) |
| Merrick (86) | Multiinstitutional US RCT - 0 vs.20 vs. 44GyEBRT + PB | 1999-2013 | 630 | 7.7y | LDR±EBRT±ADT | fIR: 46% uIR: 46% HR: 8% |
10-56% | 6mo | 99-85% for IR and HR | No benefit | 100-95% for IR and HR | No-benefit | 80-57% for IR and HR | No benefit | bPFS(iPSA, P vol.) CSS (risk groups, PPC, P vol.) OS (age, iPSA, tobacco) |
| HDR/LDR | |||||||||||||||
| Kraus (87) | William Beaumont | 1991-2004 | 1044 pts | 5y | LDR/HDR±EBRT±ADT | IR: 75% HR: 25% |
40% | 6 mo | 72% | No benefit | 98% | No benefit | 83%vs79%for+ADT | No benefit | bPFS (iPSA, GS, CS. ADT improved bPFS 11.5% p=0.02 with LDR/HDR monotherapy. ADT improved FFCF with GS>=8 and bulky local disease |
| HDR | |||||||||||||||
| Schiffmann (88) | Hamburg Germany | 1999-2009 | 392 | 4y | LDR±EBRT±ADT | IR:46% HR:53% |
56% | 3mo | 77%/65% tri vs. bi modality | ADT Benefit (11%-20%) | NR | NR | NR | NR | bPFS (ADT benefit 12% for IR and 20% in HR) |
Table 2.
Intermediate Risk Disease (IR)
| IR | Type of the study | Study years |
Number of patients |
Median FU in years |
Sub Group Risk stratification |
Treatment | % on ADT |
Median ADT duration |
Overall bPFS |
ADT benefit to bPFS |
Overall CSS |
ADT benefit to CSS |
Overall OS |
ADT benefit to OS |
Comments/factors Predictive of outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDR | |||||||||||||||
| Rosenberg (75) | Chicago | 1997-2007 | 807 | 4.5y (IQR2.7-6.2y) | NR | LDR±ADT or EBRT+LDR | 76% | 4mo (2-6 mo) | NR | NR | 98% | Benefit to ADT (2%) | NR | NR | PCSM (3.3 vs 1.1% EBRT+PB vs PB+ ADT) CSS (iPSA, GS4+3, no ADT) |
| Tran (76) | Multiinstitutional UK | 2003-2007 | 615 | 5y (0.3-8.3y) | NR | LDR±ADT | 17% | 4mo | 88% | No benefit | NR | NR | NR | NR | bPFS (iPSA) |
| Ho (77) | Mount Sinai NY 2009 | 1990-2004 | 558 | 5y | 1 IRF: 68% 2 IRF: 26% 3 IRF: 5% |
LDR±EBRT±ADT | 74% | 3-9 mo | 86% | No benefit | NR | NR | NR | NR | bPFS (BED <150Gy2, 10% benefit to ADT, p=ns) |
| Keane (78) | Harvard Boston MA | 1997-2013 | 2510 | 7,8y (IQR5.3-10.5) | fIR: 76% uIR: 24% |
LDR±ADT, or EBRT+LDR | 33% | 4mo | NR | NR | NR | Benefit ADT only in unfavourable IR (HR 0.34 CI .13-.91) | NR | NR | CSS (Year of PB, ADT (uIR and risk stratification) |
| Bittner (79) | Multiinstitutional US | 1995-2001 | 932 | 7,4y | 90%IR GS 3+4: 58% GS 4+3: 41% |
LDR+EBRT,±ADT | 29% | 6mo | 95% | No benefit | 98% | No benefit | 77% | No benefit | bPFS (GS, iPSA, stage) CSS(nil) OS(age, diabetes, tobacco, CAD) |
| Stock (80) | Mount Sinai NY | 1994-2006 | 432 | 4,6y (23-155 mo) | 1 IRF: 47% 2IRF: 41% 3IRF: 12% |
LDR+EBRT±ADT | 81% | 4mo (324) | 92% | No benefit | NR | NR | NR | NR | bPFS(iPSA, GS, CS, number of risk features) |
Table 4.
High Risk Disease (HR)
| HR | Type of the study |
Year of the study |
Number of patients |
Median FU |
Treatment | % ADT |
Median ADT duration |
Overall bPFS |
ADT benefit on bPFS |
Overall CSS | ADT benefit on CSS |
Overall OS |
ADT benefit on OS |
Comments and factors predictive of outcome bPFS, CSS and OS |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDR | |||||||||||||||
| Ohashi (89) | Japan | 2003-2009 | 206 | 5y | 1 HRf 90% 2 HRf 9% 3 HRf 0.5% |
LDR+EBRT±ADT | 4[0-9]% | 4mo | 84.4% | No benefit | 98% | NR | 97% | NR | bPFS (PPC and risk features) |
| Bittner (56) | Multiinstitutional US (very high risk) | 1995-2007 | 131 | 6.6y | GS 8/9:80% PSA>20:29% | LDR+EBRT±ADT | 9[0-9]% | 19mo (4–36) | 87% | Benefit to longer ADT (13%) | 91% | Benefit with longer ADT | 70% | No benefit | bPFS (longer ADT. PPC) CSS (longer ADT, PPC) OS (age, PPC) |
| Bittner (90) | Multiinstitutional US | 1995-2005 | 186 | 6.7y | GS8-10:76% Med iPSA:11 | LDR+EBRT (mini vs whole pelvis) ±ADT | 7[0-9]% | >6mo (75%) | 92/84% WP vs Mini P | ADT benefit | 95%/92% WPRT vs MPRT | No benefit | 79/67% WPRT vs MPRT | No benefit | bPFS (ADT) OS (age, PPC, WPRT in ADT naïve pts) |
| Wattson (91) | Multiinstitutional, US | 1991+2007 | 2234 | 4.3y | 1HRf: 83% 2HRf: 14% 3HRf:2% |
LDR±EBRT,±ADT | 7[0-9]% | 4mo | NR | NR | NR | ADT benefit | NR | NR | CSS(ADT, number of high risk factors, triple therapy vs. LDR or LDR+EBRT) |
| D'Amico (92) | Multiinstitutional US | 1991-2005 | 1342 | 5.1y | 1HRf: 5% 2HRf: 86% 3HRf:8% |
LDR±ADT or EBRT+LDR or EBRT+LDR+ADT | 6[0-9]% | 4mo (IQR 3.4-6.2mo) | NR | NR | 84% | Benefit to ADT+EBRT vs LDR alone | NR | NR | CSS (trend for better tri vs. bimodality AHR 0.32 CI 0.14-0.73) |
| Merrick (93) | Multiinstitutional US | 1995-2002 | 204 | 7y | Med iPSA 9.9 Med GS8 | EBRT+LDR±ADT | 4[0-9]% | 4 and 12mo (3–36) | 89% | ADT benefit (6-16%) | 86% | No benefit | 68% | No benefit | bPFS (PPC, ADT and ADT duration) CSS (GS) OS (GS, diabetes) |
| Shilkurt (94) | Multiinstitutional US | 1995-2010 | 448 | 5.2y | 1HRf: 84% 2HRf: 14% 3HRf: 2% |
LDR+EBRT±ADT | 7[0-9]% | 12mo (8–24) | 86% | ADT benefit (HR 0.2) | 93% | No benefit | NR | NR | From the analysis of 958 pts who received EBRT ±ADT or LDR+EBRT±ADT |
| Merrick (55) | Multiinstitutional US | 1995-2005 | 284 | 7.8y | NR | LDR+EBRT±ADT | 6[0-9]% | 4-12 mo (range 3-36) | 89% | ADT benefit if PSA>2 0 (10%) | 94% | No benefit | 69% | No benefit | bPFS(PPC, ADT) CSS(nil) OS(age, diabetes, PPC) |
| Liss (95) | Multiinstitutional US | 1998-2008 | 141 | 4.7 | GS8-10:75% Med iPSA:20 T2b-T4:40% | LDR+EBRT±ADT | 8[0-9]% | 12 mo | 80% | Benefit to ADT>1 2 mo | 94 | No benefit | 88% (with GS5) | No benefit | bPFS (iPSA, ADT, CSS(nil) MFS(iPSA, GS5, ADT OS (iPSA, GS5) |
| Fang (96) | Multiinstitution al US | 1995-2005 | 174 | 6.6y | GS 8-10 PSA<15 | LDR+EBRT±ADT | 6[0-9]% | 12mo(3–36) | 92/95% with/without ADT | No benefit | 92/95-% with/without ADT | No benefit | 66/75% with/without ADT | No benefit Detriment to OS (p=ns) | bPFS(age) CSS(iPSA, Hypertension) OS CS, Prostate Vol) NS detriment to OS with ADT |
| HDR | |||||||||||||||
| Prada (97) | Oviedo, Spain | 1998-2006 | 252 | 6.1y | 2 IRf17% 1 HRf 40% 2 HRf 35% 3 HRf8% |
HDR+EBRT±ADT | 69% | 12mo | 84%/78% 5 and 10y | No benefit | NR | NR | NR | NR | bPFS (GS, benefit to ADT 6% p=ns) |
Low Risk and Intermediate Risk Disease (Table 1)
Five studies were identified describing outcomes with LR and IR patients, treated with LDR ±ADT in 4, or LDR±ADT±EBRT in one. Three studies were multi-institutional (one included matched pair analysis) (71), 2 were Canadian single institution series. A total of 5182 patients were included. Median follow-up ranged from 4-7.5 years. ADT was used in 27-65% of the patients for a median duration of 3-6 months. ADT was most often prescribed to downsize prostate prior to PB, and in one study also for IR features (73). In all but one study, where information could not be extracted (70), IR patients had favourable IR disease (fIR)(69). Overall, bPFS was 77-95%, CSS 93%-99%, and OS 81-96%. None of the studies, including the matched-pair analysis (71) showed any benefit from ADT to bPFS. The effect of ADT on CSS was not reported in any of the studies and ADT was not associated with improved or detrimental OS in one study (73). On MVA, bPFS was associated with GS, iPSA, D90 and risk groups. OS was associated with age, PSA, GS and Clinical Stage (CS) (table 1).
Intermediate Risk Disease (Table 2)
Six studies with 5854 patients were identified describing outcomes in IR patients using LDR+ ADT or LDR±EBRT±ADT. Two were multi-institutional and 4 single institution series. Median follow-up ranged from 4.5-7.8 years. Three studies reported risk stratification. Two studies (both from the Mount Sinai group) (77,80), stratified patients by number of risk features and study from Harvard (78) stratified patients into fIR and uIR (69). ADT was used in 17-81% of the patients for a median duration of 4 months. Four out of 6 studies reported no overall benefit to bPFS with ADT. Two studies did not report on bPFS. One study reported an absolute 2% benefit to CSS with ADT (75) and one reported benefits in only the unfavourable IR subgroup (78). Ho et al. reported a benefit to ADT only if BED was <150Gy(77). Four studies did not report on an association between ADT and OS and one showed no benefit to OS with ADT (79). On MVA, bPFS was associated with GS, iPSA, BED, CS and number of risk features. CSS was associated with iPSA, GS, treatment year and a benefit to ADT in unfavourable IR patients. OS was associated with age, diabetes, tobacco use and CAD (table 2).
Intermediate Risk and High Risk Disease (Table 3)
Eight studies were identified describing outcomes in 3,485 patients with IR and HR disease; six using LDR, one HDR and one with both LDR and HDR. Patients were treated using monotherapy LDR or HDR, or with EBRT+ LDR or HDR boost, all with or without ADT. Four studies were multi-institutional, including two RCTs (20 vs. 44Gy EBRT or 0 vs. 20 vs. 44 Gy EBRT) (84,86) and 4 were single institution series. Risk stratification given in table 3 shows the predominance of IR rather than HR disease in most studies, one of which stratified IR into fIR and uIR (86). Median follow-up ranged from 3.5-10.5 years. ADT was used in 32-66 % of the patients for a median duration of 6 months (range 4-28mo). Overall bPFS was 68-95%, CSS 95-98% and OS 77-80%.
Six out of eight studies reported no benefit of ADT to bPFS, apart from ADT improving bPFS by 25%, only in patients with low D90 (81). One HDR study reported 12% and 20% bPFS benefit to adding ADT in IR and HR disease respectively (88). Kraus et al. reported no overall benefit of ADT on bPFS; however patients treated with either LDR or HDR monotherapy, had 11% improved bPFS if ADT was used. In addition, ADT improved freedom from clinical failure (FFCF) in patients with GS≥8 and bulky local disease (87). None of the studies showed overall benefit to CSS or OS with ADT. Storm et al did show a non-significant 12% improvement in OS only in HR patients with the addition of ADT (82). Factors associated with bPFS included iPSA, CS, GS, PAP and prostate volume. Factors associated with bPFS included: ADT, Risk Stratification, iPSA, D90 in ADT naïve patients, PAP and prostate volume. Factors associated with CSS included: CS, risk groups, PPC and prostate volume, and with OS: iPSA, age, diabetes and tobacco use (table 3).
High Risk (Table 4)
Eleven studies with a total of 5602 patients were identified describing outcomes in patients with HR disease, ten using EBRT with LDR, one with HDR, all treated with or without ADT. Only one study had patients treated with LDR monotherapy (91). Nine studies were multi-institutional, and 2 were single institutions (1 LDR and 1 HDR). Median follow-up ranged from 4.3-7.8 years. ADT was used in 40-91% of the patients for a median duration of 3-12 months. Overall bPFS was 65%-92%, CSS was 84-98% and OS was 69-95%. Most patients included Favourable HR patients with 1-2 HR features.
Nine studies reported an association between ADT and bPFS, 3 showed no benefit and six showed (55,56,90,93–95) benefit to ADT. One HDR study found 6% non-significant increase in bPFS with ADT (97). Bittner et al. and Lissa et al. reported up to 13% benefit to longer ADT duration (56,95). Merrick at al. reported a 10% bPFS benefit to patients with PSA>20 (55), and an overall benefit of 6-16% (93). Nine studies reported an association between ADT and CSS, six found no benefit, and 3 found a benefit to ADT (56,91,92). D’Amico et al. found a benefit to CSS with triple therapy vs. LDR+EBRT or LDR monotherapy (92). Similarly Watson et al. reported better CSS for “triple therapy” (LDR+ ADT + EBRT) vs. LDR or LDR+EBRT without ADT (91). None of the 5 studies found any increase in OS with ADT; however Fung et al. reported a non-significant detriment in OS in fIR patients (96).
Other factors associated with bPFS included: iPSA, PPC, risk stratification and age. Factors associated with CSS included: PPC, number of risk factors, GS, hypertension and prostate volume. Factors associated with OS included: age, diabetes, PPC, iPSA, GS, Gleason pattern 5 and whole pelvis radiotherapy (WPRT) in ADT naïve patients (90)
All risk categories (Table 5)
Twenty two studies with 23,180 patients were identified describing outcomes in all risk categories including LR, IR and HR disease, sixteen using LDR (20,991 patents), five using HDR (2,189 patients) and one with both. Patients were treated using monotherapy LDR or HDR ± EBRT, all with or without ADT. Eight studies were multi-institutional, and 14 are single institution series, with 4 are from the single institution (26,49,98,100). Median follow-up ranged from 3.8-10 years. ADT was used in 18-83% of the patients for median duration of 3-9months. Overall, 10 y bPFS was 57-95%, CSS 82-98% and OS% 43-98%.
Sixteen studies reported an association between ADT and bPFS, 12 found no benefit (including all 5 HDR studies), and 4 found benefit to bPFS with addition of ADT. One study reported a 15% benefit only with longer ADT duration (101). One reported a 24% benefit to ADT at 10 years, only if BED was <150Gy (98), and yet another showed a 9-15% benefit with ADT only in HR disease (104). Counterintuitively, a study from the UK showed a detriment to bPFS with the addition of ADT in IR disease (108). None of the 7 studies showed an increase in CSS with ADT. Six studies assessed the impact of ADT on OS; 3 showed no impact on OS with ADT, and 3 showed a statistically significant detriment to OS with the use of ADT (39,49,50), one showed a trend to worse OS(96). The most dramatic OS detriment was reported by Bayer at al. with a median follow-up only 4.1 years; a 20% decrease in OS was seen in those patients treated with LDR PB with up to 12 months of ADT. Worth noting is the small number of patients in analyses at the end of the OS curves, which brings into question the validity of the magnitude in OS detriment with ADT (39). Stone at al (49) reported a 5% OS detriment at 15 years post treatment with ADT, and Dosoretz et al. found an OS detriment in men >73y age (50).
Other factors associated with bPFS included: iPSA, GS, PPC, risk stratification, BED, treatment year, CAD, and positive post-treatment biopsy. Factors associated with CSS included: CS, GS, BED, positive post-treatment biopsy and hypertension, and OS: age, diabetes, tobacco use, CVD and treatment year.
ADT for Cytoreduction before PB
Since the introduction of PB, it has been a common practice to downsize the prostate prior to implant using LHRH agonists. None of the studies where ADT was used for downsizing showed an improved oncological outcome (70–74). Merrick et al. reported that instead of LHRH agonists, downsizing can be achieved with use of Dutasteride and Bicalutamide (115). This was confirmed in a recent RCT where 61 patients were randomized to receive either LHRH antagonists or Dutasteride with Bicalutamide to downsize prostate prior to brachytherapy (116). Gaudet et al reported a mean relative prostate volume reduction of 35.5% (SD 8.9) in the LHRH group and 34.6 (SD 17.2) in Dutasteride and Bicalutamide group, suggesting that 3 months of Dutasteride and Bicalutamide is non-inferior to LHRH agonist for prostate volume reduction. Due to the potential impairment of quality of life associated with ADT, in selected cases, one may consider the less toxic combination of 5alpha reductase inhibitors and oral anti-testosterone for cytoreduction instead of LHRH agonists.
Randomized Controlled Trials: ADT and Brachytherapy (Table 6)
Table 6.
Randomised Control Trials (RTCs) s in progress
| RCT | Country | Accrual | Randomization | Number of patients | Risk Groups | Primary Endpoint | Secondary Endpoints | Status |
|---|---|---|---|---|---|---|---|---|
| SHIP 0804 (120) | Japan | 2008- 2011 | PB + 3 mo neoadjuvant ADT Randomization: 0 vs. 9 mo adjuvant ADT |
420 | IR | bPFS | OS, PFS, CSS, salvage treatments, IPSS and QOL | Closed |
| SHIP 36B (121). | Japan | Closed 2012 | EBRT+PB +ADT 6mo Randomization: 0 vs. 24 mo adjuvant ADT |
340 | HR | bPFS | OS, PFS, CSS, salvage treatments and adverse effects | Closed |
| RTOG 0815 (122) | US | 2009-2016 | EBRT (79.2Gy), or HDR or LDR boost Randomization: 0 vs 6 mo ADT |
1520 (Stratified by number of risk factors and comorbidity status) | Favourable IR Excluded: T2b-T2c, PSA 10-20, and GS 7 and with > 50% PPC |
OS | bPFS, local and distant RFS, PCSM Salvage, Toxicity, QOL | Closed |
| RTOG 0924 (83,122). | US | 2011-2019? | EBRT ± HDR or LDR boost ± ADT (4,6 or 32mo) Randomization: Prostate only vs. whole pelvis RT |
Projected 2580 1175 accrued | Unfavourable IR Favourable HR | OS | bPFS, DMFS,CSS, time to CRPC toxicity QOL | Open |
| Spanish RCT(123) | Spain | 2007-2008 | EBRT+HDR boost Randomization: ADT vs no ADT |
62 | IR and HR | 6ybNED with and without ADT 83% vs.90%, P=ns | DMFS and Local Control - no difference between arms | Reported: Abstract form 2013 |
| Chinese RCT(124) | China | NR | LDR PB Randomization: 0 vs 3 mo neoadjuvant ADT |
165 | T1c-T3b (PSA 3.5-150) (all risk groups) | bNED Toxicity | NR | Reported: Abstract form 2012 |
There are 6 ongoing RCTs addressing the question of the role of ADT with PB in IR and HR patients. So far, only one completed RCT at least indirectly addresses the role of ADT in Brachytherapy (121). Denham at al published an Australian multicentre TROG 03.04 RADAR 2×2 factorial RCT in men with locally advanced prostate cancer. 1071 men were randomized to receive ADT for 6 or 18 months with dose escalated EBRT (66Gy, 70Gy, 74Gy or 46Gy+HDR 19.5Gy in three fractions), and also randomized between 0-18 months of Zoledronic Acid (4mg IV Q3 months). The primary end point of bPFS subsequently changed to a PCSM. With a median follow-up of 7.4 years, there was no significant difference in PCSM or OS between the arms. Subsequent publication shows the cumulative and composite estimates of bPFS and local control for all EBRT dose levels (n=814) and HDR boost patients (n=237) stratified by duration of ADT (6 vs. 18 months). 18 months of ADT had a positive effect on the PSA and local control outcome on all EBRT dose levels with greater benefit is seen in lower doses, and had almost no effect for patients treated with HDR boost (absolute difference 3%). This data suggest minimal if any benefit to longer ADT with the use of PB, however it does not answer the question of whether ADT in needed with PB at all (122). Three other completed Brachytherapy RCTs do not provide information on the role of ADT with dose-escalated radiation using PB (22,23)(24). Results of the ASCENDE RT trial(22) indicate that when combined with 12 months of ADT, patients treated with EBRT plus LDR boost have a significantly better bPFS compared to EBRT alone (78Gy)(83% vs 62% bPFS at 9 years in favour of PB boost arm). Two other RCTs likewise showed the superiority of dose escalation with HDR+ EBRT vs. EBRT, but both used radiation alone without ADT (23)(24).
Recently, Merrick et al. published results of 2 RTC of supplemental EBRT in addition to LDR-PB in IR patients randomized to 20 vs. 44 Gy EBRT (n=247) or 0 vs. 20Gy EBRT (n=383). ADT (<6mo) was given for downsizing or adverse features in 32% of the patients in 20/44Gy trial and 7.6% in 0/20Gy trial. The results showed a very high bPFS, and CSS for both 20/44Gy and 0/20Gy trials (biochemical failure 7.7% and 8.2%, at 8 and 13 years and CSS of 2% and 2.4% at 8 and 13 years follow-up respectively). Predictors of PSA failure were PPC and prostate volume. The trial showed no benefit of supplemental EBRT on bPFS and PCSM with high quality implants. ADT was not associated with improved outcomes. The reason for association between prostate volume and outcome is unclear (123).
Ongoing RCTs (Table 6)
SHIP 0804
SHIP 0804 (Seed and Hormone for Intermediate–Risk Prostate Cancer, ClinicalTrials.gov NCT00664456) is an ongoing multi-institutional Japanese RTC, that will be reporting outcomes on 420 IR patients treated with PB and neoadjuvant ADT for 3 months, randomized to 0 vs. 9 months adjuvant ADT. The study began recruiting in April 2008. Planned completion is March 2011. Primary endpoint is 10y bPFS. Secondary end-points include OS, clinical PFS (local, distant failures) DSS, salvage treatments, IPSS and QOL (117).
SHIP 36B
SHIP 36B (ClinicalTrials.gov: UMIN000003992) is a RTC of 340 patients with high-risk localized prostate cancer, all treated with EBRT+ PB + ADT for 6 months, randomized between additional 0 vs. 24 months of adjuvant ADT. The trial is closed for accrual in 2012. Primary endpoint is bPFS, and secondary endpoints are OS, PFS, CSS, salvage treatments and adverse effects. Results are expected in 2022 (118).
RTOG 0815
RTOG 0815 is a recently closed phase III Prospective Randomized Trial of dose-escalated radiotherapy (EBRT to 79.2Gy, or HDR or LDR) with or without 6 months ADT for patients with IR PCa. Planned accrual was 1520 pts. Primary endpoint is OS while bPFS and HRQL are some of the secondary endpoints. Patients with 3 intermediate-risk features (T2b-T2c disease, PSA >10 but ≤20, and GS 7 and with ≥ 50% PPC) were excluded from this study. Therefore, the study will not be able to answer the question whether ADT is required with dose escalated RT in unfavourable IR patients. However, patients have been stratified by Adult Comorbidity Evaluation-27 score (ACE-27) and the results will further clarify the role that comorbidity may play in risk of cardiovascular events with ADT. The study has met its target accrual and closed on March 7, 2016. (63)
RTOG 0924
RTOG 0924 is an ongoing Phase III Prospective Randomized Trial of ADT and high dose radiotherapy with or without whole-pelvic radiotherapy in unfavourable IR or favourable HR PCa. Patients are stratified, given either ADT for 6 or 32 months, treated with IMRT, or IMRT +HDR or LDR boost and randomized into IMRT to prostate or pelvis. Target accrual is 2580 pts, 1175 patients have been accrued. Primary endpoint is OS while bPFS, DM, CSS and HRQL are some of the secondary endpoints. Results will be available in 2024 (63,82).
The Spanish RCT trial
The Spanish RCT trial in “unfavourable” IR and HR prostate cancer of EBRT+ HDR ± ADT has been reported in abstract form only. With median follow-up of 60 months, there was no benefit to ADT for bPFS (83% vs. 90% P = 0.4), and no benefit to loco regional control or distant metastasis (119).
A Chinese RCT
A Chinese RCT investigated LDR monotherapy in all risk stratifications with or without ADT. The trial has been reported in abstract form only and there are no available disease outcomes published yet (120).
Discussion
This review included 52 studies and 43,303 patients, the majority treated with LDR (n=40,440). Seven HDR studies included 2863 patients. Twenty-five studies are multi-institutional and 27 are single institution. Studies are mostly retrospective in nature and most included prospective data collection with exception of two RCTs.
Overall, patients treated with brachytherapy have exceptionally good long-term disease outcomes and compare favourably with other treatment modalities (1) (Tables 1–5). For LR and favourable IR, bPFS, CSS and OS are 77-95%, 93-99% and 81-96% respectively. For IR, bPFS, CSS and OS are 88-95%, 98% and 77% respectively. For IR and HR, bPFS, CSS and OS are 68-95%, 95-98% and 57-79% respectively. For HR, bPFS, CSS and OS are 80-92%, 86-98% and 68-97% respectively.
The literature review shows significant heterogeneity of patient populations, risk categories, risk factors, follow-up time, ADT administration and duration. Inherent in all retrospective analysis is unavoidable patient selection and treatment selection bias. This has a potential to impact the results, and the conclusions, as multivariate analysis cannot always overcome the selection bias. For example, Wattson et al. reported that the number of high risk features in 2234 men with HR PCa (1 and 2 vs. 3) is strongly related to adjusted HR for PCSM (HR 0.5 95% CI 0.2-0.9 p=0.03. In many studies, patients with worse risk factors have been selected not only to receive ADT (82,83,85,86), but also to receive ADT for longer duration (55,91–94,96) (75). In addition, patients with higher risk factors are expected to do less well overall. The fact that they did have similar outcomes to patients with lower risk or fewer risk factors may indicate overall ADT benefit. It has been reported that patients with unfavourable IR and favourable HR have relatively poor outcomes with PB alone (69,99,124), however, some have speculated that with high quality brachytherapy with sufficient margins, this difference may be less significant (123).
The duration of ADT in brachytherapy studies was relatively short (median: LR 3-6 mo, IR 3-9 mo and HR 12 mo). Patients in LR and IR most often received ADT to downsize the prostate, and in some IR and most HR studies, ADT was given for high risk features, as described above. While optimal duration of ADT cannot be determined from this review, TROG 03.04 RADAR has provided some evidence that duration of ADT together with HDR-BP has less impact on bPFS and local control than when combined with EBRT (122). As most of the studies, even those with HR PCa limited ADT to median 12 months; one may consider shorted duration of ADT if PB boost is to be used (up to 12 months). This is also supported by excellent results from recently reported ASCENDE RT trial where unfavourable and IR and favourable HR patients received triple therapy with 12 months of neoadjuvant and adjuvant ADT. It is also worth noting that HR patients treated with PB tend to be in the more favourable spectrum of HR disease (table 4) (66,67). It may be for this reason that ADT duration can be limited to only 12 months. Extreme risk (ER) HR patients, or HR with multiple high risk features are few in number in the studies reviewed, as they are less likely to be offered brachytherapy boost. In studies that included Extreme Risk HR patients, ADT was given for up to 36 months (104).
The studies were grouped to reflect disease risk stratification. Advances in refining the risk stratification have been included in this review. As mentioned above, treatment selection bias is present in almost all studies presented in this review. It is clear that physicians seem to take into account the presence of multiple adverse factors and recommend more aggressive treatments, including addition of EBRT and ADT, and using ADT for longer duration (55,75,91–94,96). It is clear that further advances in refining group stratification are urgently needed in order to further refine treatment recommendations (66,68,69).
Eighty percent (n=42) of the studies have information on the effect of ADT on bPFS, 46% (n=24) on CSS and 36% (n=19) on OS (Table 7). Seventy one percent studies report no bPFS benefit with addition of ADT, while 28% reported modest, up to 15% benefit of adding ADT to PB. The lack of benefit was seen in LR and favourable IR (70–74) as well as the majority of HDR studies. Most of patients in these studies received short term ADT in order to downsize the prostate prior to brachytherapy. ADT consistently showed improved in bPFS in patients with lower BED/D90 (26,81,98,106), unfavourable IR (multiple risk factors) and majority of HR patients (55,56,88,90,93–95)(97).
Table 7.
Summary of all studies
| Total studies 52 | bPFS | CSS | OS |
|---|---|---|---|
| Reported in 42 studies (80%) |
Reported in 24 studies (46%) |
Reported in 19 studies (36%) |
|
| Benefit to ADT | 12 (28%) | 4 (16%) | 0 |
| No Benefit | 30(71%) | 19 (79%) | 16 (84%) |
| Detriment with ADT | 1(2%) | – | 3(15%) |
Only 4 studies found a small benefit to CSS with ADT; one in unfavourable IR (78) and 3 in HR PCa (56,91,92), where increase in CSS was reported with “triple-therapy” vs. monotherapy or vs. EBRT +PB without ADT(91,92). Others reported that high quality implants may derive less benefit from supplemental EBRT (123) or ADT (26,81,98,106,123). The impact of ADT on OS has not been studied well, as only 19 studies (36%) reported association of ADT and OS. Overall 16 studies found no OS benefit with ADT, however, 3 found an OS detriment with the addition of ADT to brachytherapy (39,49) and in particular in men >73y (50).
In general, most HDR studies (87,97,110–114), found no benefit to addition of ADT. The preliminary results of the Spanish HDR RCT reported no benefit to ADT (119). Only one HDR study reported 11% and 20 % improved bPFS with ADT for IR and HR patients (88). Results of RCTs in progress may provide more information on the role of ADT with HDR.
Six RCTs are in progress to further assess the role of ADT with PB (63,82,117–120). Unfortunately, RTOG 0815, the only large RTC that has an arm not receiving any ADT, excluded patients with unfavourable IR disease and will not be able to provide information regarding the role of ADT in unfavourable IR patients. Both Japanese trials (included IR and HR disease) as well as RTOG 0924 (included unfavourable IR and favourable HR disease) do not have arm treated without ADT. Therefore they will primarily test the hypothesis regarding duration of ADT, rather than whether ADT is of any benefit together with brachytherapy. RCTs that test not only the duration, but whether there is any role for ADT in unfavourable IR and favourable HR disease are urgently needed.
If there is a potential to achieve up to a 15% increase in bPFS with the use of ADT in some IR and HR patients without significant impact on CSS, will this improvement come at a price of diminished QOL, potentially increase in cardiovascular morbidity and diminished OS? Literature suggest ADT should be used with caution in older patients (50,125), and those with CVD (48,51,52,58,60). In addition, ADT may have detriment to long term OS in brachytherapy patients (39,49,50). Therefore, ADT should be prescribed only to patients likely to benefit from it. In addition, significant efforts should be directed to reducing and managing ADT side effects including appropriate life style changes, smoking cession, and referral to a family doctor or a specialist experienced in the management of CVD.
Summary.
The inherent selection bias in retrospective studies, unclear risk stratification, inconsistent use and duration of ADT, and inconsistent treatment allocation, precludes any definitive conclusions regarding use of ADT in brachytherapy treated patients. Despite these significant limitations, we can deduce that there is no clinical or biochemical benefits from addition of ADT in LR and favourable IR patients. In unfavourable IR and favourable HR patients, the use and duration of ADT was subject to considerable physician bias. Despite this, ADT was beneficial in improving bPFS in most patients with HR disease using LDR, some patients with unfavourable IR, and patients with low D90 or low BED. The very small absolute benefit (2%) to CSS was found in only few studies, and was seen predominantly with tri-modality treatment vs. PB monotherapy. No OS survival benefit was found in any study; however 3 studies had reported a detriment to OS with the use of ADT. In order to minimize morbidity and potentially excess mortality one should exercise caution in prescribing ADT to older patients and those with existing cardiovascular disease. With high quality brachytherapy, the radiation dose is sufficient that any synergistic local effect of ADT with radiation is likely to be of little benefit except, perhaps in cases with very high volume local disease. In unfavourable IR and HR disease, ADT is likely to still play a role through spatial cooperation for suppression of micrometastatic disease. The optimal duration, however, remains to be determined. RCTs testing the role of ADT in unfavourable IR and favourable HR disease are urgently needed.
Acknowledgments
Special thanks to Beth Morrison, BCCA librarian, for her help with the literature searches and in editing the manuscript.
We would also like to acknowledge the late Peter Grimm DO, who was involved in early development of this manuscript.
Abbreviations
- PB
Prostate Brachytherapy
- EBRT
External Beam Radiation therapy
- ADT
Androgen Deprivation Therapy
- HDR
High Dose-Rate
- LDR
Low Dose Rate
- BED
Biologically Effective Dose
- D90
Dose covering 90% of the prostate gland
- LR
Low Risk Prostate Cancer
- IR
Intermediate Risk Prostrate cancer
- HR
High Risk Prostate Cancer
- IRf
Intermediate Risk feature
- uIR
Unfavourable Intermediate Risk
- fIR
Favourable Intermediate Risk
- uHR
Unfavourable High Risk
- fHR
Favourable High Risk
- CS
Clinical Stage
- PSA
Prostate Specific Antigen
- PAP
Prostatic Acid Phosphatase
- PPC
Percent Positive Cores
- P Vol
Prostate Volume
- bPFS
Biochemical Progression Free Survival
- CSS
Cause Specific Survival
- DMPFS
Distant Metastasis Progression Free survival
- DM
Distant Metastasis
- PCSS
Prostate Cancer Specific Survival
- FFCF
Freedom From Clinical Failure
- OS
Overall Survival
- IQR
Inter Quartile Range
- NR
Not Recorded
- CPRPC
Castrate resistant Prostate Cancer
- QOL
Quality of Life
- Triple therapy
EBRT + PB + ADT
- CVD
Cardio Vascular Disease
- CHF
Congestive Heart Failure
- MI
Myocardial Infarction
- BX
Biopsy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Grimm PD, Billiet I, Bostwick DG, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012;109(supp 1):22–29. doi: 10.1111/j.1464-410X.2011.10827.x. [DOI] [PubMed] [Google Scholar]
- 2.Wolff RF, Ryder S, Bossi A, et al. A systematic review of randomised controlled trials of radiotherapy for localised prostate cancer. Eur J Cancer. 2015 Nov;51(16):2345–2367. doi: 10.1016/j.ejca.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Davis BJ, Horwitz EM, Lee WR, et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy. 2012 Jan-Feb;11(1):6–19. doi: 10.1016/j.brachy.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Yamada Y, Rogers L, Demanes DJ, et al. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy. 2012 Jan;11(1):20–32. doi: 10.1016/j.brachy.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Expert Panel on Radiation Oncology-Prostate. Frank SJ, Arterbery VE, Hsu IC, et al. American College of Radiology Appropriateness Criteria permanent source brachytherapy for prostate cancer. Brachytherapy. 2011 Sep-Oct;10(5):357–362. doi: 10.1016/j.brachy.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 6.NCCN Guidelines Version 2. Prostate Cancer 2016. 2016 Available at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. (Accessed September 2016)
- 7.Ash D, Flynn A, Battermann J, et al. ESTRO/EAU/EORTC recommendations on permanent seed implantation for localized prostate cancer. Radiother Oncol. 2000 Dec;57(3):315–321. doi: 10.1016/s0167-8140(00)00306-6. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs G, Potter R, Loch T, et al. GEC/ESTRO-EAU recommendations on temporary brachytherapy using stepping sources for localised prostate cancer. Radiother Oncol. 2005 Feb;74(2):137–148. doi: 10.1016/j.radonc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal SA, Bittner NH, Beyer DC, et al. American Society for Radiation Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 2011 Feb 1;79(2):335–341. doi: 10.1016/j.ijrobp.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 10.Huggins C. How Charles Huggins made his Nobel Prize winning discovery–in his own words: an historic audio recording. Interviewed by Willard Goodwin and Elmer Bell. Prostate. 2012 Dec 1;72(16):1718. doi: 10.1002/pros.22524. [DOI] [PubMed] [Google Scholar]
- 11.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002 Jul;168(1):9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 12.Zietman AL, Prince EA, Nakfoor BM, Park JJ. Androgen deprivation and radiation therapy: sequencing studies using the Shionogi in vivo tumor system. Int J Radiat Oncol Biol Phys. 1997 Jul 15;38(5):1067–1070. doi: 10.1016/s0360-3016(97)00309-x. [DOI] [PubMed] [Google Scholar]
- 13.Zietman AL. The case for neoadjuvant androgen suppression before radiation therapy. Mol Urol. 2000;4(3):203–8. 215. susson. [PubMed] [Google Scholar]
- 14.Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006 Jun;7(6):472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 15.Wo JY, Zietman AL. Why does androgen deprivation enhance the results of radiation therapy? Urol Oncol. 2008 Sep-Oct;26(5):522–529. doi: 10.1016/j.urolonc.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Cvetkovic D, Movsas B, Dicker AP, et al. Increased hypoxia correlates with increased expression of the angiogenesis marker vascular endothelial growth factor in human prostate cancer. Urology. 2001 Apr;57(4):821–825. doi: 10.1016/s0090-4295(00)01044-x. [DOI] [PubMed] [Google Scholar]
- 17.Milosevic M, Chung P, Parker C, et al. Androgen withdrawal in patients reduces prostate cancer hypoxia: implications for disease progression and radiation response. Cancer Res. 2007 Jul 1;67(13):6022–6025. doi: 10.1158/0008-5472.CAN-07-0561. [DOI] [PubMed] [Google Scholar]
- 18.Jain RK, Safabakhsh N, Sckell A, et al. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: role of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 1998 Sep 1;95(18):10820–10825. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polkinghorn WR, Parker JS, Lee MX, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013 Nov;3(11):1245–1253. doi: 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002 Aug 1;20(15):3199–3205. doi: 10.1200/JCO.2002.01.086. [DOI] [PubMed] [Google Scholar]
- 21.Pickett B, Kurhanewicz J, Pouliot J, et al. Three-dimensional conformal external beam radiotherapy compared with permanent prostate implantation in low-risk prostate cancer based on endorectal magnetic resonance spectroscopy imaging and prostate-specific antigen level. Int J Radiat Oncol Biol Phys. 2006 May 1;65(1):65–72. doi: 10.1016/j.ijrobp.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 22.Morris WJ, Tyldesley S, Pai HH, et al. ASCENDERT*: A multicenter, randomized trial of dose-escalated external beam radiation therapy (EBRTB) versus low-dose-rate brachytherapy (LDR-B) for men with unfavorable-risk localized prostate cancer. Journal of Clinical Oncology. 2015 Mar 01;33(7 SUPPL):1. 2015. [Google Scholar]
- 23.Hoskin PJ, Rojas AM, Bownes PJ, Lowe GJ, et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012 May;103(2):217–222. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Sathya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. 2005 Feb 20;23(6):1192–1199. doi: 10.1200/JCO.2005.06.154. [DOI] [PubMed] [Google Scholar]
- 25.Lo AC, Morris WJ, Pickles T, et al. Patterns of recurrence after low-dose-rate prostate brachytherapy: a population-based study of 2223 consecutive low- and intermediate-risk patients. Int J Radiat Oncol Biol Phys. 2015 Mar 15;91(4):745–751. doi: 10.1016/j.ijrobp.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Stone NN, Stock RG, Cesaretti JA, Unger P. Local control following permanent prostate brachytherapy: effect of high biologically effective dose on biopsy results and oncologic outcomes. Int J Radiat Oncol Biol Phys. 2010 Feb 1;76(2):355–360. doi: 10.1016/j.ijrobp.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 27.Stone NN, Stock RG, Unger P. Effects of neoadjuvant hormonal therapy on prostate biopsy results after (125)I and (103)Pd seed implantation. Mol Urol. 2000;4(3):163–8. 169-70. susson. [PubMed] [Google Scholar]
- 28.Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009 Aug 1;74(5):1405–1418. doi: 10.1016/j.ijrobp.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 29.Martin NE, D’Amico AV. Progress and controversies: Radiation therapy for prostate cancer. CA Cancer J Clin. 2014 Nov-Dec;64(6):389–407. doi: 10.3322/caac.21250. [DOI] [PubMed] [Google Scholar]
- 30.D’Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008 Jan 23;299(3):289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 31.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011 Jul 14;365(2):107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 32.Zapatero A, Guerrero A, Maldonado X, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015 Mar;16(3):320–327. doi: 10.1016/S1470-2045(15)70045-8. [DOI] [PubMed] [Google Scholar]
- 33.Zelefsky MJ, Pei X, Chou JF, et al. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011 Dec;60(6):1133–1139. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han K, Milosevic M, Fyles A, et al. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys. 2013 Sep 1;87(1):111–119. doi: 10.1016/j.ijrobp.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 35.Shen X, Keith SW, Mishra MV, Dicker AP, Showalter TN. The impact of brachytherapy on prostate cancer-specific mortality for definitive radiation therapy of high-grade prostate cancer: a population-based analysis. Int J Radiat Oncol Biol Phys. 2012 Jul 15;83(4):1154–1159. doi: 10.1016/j.ijrobp.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 36.Amini A, Jones B, Jackson MW, et al. Survival Outcomes of Dose-Escalated External Beam Radiotherapy versus Combined Brachytherapy for Intermediate and High Risk Prostate Cancer Using the National Cancer Data Base. J Urol. 2015 Nov 11;195(5):1453–8. doi: 10.1016/j.juro.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Voog JC, Paulus R, Shipley WU, et al. Cardiovascular Mortality Following Short-term Androgen Deprivation in Clinically Localized Prostate Cancer: An Analysis of RTOG 94-08. Eur Urol. 2016 Feb;69(2):204–210. doi: 10.1016/j.eururo.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008 Mar 20;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 39.Beyer DC, McKeough T, Thomas T. Impact of short course hormonal therapy on overall and cancer specific survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2005 Apr 1;61(5):1299–1305. doi: 10.1016/j.ijrobp.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 40.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006 Sep 20;24(27):4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 41.Green HJ, Pakenham KI, Headley BC, et al. Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: a randomized controlled trial. BJU Int. 2002 Sep;90(4):427–432. doi: 10.1046/j.1464-410x.2002.02917.x. [DOI] [PubMed] [Google Scholar]
- 42.Nelson CJ, Lee JS, Gamboa MC, Roth AJ. Cognitive effects of hormone therapy in men with prostate cancer: a review. Cancer. 2008 Sep 1;113(5):1097–1106. doi: 10.1002/cncr.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bosco C, Crawley D, Adolfsson J, et al. Quantifying the evidence for the risk of metabolic syndrome and its components following androgen deprivation therapy for prostate cancer: a meta-analysis. PLoS One. 2015 Mar 20;10(3):e0117344. doi: 10.1371/journal.pone.0117344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004 Apr;63(4):742–745. doi: 10.1016/j.urology.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 45.Hatakeyama S, Yamamoto H, Imai A, et al. Type of androgen deprivation therapy affects metabolic condition and adipose tissue distribution. Journal of Urology; 2015 Annual Meeting of the American Urological Association; AUA New Orleans, LA United States. 2015. p. e933. [Google Scholar]
- 46.Levine GN, D’Aamico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: A science advisory from the American heart association American cancer society, and American urological association. CA Cancer J Clin. 2010 May-Jun;60(3):194–201. doi: 10.3322/caac.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006 Apr;91(4):1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 48.Saigal CS, Gore JL, Krupski TL, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007 Oct 1;110(7):1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 49.Stone NN, Stock RG. 15-year cause specific and all-cause survival following brachytherapy for prostate cancer: negative impact of long-term hormonal therapy. J Urol. 2014 Sep;192(3):754–759. doi: 10.1016/j.juro.2014.03.094. [DOI] [PubMed] [Google Scholar]
- 50.Dosoretz AM, Chen MH, Salenius SA, et al. Mortality in men with localized prostate cancer treated with brachytherapy with or without neoadjuvant hormone therapy. Cancer. 2010 Feb 15;116(4):837–842. doi: 10.1002/cncr.24750. [DOI] [PubMed] [Google Scholar]
- 51.Tsai HK, D’Amico AV, Sadetsky N, et al. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007 Oct 17;99(20):1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 52.Bosco C, Bosnyak Z, Malmberg A, et al. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol. 2015 Sep;68(3):386–396. doi: 10.1016/j.eururo.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 53.D’Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007 Jun 10;25(17):2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen PL, Je Y, Schutz FA, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011 Dec 7;306(21):2359–2366. doi: 10.1001/jama.2011.1745. [DOI] [PubMed] [Google Scholar]
- 55.Merrick GS, Butler WM, Galbreath RW, et al. Prostate cancer death is unlikely in high-risk patients following quality permanent interstitial brachytherapy. BJU Int. 2011 Jan;107(2):226–232. doi: 10.1111/j.1464-410X.2010.09486.x. [DOI] [PubMed] [Google Scholar]
- 56.Bittner N, Merrick GS, Butler WM, et al. Long-term outcome for very high-risk prostate cancer treated primarily with a triple modality approach to include permanent interstitial brachytherapy. Brachytherapy. 2012 Jul;11(4):250–255. doi: 10.1016/j.brachy.2012.02.002. 2012. [DOI] [PubMed] [Google Scholar]
- 57.Bittner N, Merrick GS, Galbreath RW, et al. Primary causes of death after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2008 Oct 1;72(2):433–440. doi: 10.1016/j.ijrobp.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Nanda A, Chen MH, Braccioforte MH, et al. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009 Aug 26;302(8):866–873. doi: 10.1001/jama.2009.1137. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen PL, Chen MH, Beckman JA, et al. Influence of androgen deprivation therapy on all-cause mortality in men with high-risk prostate cancer and a history of congestive heart failure or myocardial infarction. Int J Radiat Oncol Biol Phys. 2012 Mar 15;82(4):1411–1416. doi: 10.1016/j.ijrobp.2011.04.067. [DOI] [PubMed] [Google Scholar]
- 60.Ziehr DR, Chen MH, Zhang D, et al. Association of androgen-deprivation therapy with excess cardiac-specific mortality in men with prostate cancer. BJU Int. 2015 Sep;116(3):358–365. doi: 10.1111/bju.12905. [DOI] [PubMed] [Google Scholar]
- 61.Kohutek Z, Steinberger E, Pei X, et al. Long-term impact of androgen deprivation therapy on cardiovascular morbidity after radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys: 56th Annual Meeting of the American Society for Radiation Oncology, ASTRO 2014 San Francisco, CA United States. 2014;90(1 SUPPL. 1):S15. [Google Scholar]
- 62.Taira AV, Merrick GS, Galbreath RW, et al. Factors impacting all-cause mortality in prostate cancer brachytherapy patients with or without androgen deprivation therapy. Brachytherapy. 2010 Jan-Mar;9(1):42–49. doi: 10.1016/j.brachy.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 63.RTOG. RTOG://www.rtog.org/ClinicalTrials/ProtocolTable.aspx. (Accessed September 2016)
- 64.Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014 Mar;65(3):565–573. doi: 10.1016/j.eururo.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 65.Klotz L, Miller K, Crawford ED, et al. Disease control outcomes from analysis of pooled individual patient data from five comparative randomised clinical trials of degarelix versus luteinising hormone-releasing hormone agonists. Eur Urol. 2014 Dec;66(6):1101–1108. doi: 10.1016/j.eururo.2013.12.063. [DOI] [PubMed] [Google Scholar]
- 66.Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013 Dec;64(6):895–902. doi: 10.1016/j.eururo.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen PL, Chen MH, Catalona WJ, et al. Predicting prostate cancer mortality among men with intermediate to high-risk disease and multiple unfavorable risk factors. Int J Radiat Oncol Biol Phys. 2009 Mar 1;73(3):659–664. doi: 10.1016/j.ijrobp.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Muralidhar V, Xiang M, Orio PF, 3rd, et al. Brachytherapy boost and cancer-specific mortality in favorable high-risk versus other high-risk prostate cancer. J Contemp Brachytherapy. 2016 Feb;8(1):1–6. doi: 10.5114/jcb.2016.58080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodrigues G, Lukka H, Warde P, et al. The prostate cancer risk stratification project: database construction and risk stratification outcome analysis. J Natl Compr Canc Netw. 2014 Jan;12(1):60–69. doi: 10.6004/jnccn.2014.0007. [DOI] [PubMed] [Google Scholar]
- 70.Ciezki JP, Klein EA, Angermeier K, et al. A retrospective comparison of androgen deprivation (AD) vs. no AD among low-risk and intermediate-risk prostate cancer patients treated with brachytherapy, external beam radiotherapy, or radical prostatectomy. Int J Radiat Oncol Biol Phys. 2004 Dec 1;60(5):1347–1350. doi: 10.1016/j.ijrobp.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 71.Potters L, Morgenstern C, Calugaru E, et al. 12-Year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. Journal of Urology. 2005;173(5):1562–1566. doi: 10.1097/01.ju.0000154633.73092.8e. [DOI] [PubMed] [Google Scholar]
- 72.Ohashi T, Yorozu A, Saito S, et al. Outcomes following iodine-125 prostate brachytherapy with or without neoadjuvant androgen deprivation. Radiother Oncol. 2013 Nov;109(2):241–245. doi: 10.1016/j.radonc.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 73.Morris WJ, Keyes M, Spadinger I, et al. Population-based 10-year oncologic outcomes after low-dose-rate brachytherapy for low-risk and intermediate-risk prostate cancer. Cancer. 2013 Apr 15;119(8):1537–1546. doi: 10.1002/cncr.27911. [DOI] [PubMed] [Google Scholar]
- 74.Martin AG, Roy J, Beaulieu L, et al. Permanent prostate implant using high activity seeds and inverse planning with fast simulated annealing algorithm: A 12-year Canadian experience. Int J Radiat Oncol Biol Phys. 2007 Feb 1;67(2):334–341. doi: 10.1016/j.ijrobp.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 75.Rosenberg JE, Chen M, Nguyen PL, et al. Hormonal therapy or external-beam radiation with brachytherapy and the risk of death from prostate cancer in men with intermediate risk prostate cancer. Clinical Genitourinary Cancer. 2012 Mar;10(1):21–25. doi: 10.1016/j.clgc.2011.10.003. 2012. [DOI] [PubMed] [Google Scholar]
- 76.Tran AT, Mandall P, Swindell R, et al. Biochemical outcomes for patients with intermediate risk prostate cancer treated with I-125 interstitial brachytherapy monotherapy. Radiother Oncol. 2013 Nov;109(2):235–240. doi: 10.1016/j.radonc.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 77.Ho AY, Burri RJ, Cesaretti JA, et al. Radiation dose predicts for biochemical control in intermediate-risk prostate cancer patients treated with low-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2009 Sep 1;75(1):16–22. doi: 10.1016/j.ijrobp.2008.10.071. [DOI] [PubMed] [Google Scholar]
- 78.Keane FK, Chen MH, Zhang D, et al. Androgen deprivation therapy and the risk of death from prostate cancer among men with favorable or unfavorable intermediate-risk disease. Cancer. 2015 Aug 15;121(16):2713–2719. doi: 10.1002/cncr.29420. [DOI] [PubMed] [Google Scholar]
- 79.Bittner N, Merrick GS, Butler WM, et al. Gleason score 7 prostate cancer treated with interstitial brachytherapy with or without supplemental external beam radiation and androgen deprivation therapy: is the primary pattern on needle biopsy prognostic? Brachytherapy. 2013 Jan-Feb;12(1):14–18. doi: 10.1016/j.brachy.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Stock RG, Yalamanchi S, Hall SJ, Stone NN. Impact of hormonal therapy on intermediate risk prostate cancer treated with combination brachytherapy and external beam irradiation. J Urol. 2010 Feb;183(2):546–550. doi: 10.1016/j.juro.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 81.Lee LN, Stock RG, Stone NN. Role of hormonal therapy in the management of intermediate-to high-risk prostate cancer treated with permanent radioactive seed implantation. Int J Radiat Oncol Biol Phys. 2002 Feb 1;52(2):444–452. doi: 10.1016/s0360-3016(01)02598-6. [DOI] [PubMed] [Google Scholar]
- 82.Strom TJ, Hutchinson SZ, Shrinath K, et al. External beam radiation therapy and a low-dose-rate brachytherapy boost without or with androgen deprivation therapy for prostate cancer. Int Braz J Urol. 2014 Jul-Aug;40(4):474–483. doi: 10.1590/S1677-5538.IBJU.2014.04.05. [DOI] [PubMed] [Google Scholar]
- 83.Merrick GS, Galbreath RW, Butler WM, et al. Primary Gleason pattern does not impact survival after permanent interstitial brachytherapy for gleason score 7 prostate cancer. Cancer. 2007 Jul 15;110(2):289–296. doi: 10.1002/cncr.22793. 2007. [DOI] [PubMed] [Google Scholar]
- 84.Merrick GS, Wallner KE, Butler WM, et al. 20 Gy versus 44 Gy of supplemental external beam radiotherapy with palladium-103 for patients with greater risk disease: results of a prospective randomized trial. Int J Radiat Oncol Biol Phys. 2012 Mar 1;82(3):e449–55. doi: 10.1016/j.ijrobp.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 85.Dattoli M, Wallner K, True L, et al. Long-term outcomes for patients with prostate cancer having intermediate and high-risk disease, treated with combination external beam irradiation and brachytherapy. J Oncol. 2010;2010 doi: 10.1155/2010/471375. pii: 471375. Epub 2010 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Merrick GS, Wallner KE, Galbreath RW, et al. Is supplemental external beam radiation therapy essential to maximize brachytherapy outcomes in patients with unfavorable intermediate-risk disease? Brachytherapy. 2016 Jan-Feb;15(1):79–84. doi: 10.1016/j.brachy.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 87.Krauss D, Kestin L, Ye H, et al. Lack of benefit for the addition of androgen deprivation therapy to dose-escalated radiotherapy in the treatment of intermediate- and high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2011 Jul 15;80(4):1064–1071. doi: 10.1016/j.ijrobp.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Schiffmann J, Lesmana H, Tennstedt P, et al. Additional androgen deprivation makes the difference: Biochemical recurrence-free survival in prostate cancer patients after HDR brachytherapy and external beam radiotherapy. Strahlenther Onkol. 2015 Apr;191(4):330–337. doi: 10.1007/s00066-014-0794-y. [DOI] [PubMed] [Google Scholar]
- 89.Ohashi T, Yorozu A, Saito S, et al. Combined brachytherapy and external beam radiotherapy without adjuvant androgen deprivation therapy for high-risk prostate cancer. Radiat oncol. 2014;9:13. doi: 10.1186/1748-717X-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bittner N, Merrick GS, Wallner KE, et al. Whole-pelvis radiotherapy in combination with interstitial brachytherapy: does coverage of the pelvic lymph nodes improve treatment outcome in high-risk prostate cancer? Int J Radiat oncol Biol Phys. 2010 Mar 15;76(4):1078–1084. doi: 10.1016/j.ijrobp.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 91.Wattson DA, Chen MH, Moul JW, et al. The number of high-risk factors and the risk of prostate cancer-specific mortality after brachytherapy: implications for treatment selection. Int J Radiat oncol Biol Phys. 2012 Apr 1;82(5):e773–9. doi: 10.1016/j.ijrobp.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 92.D’Amico AV, Moran BJ, Braccioforte MH, et al. Risk of death from prostate cancer after brachytherapy alone or with radiation, androgen suppression therapy, or both in men with high-risk disease. J Clin Oncol. 2009 Aug 20;27(24):3923–3928. doi: 10.1200/JCO.2008.20.3992. [DOI] [PubMed] [Google Scholar]
- 93.Merrick GS, Butler WM, Wallner KE, et al. Androgen deprivation therapy does not impact cause-specific or overall survival in high-risk prostate cancer managed with brachytherapy and supplemental external beam. Int J Radiat oncol Biol Phys. 2007 May 1;68(1):34–40. doi: 10.1016/j.ijrobp.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 94.Shilkrut M, Merrick GS, McLaughlin PW, et al. The addition of low-dose-rate brachytherapy and androgen-deprivation therapy decreases biochemical failure and prostate cancer death compared with dose-escalated external-beam radiation therapy for high-risk prostate cancer. Cancer. 2013 Feb 1;119(3):681–690. doi: 10.1002/cncr.27784. [DOI] [PubMed] [Google Scholar]
- 95.Liss AL, Abu-Isa EI, Jawad MS, et al. Combination therapy improves prostate cancer survival for patients with potentially lethal prostate cancer: The impact of Gleason pattern 5. Brachytherapy. 2015 Jul-Aug;14(4):502–510. doi: 10.1016/j.brachy.2015.02.389. [DOI] [PubMed] [Google Scholar]
- 96.Fang LC, Merrick GS, Butler WM, et al. High-Risk Prostate Cancer With Gleason Score 8-10 and PSA Level </=15 ng/mL Treated With Permanent Interstitial Brachytherapy. Int J Radiat oncol Biol Phys. 2010 Oct 5; doi: 10.1016/j.ijrobp.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 97.Prada PJ, Mendez L, Fernandez J, et al. Long-term biochemical results after high-dose-rate intensity modulated brachytherapy with external beam radiotherapy for high risk prostate cancer. Radiat oncol. 2012 Mar 7;7:31. doi: 10.1186/1748-717X-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stock RG, Buckstein M, Liu JT, Stone NN. The relative importance of hormonal therapy and biological effective dose in optimizing prostate brachytherapy treatment outcomes. BJU Int. 2013 Jul;112(2):E44–50. doi: 10.1111/bju.12166. [DOI] [PubMed] [Google Scholar]
- 99.Hinnen KA, Battermann JJ, van Roermund JG, et al. Long-term biochemical and survival outcome of 921 patients treated with I-125 permanent prostate brachytherapy. Int J Radiat oncol Biol Phys. 2010 Apr;76(5):1433–1438. doi: 10.1016/j.ijrobp.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 100.Burri RJ, Ho AY, Forsythe K, et al. Young men have equivalent biochemical outcomes compared with older men after treatment with brachytherapy for prostate cancer. Int J Radiat oncol Biol Phys. 2010;77(5):1315–1321. doi: 10.1016/j.ijrobp.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 101.Merrick GS, Butler WM, Wallner KE, et al. Androgen-deprivation therapy does not impact cause-specific or overall survival after permanent prostate brachytherapy. Int J Radiat oncol Biol Phys. 2006;65(3):669–677. doi: 10.1016/j.ijrobp.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 102.Taira AV, Merrick GS, Butler WM, et al. Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy. Int J Radiat oncol Biol Phys. 2011 Apr 1;79(5):1336–1342. doi: 10.1016/j.ijrobp.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 103.Potters L, Torre T, Ashley R, Leibel S. Examining the role of neoadjuvant androgen deprivation in patients undergoing prostate brachytherapy. J Clin Oncol. 2000 Mar;18(6):1187–1192. doi: 10.1200/JCO.2000.18.6.1187. [DOI] [PubMed] [Google Scholar]
- 104.Merrick GS, Butler WM, Wallner KE, et al. Impact of supplemental external beam radiotherapy and/or androgen deprivation therapy on biochemical outcome after permanent prostate brachytherapy. Int J Radiat oncol Biol Phys. 2005 Jan 1;61(1):32–43. doi: 10.1016/j.ijrobp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 105.Kollmeier MA, Stock RG, Stone N. Biochemical outcomes after prostate brachytherapy with 5-year minimal follow-up: Importance of patient selection and implant quality. 2003 Nov 01;2003 doi: 10.1016/s0360-3016(03)00627-8. [DOI] [PubMed] [Google Scholar]
- 106.Senzaki T, Fukumori T, Mori H, et al. Clinical Significance of Neoadjuvant Combined Androgen Blockade for More Than Six Months in Patients with Localized Prostate Cancer Treated with Prostate Brachytherapy. Urol Int. 2015;95(4):457–464. doi: 10.1159/000439573. [DOI] [PubMed] [Google Scholar]
- 107.Wilson C, Waterhouse D, Lane SE, et al. Ten-year outcomes using low dose rate brachytherapy for localised prostate cancer: An update to the first Australian experience. J Med Imaging Radiat oncol. 2016 Mar 28; doi: 10.1111/1754-9485.12453. [DOI] [PubMed] [Google Scholar]
- 108.Henry AM, Al-Qaisieh B, Gould K, et al. Outcomes following iodine-125 monotherapy for localized prostate cancer: the results of Leeds 10-year single-center brachytherapy experience. Int J Radiat oncol Biol Phys. 2010 Jan 1;76(1):50–56. doi: 10.1016/j.ijrobp.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 109.Zelefsky MJ, Chou JF, Pei X, et al. Predicting biochemical tumor control after brachytherapy for clinically localized prostate cancer: The Memorial Sloan-Kettering Cancer Center experience. Brachytherapy. 2012 Jul-Aug;11(4):245–249. doi: 10.1016/j.brachy.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 110.Tselis N, Tunn UW, Chatzikonstantinou G, et al. High dose rate brachytherapy as monotherapy for localised prostate cancer: a hypofractionated two-implant approach in 351 consecutive patients. Radiat oncol. 2013;8:115. doi: 10.1186/1748-717X-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Demanes DJ, Brandt D, Schour L, Hill DR. Excellent results from high dose rate brachytherapy and external beam for prostate cancer are not improved by androgen deprivation. Am J Clin Oncol. 2009 Aug;32(4):342–347. doi: 10.1097/COC.0b013e31818cd277. [DOI] [PubMed] [Google Scholar]
- 112.Galalae RM, Martinez A, Mate T, et al. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat oncol Biol Phys. 2004 Mar 15;58(4):1048–1055. doi: 10.1016/j.ijrobp.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 113.Phan TP, Syed AM, Puthawala A, et al. High dose rate brachytherapy as a boost for the treatment of localized prostate cancer. J Urol. 2007 Jan;177(1):123–7. doi: 10.1016/j.juro.2006.08.109. discussion 127. [DOI] [PubMed] [Google Scholar]
- 114.Martinez A, Galalae R, Gonzalez J, et al. No apparent benefit at 5 years from a course of neoadjuvant/concurrent androgen deprivation for patients with prostate cancer treated with a high total radiation dose. J Urol. 2003 Dec;170(6 Pt 1):2296–2301. doi: 10.1097/01.ju.0000096709.05800.48. [DOI] [PubMed] [Google Scholar]
- 115.Merrick GS, Butler WM, Wallner KE, et al. Efficacy of neoadjuvant bicalutamide and dutasteride as a cytoreductive regimen before prostate brachytherapy. Urology. 2006 Jul;68(1):116–120. doi: 10.1016/j.urology.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 116.Gaudet M, Vigneault E, Meyer F, et al. Randomized trial of bicalutamide and dutasteride versus LHRH agonists for prostate volume reduction prior to I-125 permanent implant brachytherapy for prostate cancer. Brachytherapy. 2015 May-Jun;14:S33–S34. doi: 10.1016/j.radonc.2015.11.022. 2015. [DOI] [PubMed] [Google Scholar]
- 117.Miki K, Kiba T, Sasaki H, et al. Transperineal prostate brachytherapy, using I-125 seed with or without adjuvant androgen deprivation, in patients with intermediate-risk prostate cancer: study protocol for a phase III, multicenter, randomized, controlled trial. BMC Cancer. 2010;10:572. doi: 10.1186/1471-2407-10-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Konaka H, Egawa S, Saito S, et al. Tri-Modality therapy with I-125 brachytherapy, external beam radiation therapy, and short- or long-term hormone therapy for high-risk localized prostate cancer (TRIP): study protocol for a phase III, multicenter, randomized, controlled trial. BMC Cancer. 2012;12:110. doi: 10.1186/1471-2407-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garcia Blanco A, Anchuelo Latorre J, Paya Barcela G, et al. Brachytherapy in localized prostate cancer with or without androgen deprivation. Reports of Practical Oncology and Radiotherapy. 2013;18:S142. [Google Scholar]
- 120.Cui X, Li Q, Xu JJ, et al. Application of neoadjuvant hormonal therapy in (125)I permanent seed implantation for prostate cancer. Zhonghua Yi Xue Za Zhi. 2012 Oct 16;92(38):2710–2712. [PubMed] [Google Scholar]
- 121.Denham JW, Joseph D, Lamb DS, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): an open-label, randomised, phase 3 factorial trial. Lancet Oncol. 2014 Sep;15(10):1076–1089. doi: 10.1016/S1470-2045(14)70328-6. [DOI] [PubMed] [Google Scholar]
- 122.Denham JW, Steigler A, Joseph D, et al. Radiation dose escalation or longer androgen suppression for locally advanced prostate cancer? Data from the TROG 03.04 RADAR trial. Radiother Oncol. 2015 Jun;115(3):301–307. doi: 10.1016/j.radonc.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 123.Merrick GS, Wallner KE, Galbreath RW, et al. Is supplemental external beam radiation therapy necessary for patients with higher risk prostate cancer treated with 103Pd? Results of two prospective randomized trials. Brachytherapy. 2015 Sep-Oct;14(5):677–685. doi: 10.1016/j.brachy.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 124.Kittel JA, Reddy CA, Smith KL, et al. Long-Term Efficacy and Toxicity of Low-Dose-Rate Prostate Brachytherapy as Monotherapy in Low-, Intermediate-, and High-Risk Prostate Cancer. Int J Radiat oncol Biol Phys. 2015 Jul 15;92(4):884–893. doi: 10.1016/j.ijrobp.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 125.Kohutek ZA, Weg ES, Pei X, et al. Long-term Impact of Androgen-deprivation Therapy on Cardiovascular Morbidity After Radiotherapy for Clinically Localized Prostate Cancer. Urology. 2016 Jan;87:146–152. doi: 10.1016/j.urology.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


