Abstract
The nutritional status of women and men before conception has profound implications for the growth, development and long-term health of their offspring. Evidence of the effectiveness of preconception interventions in improving outcomes for mothers or babies is scarce, though given the large potential health return, relatively low costs and risk of harm, intervention is still warranted. We identify three promising strategies for intervention that are likely to be scalable and to have positive effects on a range of health outcomes: supplementation and fortification; cash transfers; and behaviour change interventions. Based on this, we suggest a model specifying pathways to effect. Pathways are incorporated into a lifecourse framework using individual motivation and receptiveness at different ‘preconception action phases’ to guide design and targeting of preconception interventions. Interventions with those not planning immediate pregnancy take advantage of settings and implementation platforms outside the maternal and child health arena, since this group is unlikely to be engaged with maternal health services. Interventions to improve women’s nutritional status and health behaviours at all preconception action phases need to take account of social and environmental determinants to avoid exacerbating health and gender inequalities, and should be underpinned by a social movement that touches the whole population. A dual strategy that targets specific groups actively planning a pregnancy, while improving the health of the population more broadly, is proposed. The engagement of modern marketing techniques points to a social movement based on an emotional and symbolic connection between improved maternal nutrition and health prior to conception and offspring health. We suggest that speedy and scalable public health benefit might be achieved through strategic engagement with the private sector. Political theory supports the development of an advocacy coalition of groups interested in preconception health, to harness the political will and leadership necessary to turn high-level policy into effective co-ordinated action.
Introduction
In 2016 the United Nations declared a ‘Decade of Action on Nutrition’ and committed to ‘end all forms of malnutrition, including internationally agreed targets on stunting and wasting in children under 5 years, and addressing the nutritional needs of adolescent girls, pregnant and lactating women’(p. 1).(1) There is increasing evidence from epidemiological and developmental biology research that these growth and development targets for children and the consequent reduction in their risk of non-communicable disease in adulthood could be achieved through improving women’s nutritional status and health behaviour before conception. (Reference Stephenson et al; Fleming et al; (2)) Two previous Lancet series have called for innovation in the design and delivery of affordable, scalable nutrition interventions to improve maternal and child health.(3, 4) In this paper, we review what is known about the effectiveness of nutrition and behavioural interventions before conception, and propose a strategy of aligning interventions to individual motivation and receptiveness at different ‘preconception action phases’ during the lifecourse. We propose a dual strategy targeting health improvement in both men and women planning a pregnancy and in the general population. This is on the basis that improvements in preconception health require a supportive environment, underpinned by a ‘social movement’ and policy initiatives, and of necessity engaging big business.
Method
We conducted a quasi-systematic review of trials of preconception nutrition and health behaviour interventions to identify effective interventions and specify pathways to effect. All search details are in a web appendix. (Provide link to web appendix.) We included interventions assessing nutritional status and body composition outcomes only, excluding other clinical outcomes such as improved glycaemic control. Pathways to effect were then incorporated into a lifecourse framework to aid targeting of interventions. Current preconception interventions were reviewed using the REAIM framework to assess, where possible, their reach, efficacy/effectiveness, adoption, implementation and maintenance.(5) Finally, we applied a consumer marketing approach to the challenge of creating a social movement to strengthen political resolve for wide-scale intervention
Current strategies for preconception nutrition intervention
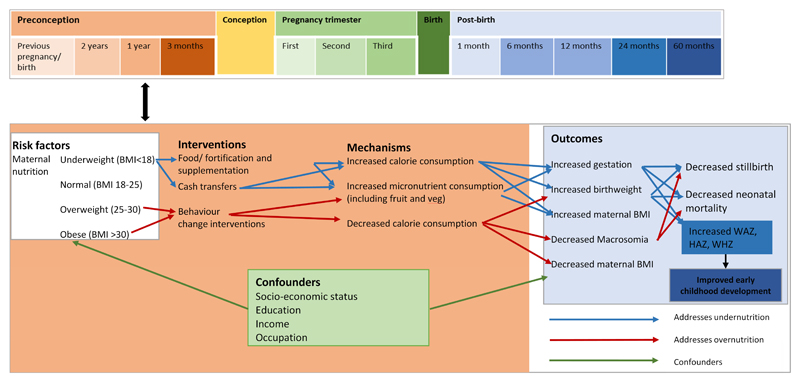
We identified 14 controlled primary studies evaluating three strategies: supplementation/fortification, cash transfers or incentives, and behaviour change intervention. Too few good quality studies conducted in the preconception period were identified to enable firm conclusions about effectiveness or meta-analysis. Current epidemiological and biological evidence points, however, to the value of intervening prior to conception. Intervention strategies were therefore selected for review on the basis of scalability, likely benefit to a nutritional outcomes in the preconception period and being low risk. Using these strategies, we developed a model identifying the key pathways to be quantified once more high quality data from randomised trials are available (Figure 1).
Figure 1. Conceptual model of pathways between interventions to improve maternal nutritional status and maternal and infant outcomes.
(Figure is colour coded such that brown box containing pathways relates to the preconception period and the blue box refers to the period post-birth.)
Supplementation and food fortification
The majority of evidence for the benefit of improving preconception nutrition and health comes from ‘supplementation trials’. These trials examine the effects of micronutrient and energy supplementation. The Bacon Chow study in Taiwan found that supplementing undernourished women’s diets with 800kcal and 40g protein per day after their first baby increased birthweight in the second baby when compared to a control group given just 80kcal per day.(6) A similar study in the USA also found increased birthweight of subsequent babies among women given supplements for five to seven months following first birth, compared with those given supplements for up to two months.(7) The Mumbai Maternal Nutrition Project showed that a locally-sourced micronutrient-rich snack, given daily before conception and during pregnancy, reduced the likelihood of gestational diabetes, and increased birthweight in a high-risk Indian population but only among mothers who were not underweight.(8, 9) These studies currently represent the best available evidence for preconception nutritional supplementation. Efficient and effective strategies to improve access to additional calories before conception still need to be identified in contexts where maternal undernutrition is common.
Supplementation interventions are acceptable to women but uptake is often hampered by poor adherence. Several solutions have been proposed, including a contraceptive pill containing folic acid currently available in the USA.(10, 11) The impact of this ingenious solution depends, however, on contraceptive pill use which varies widely between countries. Fortifying foods such as flour or rice has wide potential reach and is currently mandated in 87 countries.(12) The WHO has also issued a guideline for the fortification of salt with iodine, which can prevent irreversible mental impairment of the fetus.(13) Reductions in the prevalence of neural tube defects have been observed following mandatory folic acid fortification in the USA, Canada, Chile, Costa Rica and South Africa.(14, 15) However folic acid fortification is not mandatory in Europe. In the UK, there are concerns about increasing cancer risk in older populations, potential masking of vitamin B12 deficiency anaemia, and removing individual choice.(14) Despite these concerns, is little evidence of negative consequences from the implementation of folic acid fortification.(16) The UK’s Scientific Advisory Committee on Nutrition continues to recommend mandatory folic acid fortification to improve the folate status of women most at risk of neural tube defect-affected pregnancies.
Cash transfers/incentives
None of the studies we found explicitly investigated the effects of preconception cash transfers on birth or nutritional outcomes. This strategy was included in the model, however, because in low income settings, cash transfers are effective in improving i) school enrolment and attendance among girls, ii) access to preventive healthcare and iii) household food consumption.(17–19) As these are all risk factors for poor birth and nutritional outcomes, preconception cash transfers may be useful.(20, 21) Further work is needed to demonstrate effectiveness and acceptability of combatting overweight and obesity through incentivising the purchase of healthy foods in high income settings.
Behaviour change interventions
Two systematic reviews examining 12 preconception trials identified possible improvements in i) health behaviours including reducing alcohol consumption and smoking, and ii) psychological mediators of intervention effects, such as maternal self-efficacy and perceived control.(22, 23). Neither review reported on maternal nutritional status as an outcome. Two studies tested the effect of preconception nutritional and/or behavioural interventions on birth outcomes: a study in the Netherlands found no effect on pregnancy outcomes when general practitioners counselled couples on health behaviours (24); and a study in Australia found a negative effect on birthweight of counselling on risk factors including diet, timing of next pregnancy, and specialist referrals.(25) The authors speculate that improved preconception health meant that previously unsustainable pregnancies were sustained for longer, resulting in more pre-term births and lower birth weights. If true, this would be an unexpected and adverse effect of preconception intervention.
The challenges of addressing preconception under-nutrition in low income settings may require broader behavioural strategies than those tackling over-nutrition in high income settings. Low resource households cannot simply change their behaviour if food is unavailable and so strategies must combine behaviour change with food access in the way that the CARING Trial has recently evaluated in eastern India.(26, 27) This trial also used a healthcare approach that has successfully engaged women and reduced maternal and neonatal mortality in rural, low-resource settings: participatory learning and action through women’s groups.(28) This facilitated group-based problem-solving approach involves women of all ages and tackles a variety of maternal and newborn problems including nutrition. Although the original trials of this approach do not report on nutritional outcomes, the CARING trial has found that although the approach was not able to significantly increase child length, it did improve key secondary outcomes including dietary diversity and handwashing. The LBWSAT trial is due to report soon.(29) (See Panel 2 for details of ongoing trials.) Interventions in high resource contexts can focus on individual choice but evidence suggests that multi-level interventions may be more effective.(30) The recently-announced intervention trials developed as part of the Canadian governments Healthy Life Trajectory Initiative are good examples of such multi-level interventions that aim to address preconception nutrition and health behaviour but also wider health and social determinants (http://www.cihr-irsc.gc.ca/e/49511.html). These trials will provide gold standard evidence of the effectiveness and cost-effectiveness of multicomponent preconception interventions in improving outcomes for children.
Panel 2 - Ongoing trials of preconception nutrition interventions.
NCT02509988 Nutritional Intervention Preconception and During Pregnancy to Maintain Healthy Glucose Metabolism and Offspring Health (NiPPeR study). The study aims to assess whether a nutritional drink taken before conception and continuing through pregnancy, assists in the maintenance of healthy glucose metabolism in the mother and promotes offspring health. N=1800 women, estimated completion Oct 2018. UK, Singapore and New Zealand sites.
NCT02989142 Inter-pregnAncy Coaching for a Healthy fuTure (inter-act). This intervention targets women with excessive weight gain in their first pregnancy, and attempts to reduce complications in the second pregnancy through an inter-partum programme of coaching combining face-to-face counselling with the use of a mobile App connected to medical devices (scale and pedometer). N=1100, estimated completion Sept 2020. Belgium.
NCT01883193 Women First: Preconception Maternal Nutrition (WF). Multi-country three-arm, individually randomized, non-masked, controlled trial to ascertain the benefits of ensuring optimal maternal nutrition before conception and providing an evidence base for programmatic priority directed to minimizing the risk of malnutrition in all females of reproductive age. Women required to take a lipid-based micronutrient supplement. Running in Pakistan, India, Guatemala and the Congo. N=7374, Run from University of Colorado, Denver, United States. Completion date October 2019.
NCT02617693 Development of Pre-pregnancy Intervention to Reduce the Risk of Diabetes and Prediabetes (Jom Mama). The aim of this study is to assess the efficacy of a pre-pregnancy intervention to reduce the risk of diabetes and prediabetes. A lifestyle intervention combines behaviour change counselling from community health promoters (CHPs) trained in skills to support behaviour change and utilisation of an e-Health platform providing preconception information and support. N=660, estimated completion November 2017. Malaysia.
NTR4150 Erasmus MC Care Innovation for a healthy pregnancy. Efficacy of "Smarter Pregnant", an interactive food and lifestyle coaching program on the mobile phone. To rest whether use of the "Smarter Pregnant" intervention leads to an improvement in unhealthy food habits (vegetables, fruit, folic acid use) after 6 months’ intervention, measured as a decrease in the Food Risk Score of women and men with a wish to become pregnant. N = 3000, estimated completion date January 2017. Netherlands.
Four inter-linked preconception nutrition intervention trials are currently being planned by a consortium of the Canadian Institute for Health Research, the World Health Organisation, the governments of Canada, India, South Africa and China, and academic partners in each country. These randomised controlled trials which are part of the Healthy Lifestyles Trajectory Initiative (HeLTI) aim to test the effect of a package of nutritional and lifestyle interventions before conception on body composition of the offspring. They are currently in planning with the aim of beginning in October 2017.
An additional trial below which is not a preconception trial but which will have implications for understanding the value of cash transfers and participatory women’s groups in improving the nutritional status of women of childbearing age:
ISRCTN75964374 The Low Birth Weight in South Asia Trial (LBWSAT) This cluster randomised controlled trial aims to identify the most cost effective means of increasing birthweight by comparing birthweight in current programme areas with birthweight in areas where one of three combinations of interventions is conducted:(i) a behaviour change strategy (BCS) involving working with participatory women’s groups and other community members to change pregnant women’s eating behaviour to increase their intake of nutritious food; (ii) and (iii) combine this BCS with provision of a food supplement or a cash payment respectively. The primary outcome of the trial is birthweight accurate to 10g measured within 72 hours of birth. N = 17,000 pregnant women; 13,000 babies from 80 study areas in southern Nepal. Completion date unknown.
Preconception interventions often require engagement from individuals who are not thinking about becoming pregnant in the near future and are unlikely to be using maternal health services. Interventions to improve health behaviours of adolescents and young adults may therefore have to be placed outside maternal and child health services and appeal to motivations unrelated to health, such as attractiveness.(31–33)
Motivation for, and engagement in, preconception nutrition and behavioural interventions
The complexities involved in changing individual and population health behaviours are well recognised. It is usually not enough to simply educate or give advice. Knowing something is ‘good for you’ is rarely sufficient to change behaviour. Successful behaviour change requires the target population to i) engage with the need to change, ii) sustain the motivation to maintain the change, and iii) be supported by contexts (service providers, society, social networks, environments) that facilitate change.(34)
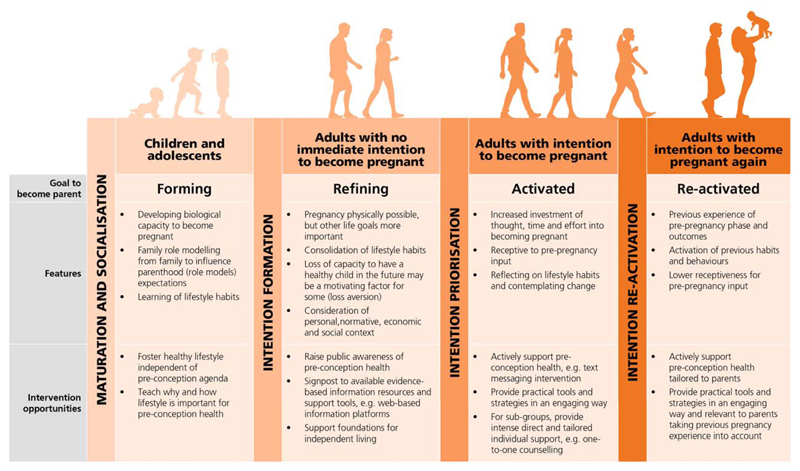
Figure 2 displays a model of Preconception Action Phases adapted from the Rubicon Model of Action Phases and the Action Phase Model of Developmental Regulation, and applied to preconception motivations and interventions.(35, 36) It is based on five assumptions:
Most young adults intend to become parents at some point and this goal begins to form in childhood.
Young adults have the adaptive capacity to pursue this goal amongst their other developmental lifecourse goals and to translate it into action.
The goal to become a parent is nested within other facilitating and conflicting developmental lifecourse goals, which are pursued as opportunities evolve over time.
Motivation to become a parent is the driver that translates that goal into relevant preconception behaviours.
Translating the goal to become a parent into conception and pregnancy outcomes is imperfect.
Figure 2. Model of Pre-conception Action Phases.
(after Heckhausen)
The model distinguishes four phases characterised by overarching biological or psychological agendas and motives in relation to the goal to become a parent. From left to right across the figure interventions become less general and more targeted towards specific populations, in keeping with the dual strategy for promoting preconception health proposed in this paper. Intervention reach will be greater in the earlier phases of the model though effect sizes are likely to be smaller due to lower intensity. Benefits of interventions in these early phases will be general; healthier diets will benefit both the individual and society and enhance motivation in those not planning imminent pregnancy. Creating a social movement would raise awareness of the significance of preconception nutrition and generate a supportive social environment for preconception health. It would help build engagement at each phase and facilitate preparation for pregnancy as a normal part of ‘having a baby', and standard healthcare practice.(37)
Intervening with children and adolescents
In the first phase of Preconception Action, motivation to become a parent, forms without any physical capability for childbearing, which changes as children develop into adolescents. Laying foundations for a healthy life is essential at this time for reasons independent of any preconception health agenda, but given the general low level of awareness of healthy preparation for pregnancy as a concept, awareness-raising is needed from an early age.
Recent recognition of the ‘triple benefit’ from investment in adolescent health – their health now, their health in the future and the health of the next generation – has focused attention on this lifecourse phase.(38–40) Ninety percent of the world’s 1.8 billion adolescents live in low- and middle-income countries (LMICs); up to half are stunted and pregnancy is common.(39) For this group, a key intervention in improving outcomes for mothers and babies is to delay first pregnancy beyond 18 years, when nutrients are no longer needed to support maternal growth.(41) In high income countries (HICs), adolescents have the poorest diets of any age group.(42) Both physiological responses and health behaviours established during adolescence are known to track into adulthood, and neurological and epigenetic changes in adolescence suggest it as a critical period for establishing long-term health risk.(43, 44) Adolescents typically disengage with traditional health messages, prioritising the immediate over the long-term; having a strong desire for autonomy causes them to reject instructive health education.(45, 46) Effective interventions with adolescents need to empower and encourage by giving rather than taking away responsibility.
The LifeLab programme (see web appendix for details) is an example of a school-based intervention aimed at developing adolescents’ motivations for improving their diets and physical activity levels through engagement with science, with an emphasis on their health but with reference to benefits for their future children.(47, 48) The students report that being good parents in the future is important to them. Learning about preconception health motivates them to improve their diets and physical activity. LifeLab has potential to help children and adolescents develop a concept of preconception and parenthood, but this may not motivate change because it is not an immediate imperative. Motivation is a necessary but not sufficient condition for behaviour change.(49) The addition of an in-person intervention to LifeLab would support students’ capabilities (ie. ‘you can do this!’, ‘I believe in you!’) and opportunities for behaviour change (ie. ‘how are you going to exercise more?’, ‘what is your plan for eating healthily?’). In settings where female participation in formal schooling is low, alternative approaches are needed to ensure engagement of adolescent boys and girls.
In rural South Africa, where there are high rates of overweight and obesity amongst adolescents, the ‘Ntshembo’ (‘Hope’) intervention aims to achieve a healthy body mass index in 14-19 year olds through a two-year programme of behaviour change support. Working with adolescents, their carers and village leaders, Ntshembo is explicitly designed to address individual and community motivations and capabilities and to restructure opportunities for adolescents to eat well and exercise more.(50) It harnesses the power of social influence on adolescent behaviour through peer support, and employs community health workers trained to support problem-solving and capitalise on adolescents’ need for autonomy; the development of an adolescent-friendly health service to deliver gender and context-specific interventions is widely supported.(51) As in LifeLab, the preconception agenda in Ntshembo is largely that of the intervention developers, who will need to engage with adolescent’s own imperatives for the intervention to succeed. The results of the current pilot trial are eagerly awaited.
Interventions with adults not immediately intending to become pregnant
In this second phase, the goal to become a parent is refined and shaped by the individuals’ psychological, social, economic and biological status.(52–54) As young adults mature, developmental goals such as completing education, obtaining employment and forming intimate relationships generally take priority over becoming a parent. Consequently, preconception health will have little ‘motivational currency’ during this phase. Effective methods of engagement at this stage will be highly context specific.
In some cultures, marriage offers an opportunity to engage couples in thinking about their nutrition and health before conception, particularly in countries where pre-marital testing aimed at reducing transmission of inherited disorders is mandatory. The Jom Mama project, supported by the Malaysian government, uses an existing pre-marital HIV screening and wellness programme to provide preconception nutrition support to couples, using a combination of a web-based platform and in-person behaviour change support. (55) (See web appendix for more details.) Newly-married Malaysian women said that having a healthy baby in the future was a major motivation for improving their diets and physical activity. (See Panel 3 for details of these conversations.) Other lifecourse goals however, such as work, were a barrier to eating well and being active. The effectiveness of this intervention is not yet known but may be constrained by its focus on individual responsibility and the fact that it does not directly address the challenge of social influences or an obesogenic environment.
Panel 3 - Motivations for engagement in interventions to improve preconception health.
In the development of the Jom Mama intervention, 18 couples were interviewed about their motivations to engage with the intervention programme and to improve their health before conception. Having a healthy pregnancy and a healthy child were clear motives for improving diet and lifestyle:
Because I want to conceive as I’ve never conceived before. So getting pregnant will motivate us. Respondent 12
I wanted to be healthy for myself and for my child…I think my commitment as a wife and mother is important. Respondent 10
Interviewees suggested a range of incentives including financial and personalised support from healthcare staff would sustain their engagement in a programme of diet and lifestyle improvement, as would stories from others at the same stage of life. They also proposed that programme content should be simple, attractive, and specifically targeted to them and that it should not interfere with their working hours, suggesting therefore that delivery be on a digital platform, accessible at their convenience.
Participants in Jom Mama described a number of features of their lives as young, working people that acted as barriers to improving their diets and physical activity levels in preparation for pregnancy.
Working patterns:
I usually don’t take breakfast …and then I start work, rest at 12.30pm, but if I’m too busy I don’t rest until the evenings, sometimes at 6pm, sometimes until 8, 9pm only then I go home. Respondent 8
Eating habits:
Sometimes I have lunch at 12 noon…sometimes at 3pm…it’s uncertain Respondent 13
Exercise:
Not after marriage…can’t make it in the evening. No time Respondent 01
In the UK, women who had recently had a child and were attending routine appointments with health visitors were approached and asked whether they would be planning another pregnancy in the following 12 months. Those who indicated they would be interested were invited to participate in a pilot study of the effectiveness of the Smarter Pregnancy intervention and subsequently provide an in-depth interview. Fifteen women were interviewed and their views of preconception care were sought.
Women felt that just because they had already had a baby did not mean they were aware of what was required for a healthy conception and pregnancy. Because of their involvement in the inter-conception study, they accepted that preconception care was important, something they may not have considered before:
We’ve not had something like this before and I felt like, at that time when I wanted to get pregnant… you don’t know, even though you’ve had three kids already before. You just forget everything. (Woman 31, married with 3 children aged 14, 8 and 4)
I know [now] that our body has to be ready before we get pregnant. You need to be prepared. Everything has to be enough. Since then, I know, I start to understand you have to eat enough vitamins to get pregnant. (Woman 31, married with 2 children - a baby and a 10 year old.)
When they discussed the implications of their new understanding, women highlighted the importance of improving their health prior to conceiving, with specific focus on improving their diet and being a healthy weight:
In terms of...sometimes, you lose track of what is healthy. So that is when I had to relook at my diet in terms of having more vegetables and then taking my folic acid and looking at all of these healthy things. (Woman 40, previous still birth, currently pregnant)
Key sources of information for preconception care were the internet and friends and family. There was a desire for reliable and accredited sources of information to put couples’ minds at ease. What the women said suggests there is an evident gap in current provision of preconception health information:
I think the problem is if people don't know, they go to Google. And you go to Google, and you get some chat on Mumsnet. And it's a load of women feeding other women garbage... there's so much false information out there. But if you don't know that, you go “This is what it means.” Stuff like this [the intervention material] just keeping people on the straight and narrow is quite helpful. (Woman 32, one child aged 1 year, recent miscarriage)
There was agreement amongst women that healthier lifestyles can contribute to healthier pregnancies, a reflection that they had not considered this for their previous pregnancy and an intention to improve their nutritional status in preparation for the next pregnancy. All of which suggests that the inter-partum period might be a fruitful time to engage women in preconception health care.
Footnote:
In the UK, women are under the care of the community health visiting services from pregnancy up to 5 years of age of the child.
The absence of dedicated preconception healthcare in many countries means interventions to improve preconception nutritional status need to take advantage of routine contact between young adults and healthcare providers.(56) Offering support in reproductive health clinics, for example, has the potential to improve the preconception nutritional status of women who may or may not be actively planning pregnancies. This requires healthcare professionals to be aware of its significance, have skills to intervene and see it as part of their job; none of which is currently the case. To help raise awareness, the USA’s Centre for Disease Control promotes a ‘Reproductive Life Plan’ intended to encourage people of child-bearing age to prepare for pregnancy and maximise the preconception benefit of interactions with healthcare professionals.(57)
Training for healthcare professionals of all types in skills to support behaviour change is available in the form of Healthy Conversation Skills. This set of easily-acquired, theory-based skills for practitioners is designed to engage and motivate patients and clients during brief consultations. Unlike giving information and advice, Healthy Conversation Skills training promotes use of open discovery questions, listening, reflecting and goal-setting to enable a woman or couple to prepare for pregnancy and support them in finding their own solutions to challenges. The skills have been used in maternal and child health contexts around the world and their use is both acceptable and feasible.(58–60)
Armed with such skills, practice and community nurses, sexual and reproductive health clinic staff, those working in Early Pregnancy Units who see women who have miscarried, and staff providing weight management services are all potential agents for delivering appropriate, timely, and culturally-sensitive support to improve preconception nutritional status at scale. Extending training in skills to support behaviour change to community health workers has potential for widespread impact on preconception health; evidence from other contexts suggests this can improve health outcomes in a range of public health and primary care settings.(61, 62) Local and national policies would be helpful to support the implementation of such training for community health workers. An approach such as Healthy Conversation Skills enables healthcare professionals to provide care that is responsive to women’s personal, social and cultural milieux.(56)
In contexts outside healthcare, supermarkets represent an unexploited opportunity for promoting preconception nutrition. Supermarkets have an unparalleled reach into communities and expertise in customer engagement. Women do the majority of family food shopping and in HICs these choices are made in supermarkets.(63) Recent research indicates that the food choices of disadvantaged women are particularly susceptible to the supermarket environment, suggesting that modifications which encourage the purchasing of healthier foods might have greatest impact on women with the poorest diets.(64) In LMICs, the role of supermarkets as food purveyors is rapidly increasing although this is less the case in remote and rural areas where increasing the accessibility of nutrient-dense food remains a priority.(65, 66) A model whereby supermarkets offer preconception nutritional support organised around sales of folic acid and other supplements is one that could be developed in HICs and, if successful, translated to LMICs as supermarkets become more widespread.
Interventions with adults intending to become pregnant
In the third phase, the goal to become a parent has been activated through a combination of social (e.g. subjective norms), situational (e.g. marriage) and biological (e.g. age) factors and is now actively pursued. This phase is characterised by an increased investment of thought, time and effort into becoming pregnant. Willingness to engage in interventions increases and can range from passive (e.g. reduced investment in contraception) to active behaviours. Preconception interventions are likely to be attended to and, with support, translated into behaviour change. Interventions need to allow for swift and discrete implementation, given the sensitive nature of couples’ plans for conception, and active promotion through channels such as contraception counselling.
Since this group is likely to be engaged and seeking information, preconception health services in primary care, with a focus on nutrition, may be appropriate. There is evidence that interventions offered in this setting can improve preconception health behaviours in women who are planning to become pregnant.(23, 67, 68) Screening for pregnancy intention (ref Stephenson et al paper 1 in this series) would enable practitioners in sexual and reproductive health clinics to offer preconception support to women attending for removal of implants and IUDs, for example.
Digital interventions, web or smartphone-based, offer privacy and easy access for disadvantaged or disenfranchised groups less likely to engage with more formal services. ‘Smarter Pregnancy’, or Slimmer Zwanger in Dutch, is a rare example of a digital intervention designed specifically to support improvements in preconception nutrition and health behaviours. It has had some success with couples who are actively preparing for pregnancy.(69, 70) (See web appendix for details.) Mobile phone interventions to improve maternal and child health in LMICs have delivered tailored information and supported improved infant feeding outcomes.(71) Evidence is accumulating that combining digital interventions with motivational human interaction increases engagement with, and effectiveness of, behaviour change interventions.(72) An accessible, population-wide preconception healthcare service could be offered to women via a digital intervention combined with face-to-face or telephone contact with healthcare staff trained in a motivational approach such as Healthy Conversation Skills.
Interventions with adults intending to become pregnant again
In the fourth phase, the goal to become a parent is re-activated. Preparation for this pregnancy is likely to be influenced by couples’ previous preconception experiences. Previously uncomplicated pregnancies might decrease receptiveness for preconception input; if their first baby was healthy why would couples change their preparations?
Women and their families have intensive contact with health services and health professionals during pregnancy and are motivated to make dietary changes. Evidence shows that interventions can support maternal dietary behaviour change (ref Stephenson et al paper 1 in this series), and reduce postnatal weight gain. (73–77)
In LMICs, interest has focussed mainly on maternal underweight and micronutrient deficiencies. Numerous supplementation studies have shown women’s willingness to take nutritional supplements during pregnancy, with consequent reductions in low birth weight. Few have addressed under-nutrition during pregnancy by supporting change in habitual dietary behaviour, probably because choices tend to be limited in undernourished settings. Qualitative studies have suggested modifiable dietary behaviours in LMIC populations however, and this is ripe for more research.(78)Young rural Indian women report avoiding specific nutritious foods because of fears they could harm a pregnancy, ‘eat down’ in the belief that this will make delivery easier, eat the least nutritious foods after other family members have eaten because of household hierarchies, and observe women’s cultural fasting days, eating predominantly low nutrient foods.(79, 80) These data provide further support for embedding preconception nutritional interventions in those that support wider social and cultural change.
Maternal and child healthcare systems offer some post- or inter-partum opportunities for working with women to support dietary behaviour change. Women interviewed following an inter-partum intervention at a health visitor clinic in London, UK, had a new awareness that their nutritional status during and between pregnancies had an impact on the baby (see Panel 3). In HICs, post-partum studies have focussed mainly on limiting weight retention among normal or overweight women and/or improving glucose tolerance among women with a history of gestational diabetes.(81, 82) Reviews suggest that interventions to address both diet and physical activity which include self-monitoring of progress may be more effective than others.(83, 84) Some studies have successfully used education programmes or financial incentives to improve dietary quality by reducing energy intake and increasing fruit, vegetable and whole grain intake.
Many post-partum randomised studies report low recruitment or retention rates however; post-partum mothers report multiple barriers to participation, including little spare time, stress and sleep deprivation. Interventions may need to take a supportive approach involving home visits, provision of foods and/or childcare, and/or self-monitoring facilities such as weighing scales.(85) One solution may be to integrate in-person support for inter-partum behaviour change with a digital service. Postpartum weight retention is associated with lifetime obesity risk and adverse outcomes in the next pregnancy. A recent, cluster randomised trial of an internet-based weight loss programme coupled with face-to-face support (Fit Moms/Mamás Activas) in low-income women in California, USA, found that women in the intervention group maintained significantly greater weight loss at 12 months than women who were not randomised to the intervention (3.2kg versus 0.9kg; difference 2.3 kg (95% CI, 1.1 to 3.5). (86)
Creating a social movement for preconception nutrition
A social movement to optimise preconception health, nutritional status and health behaviours needs to involve the whole population and harness political will and leadership. A social movement in Brazil led to significant improvements in preconception nutrition for women and virtual eradication of undernutrition and wasting among children under-five between 1994 and 2006.(87, 88) The movement involved i) a ‘National Campaign against Hunger’ that raised public awareness of the need to tackle malnutrition and ii) development of an advocacy coalition with political affinities that created a critical mass of activists and monitored government’s progress in reducing malnutrition. Eradicating malnutrition became a high-profile social responsibility, prompting strong leadership from central government in addressing food security. Underpinning Brazil’s approach was an appreciation that how women feed themselves and their children is not solely an individual responsibility but involves wider determinants.
Social movements are distinct from social marketing campaigns. The latter would classically attempt to improve nutrition and health behaviour through providing information and recommending behaviour change, but may fail to reach the neediest groups and inadvertently widen inequalities.(89, 90) The UK’s ‘Change4Life’ intervention adopted this approach, with little evidence of effectiveness.(91) Social practice theory provides some insight as to why such campaigns are insufficient; individuals and communities require not only knowledge but also resources to enact change, and a purpose or meaning to provide motivation.(92) A social movement which would provide these might best be founded in socially-constructed ideas of human action and allied therefore to the field of consumer marketing and brand creation.
Consumer marketing recognises that individual behaviour and choices are a function of self-image, and brands must develop an emotional and symbolic connection with consumers, making the brand a form of self-expression.(93) A campaign using current brand development practice would target emotions that are central to an individual’s identity. This approach is epitomised in such campaigns as the ‘handwashing with soap’ social movement, which applied brand marketing practices and an advocacy campaign to address infant mortality under the tag-line “Help a Child Reach 5”. The media campaign follows the principles of being personally relevant, emotionally engaging and easy to understand.(94) The evidence-based rationale for handwashing is given only after the other appeals have been made. The campaign was driven by a multi-national company (Unilever), supported by an alliance of public health activists and academics. It has received strong endorsement by the inclusion of handwashing with soap as an indicator in the Sustainable Development Goals, and government policy initiatives to improve washing facilities.
The handwashing movement is an example of mutual benefit for public health and for private sector profit that can come from a joint social purpose. Companies are much more likely to ‘do the right thing’ and to do it sustainably if public health benefit is accompanied by commercial gain.(94)
Black and colleagues in the Lancet in 2013 declare that ‘the private sector is an important force in shaping nutrition outcomes and has the potential to do more’ to improve maternal and child nutrition (p.374).(3) There is a growing recognition of the importance of engaging with the food industry in recognition of their reach and power to shape consumer behaviour. A major difficulty with applying the ‘mutual benefit’ approach to improving preconception nutrition and lifestyle through a relationship with the food industry is their history of malpractice in respect of infant feeding and their role in generating and sustaining an obesogenic environment. Whether commercial and public health interests can be aligned in the way they have been for handwashing remains to be seen. One attempt is Unilever’s campaign to market stock cubes fortified to reduce iron-deficiency anaemia amongst women in Nigeria.(95) Current lobbying by industry against sugar-sweetened beverage taxes does suggest, however, that caution is required to ensure the legitimacy of health actions and lobbying by food industry. Independent monitoring of food industry activities by academia and the public is crucial to building societal support that will catalyse government and industry actions in respect of preconception health.(96)
Applying marketing principles to generating a preconception social movement suggests that it should be emotionally engaging and positively framed. The voice of a child not yet born, speaking from the future, thanking parents, grandparents, aunts, etc. for looking after her health from before conception is the kind of emotional appeal applicable to a preconception campaign. The call to action would target the whole population and would ask people to, for example, support young women or couples to achieve an optimal pre-pregnancy weight or eat a variety of fruit and vegetables. The challenge is to identify simple actions around which the campaign could be built.
Building advocacy coalitions for preconception nutrition
Political science suggests that we need to develop a strong advocacy coalition within international, national and local policy subsystems to place preconception nutrition firmly on government agendas to incite global policy action.(97) International organisations are already engaged in advocacy to promote improved preconception healthcare. In 2012, WHO coordinated a global consensus on ‘Preconception Care to Reduce Maternal and Childhood Mortality and Morbidity’ and provided a package of evidence-based interventions, including nutritional interventions.(98) Following this, preconception nutrition was integrated into a number of transnational organisation initiatives. With the notable exception of the Netherlands, only LMICs provide examples of political support for the adoption of strategies to address social, environmental and economic determinants of maternal and child malnutrition and grass roots demand for action.(87, 99) Political debate in the Netherlands was sparked by academics drawing attention to high national perinatal mortality rates, especially among poor immigrant communities. The promotion of preconception health to reach the poorest has since become a Dutch priority and includes addressing social deprivation and broad determinants of maternal ill-health.
Policy change is more likely if advocacy coalitions are developed to focus on a specific policy subsystem and engage multiple participants (i.e. government agencies, research institutions, non-government organisations, the media, commercial interests and influential individuals) to build critical mass.(97) Strong leadership, adequate resources, and coordinated infrastructure are required to ensure advocacy coalitions sustain engagement over the potentially lengthy period of time necessary to achieve high-level, coordinated policy action particularly in competitive policy subsystems with opposing advocacy coalitions. Initiatives such as sugar taxes or marketing restrictions to curb sugar intake have recently gained policy traction in some countries following decades of increasing evidence, advocacy and public awareness and in spite of strong opposition from food companies.(100) One major advantage of campaigning for better preconception nutrition is that the focus is building stronger mothers and babies and reducing non-communicable disease in the next generation. These are uncontroversial messages, easy for the public to engage with emotionally.
Conclusions
A dual strategy of simultaneously targeting women and couples most likely to be planning a pregnancy, while promoting the health of all women of child-bearing age may be the most effective approach to improving preconception health. Sparse evidence from robust and context-relevant trials of preconception nutrition and health behaviour interventions makes it hard to draw firm conclusions about their effectiveness in improving outcomes for mothers and babies on a large scale. Trials of preconception interventions are far fewer than those conducted during pregnancy, because recruitment is more difficult and outcomes can be assessed only in women who subsequently become pregnant. Fortunately several such trials are underway. Meanwhile, public health strategies to improve nutritional status in children and those of reproductive age should be strengthened without delay.
Best evidence suggests that interventions will be more effective for longer if they use existing delivery platforms within a systems approach. System-wide changes to accommodate preconception healthcare will need support from a social movement that establishes its importance for the health of the next generation, stresses societal responsibility and requires strong local, national and international leadership. The strength of this social movement and the capacity to deliver effective nutrition and behavioural interventions may be enhanced through carefully negotiated engagement with commercial interests.
Panel 1 - Key messages.
-
-
Epidemiological data and findings from developmental biology suggest that intervening to improve men and women’s nutritional status before pregnancy improves longterm outcomes for mothers and babies
-
-
Trials of interventions to improve nutritional status before conception and birth outcomes are scarce, but new trials are underway
-
-
Existing evidence of effectiveness of preconception nutritional interventions endorses the provision of micronutrients, mainly through supplementation or food fortification, particularly folic acid and iodine
-
-
To maximise benefit and achieve health growth trajectories in the next generation, preconception strategies should be broader than supplementation or fortification and address the wider determinants of health
-
-
Motivations to engage with preconception nutrition differ according to age and phase of life stage; understanding and harnessing these motivations is key to successful intervention
-
-
Interventions need also to be context specific and to make best use of existing platforms for delivery
-
-
Preconception interventions need to be supported by a social movement and political will, both of which may of necessity require skilful engagement with powerful commercial interests.
Acknowledgments
The idea for this series was conceived by Judith Stephenson and developed during a four day symposium, led by Mary Barker and Judith Stephenson and funded by The Rank Prize Funds, on ‘Preconception Nutrition and Lifelong Health’ in Grasmere, UK, February 2016. We thank a number of individuals who have contributed their thoughts and time to this paper: Nicola Heslehurst for her contribution to an early draft of this paper; Zulfi Bhutta for his perspectives on interventions in LMICs; Chandni Jacob and Mark Hanson for their contribution to the review; Jayne Hutchinson and Janet Cade for advice on fortification; Matthijs van Dijk for data from Smarter Pregnancy; Julius Cheah and the Ministry of Health Malaysia for data from the Jom Mama Project; Mike Kelly for discussions on the value of social practices; and Andy Last for his insights into how a social movement might be created around preconception health.
Footnotes
Contributors
MB conceptualised the review in consultation with all authors and wrote the first draft of the paper with substantial inputs from TC, JSW, GN, SUD, FFS, CHDF, SN, CV, NMK, WL and JS. TC, JSW and GN carried out the review and produced the pathways model of intervention effects. The analysis of preconception action phases was developed by SUD and FFS. SN, RST, DP, KWT provided data and wrote descriptions of exemplar intervention studies. CHDF wrote the first draft of the section on interventions with adults intending to become pregnant again. Sections on the creation of a social movement and advocacy coalitions were produced by CV and NMK. JS oversaw and advised on all aspects of producing and editing this paper. All authors saw successive drafts of the paper and provided input. MB finalized the paper and is the overall guarantor.
Declaration of interests
We declare no competing interests.
References
- 1.World Health Organisation. Work Programme of the UN Decade of Action on Nutrition, 2016-2025. Geneva: WHO; 2017. Available from: http://www.who.int/nutrition/decade-of-action/workprogramme-2016to2025/en/ [Google Scholar]
- 2.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends in endocrinology and metabolism: TEM. 2010;21(4):199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Black RE, Alderman H, Bhutta ZA, Gillespie S, Haddad L, Horton S, et al. Maternal and child nutrition: building momentum for impact. The Lancet. 2013;382(9890):372–5. doi: 10.1016/S0140-6736(13)60988-5. [DOI] [PubMed] [Google Scholar]
- 4.Ceschia A, Horton R. Maternal health: time for a radical reappraisal. The Lancet. 2016;388(10056):2064–6. doi: 10.1016/S0140-6736(16)31534-3. [DOI] [PubMed] [Google Scholar]
- 5.Gaglio B, Shoup JA, Glasgow RE. The RE-AIM Framework: A Systematic Review of Use Over Time. American Journal of Public Health. 2013;103(6):e38–e46. doi: 10.2105/AJPH.2013.301299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald EC, Pollitt E, Mueller W, Hsueh AM, Sherwin R. The Bacon Chow study: maternal nutrition supplementation and birth weight of offspring. Am J Clin Nutr. 1981;34(10):2133–44. doi: 10.1093/ajcn/34.10.2133. [DOI] [PubMed] [Google Scholar]
- 7.Caan B, Horgen DM, Margen S, King JC, Jewell NP. Benefits associated with WIC supplemental feeding during the interpregnancy interval. Am J Clin Nutr. 1987;45(1):29–41. doi: 10.1093/ajcn/45.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Potdar RD, Sahariah SA, Gandhi M, Kehoe SH, Brown N, Sane H, et al. Improving women's diet quality preconceptionally and during gestation: effects on birth weight and prevalence of low birth weight--a randomized controlled efficacy trial in India (Mumbai Maternal Nutrition Project) Am J Clin Nutr. 2014;100(5):1257–68. doi: 10.3945/ajcn.114.084921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahariah SA, Potdar RD, Gandhi M, Kehoe SH, Brown N, Sane H, et al. A Daily Snack Containing Leafy Green Vegetables, Fruit, and Milk before and during Pregnancy Prevents Gestational Diabetes in a Randomized, Controlled Trial in Mumbai, India. The Journal of Nutrition. 2016;146(7):1453S–60S. doi: 10.3945/jn.115.223461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pena-Rosas JP, De-Regil LM, Dowswell T, Viteri FE. Daily oral iron supplementation during pregnancy. The Cochrane database of systematic reviews. 2012;12 doi: 10.1002/14651858.CD004736.pub4. Cd004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassi ZS, Bhutta ZA. Clinical utility of folate-containing oral contraceptives. International Journal of Women's Health. 2012;4:185–90. doi: 10.2147/IJWH.S18611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food Fortification Initiative. Say Hello to a Fortified Future: 2016 YEAR IN REVIEW. 2016 Available from: http://ffinetwork.org/about/stay_informed/publications/documents/FFI2016Review.pdf.
- 13.World Health Organisation. Fortification of food-grade salt with iodine for the prevention and control of iodine deficiency disorders: Guideline. Geneva, Switzerland: World Health Organisation; 2014. [PubMed] [Google Scholar]
- 14.Crider KS, Bailey LB, Berry RJ. Folic Acid Food Fortification—Its History, Effect, Concerns, and Future Directions. Nutrients. 2011;3(3):370–84. doi: 10.3390/nu3030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mastroiacovo P, Leoncini E. More folic acid, the five questions: why, who, when, how much, and how. BioFactors (Oxford, England) 2011;37(4):272–9. doi: 10.1002/biof.172. [DOI] [PubMed] [Google Scholar]
- 16.Scientifc Advisory Committee on Nutrition. Update on folic acid. 2017. Jul, [Google Scholar]
- 17.Manley J, Gitter S, Slavchevska V. How Effective are Cash Transfers at Improving Nutritional Status? A Rapid Evidence Assessment of Programmes’ Effects on Anthropometric Outcomes. London: EPPI Centre, Social Sciences Research Unit, Institute of Education, University of London; 2012. [Google Scholar]
- 18.Fenn B, Colbourn T, Dolan C, Pietzsch S, Sangrasi M, Shoham J. Impact evaluation of different cash-based intervention modalities on child and maternal nutritional status in Sindh Province, Pakistan, at 6 mo and at 1 y: A cluster randomised controlled trial. PLOS Medicine. 2017;14(5):e1002305. doi: 10.1371/journal.pmed.1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenn B, Pietzsch S, Morel J, Ait-Aissa M, Calo M, Grootenhuis F, et al. Research on Food Assistance for Nutritional Impact (REFANI): Literature Review. New York, USA: Action Against Hunger; 2015. [Google Scholar]
- 20.Ruel MT, Alderman H. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet. 2013;382(9891):536–51. doi: 10.1016/S0140-6736(13)60843-0. [DOI] [PubMed] [Google Scholar]
- 21.Rawlings LB, Rubio GM. Evaluating the Impact of Conditional Cash Transfer Programs. The World Bank Research Observer. 2005;20(1):29–55. [Google Scholar]
- 22.Whitworth MK, Dowswell T. Routine pre-pregnancy health promotion for improving pregnancy outcomes. Cochrane Database of Systematic Reviews. 2009;(4) doi: 10.1002/14651858.CD007536.pub2. Art. No.: CD007536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussein N, Kai J, Qureshi N. The effects of preconception interventions on improving reproductive health and pregnancy outcomes in primary care: A systematic review. The European journal of general practice. 2016;22(1):42–52. doi: 10.3109/13814788.2015.1099039. [DOI] [PubMed] [Google Scholar]
- 24.Elsinga J, de Jong-Potjer LC, van der Pal-de Bruin KM, le Cessie S, Assendelft WJ, Buitendijk SE. The effect of preconception counselling on lifestyle and other behaviour before and during pregnancy. Womens Health Issues. 2008;18(6 Suppl):S117–25. doi: 10.1016/j.whi.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Lumley J, Donohue L. Aiming to increase birth weight: a randomised trial of pre-pregnancy information, advice and counselling in inner-urban Melbourne. BMC Public Health. 2006;6:299. doi: 10.1186/1471-2458-6-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair N, Tripathy P, Sachdev HS, Bhattacharyya S, Gope R, Gagrai S, et al. Participatory women’s groups and counselling through home visits to improve child growth in rural eastern India: protocol for a cluster randomised controlled trial. BMC Public Health. 2015;15(1):384. doi: 10.1186/s12889-015-1655-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nair N, Tripathy P, Sachdev HS, Pradhan H, Bhattacharyya S, Gope R, et al. Effect of participatory women's groups and counselling through home visits on children's linear growth in rural eastern India (CARING trial): a cluster-randomised controlled trial. The Lancet Global health. 2017;5(10):e1004–e16. doi: 10.1016/S2214-109X(17)30339-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prost A, Colbourn T, Seward N, Azad K, Coomarasamy A, Copas A, et al. Women's groups practising participatory learning and action to improve maternal and newborn health in low-resource settings: a systematic review and meta-analysis. Lancet. 2013;381(9879):1736–46. doi: 10.1016/S0140-6736(13)60685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saville NM, Shrestha BP, Style S, Harris-Fry H, Beard BJ, Sengupta A, et al. Protocol of the Low Birth Weight South Asia Trial (LBWSAT), a cluster-randomised controlled trial testing impact on birth weight and infant nutrition of Participatory Learning and Action through women’s groups, with and without unconditional transfers of fortified food or cash during pregnancy in Nepal. BMC pregnancy and childbirth. 2016;16:320. doi: 10.1186/s12884-016-1102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Compernolle S, De Cocker K, Lakerveld J, Mackenbach JD, Nijpels G, Oppert J-M, et al. A RE-AIM evaluation of evidence-based multi-level interventions to improve obesity-related behaviours in adults: a systematic review (the SPOTLIGHT project) International Journal of Behavioral Nutrition and Physical Activity. 2014;11(1):147. doi: 10.1186/s12966-014-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos I, Sniehotta FF, Marques MM, Carraça EV, Teixeira PJ. Prevalence of personal weight control attempts in adults: a systematic review and meta-analysis. Obesity Reviews. 2017;18(1):32–50. doi: 10.1111/obr.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhutta Z, Das J, Rizvi A, Gaffey M, Walker N, Horton S, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382(9890):452–77. doi: 10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
- 33.De-Regil LM, Harding KB, Roche ML. Preconceptional Nutrition Interventions for Adolescent Girls and Adult Women: Global Guidelines and Gaps in Evidence and Policy with Emphasis on Micronutrients. The Journal of Nutrition. 2016;146(7):1461S–70S. doi: 10.3945/jn.115.223487. [DOI] [PubMed] [Google Scholar]
- 34.Kwasnicka D, Dombrowski SU, White M, Sniehotta F. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health Psychol Rev. 2016;10(3):277–96. doi: 10.1080/17437199.2016.1151372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heckhausen H. Motivation and Action. Berlin: Springer-Verlag; 1991. [Google Scholar]
- 36.Heckhausen J. Developmental regulation in adulthood: Age-normative and sociostructural constraints as adaptive challenges. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 37.Steegers EA, Barker ME, Steegers-Theunissen RP, Williams MA. Societal Valorization of New Knowledge to Improve Perinatal Health: Time to Act. Paediatric and Perinatal Epidemiology. 2016;30:201–4. doi: 10.1111/ppe.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United Nations Secretary-General. Global Strategy for Women’s, Children’s and Adolescent’s Health (2016-2030) New York: 2015. [Google Scholar]
- 39.World Health Organisation. Global Accelerated Action for the Health of Adolescents (AA-HA!): guidance to support country implementation. Geneva: World Health Organisation; 2017. Contract No.: WHO/FWC/MCA/17.05. [Google Scholar]
- 40.Patton GC, Sawyer SM, Santelli JS, Ross DA, Afifi R, Allen NB, et al. Our future: a Lancet commission on adolescent health and wellbeing. The Lancet. 2016;387(10036):2423–78. doi: 10.1016/S0140-6736(16)00579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King JC. A Summary of Pathways or Mechanisms Linking Preconception Maternal Nutrition with Birth Outcomes. The Journal of Nutrition. 2016 doi: 10.3945/jn.115.223479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bates B, Lennox A, Prentice A, Bates C, Page P, Nicholson S, et al. National Diet and Nutrition Survey: Results from Years 1-4 (combined) of the Rolling Programme (2008/2009 – 2011/12) London: Public Health England and Food Standards Agency; 2014. [Google Scholar]
- 43.Craigie AM, Lake AA, Kelly SA, Adamson AJ, Mathers JC. Tracking of obesity-related behaviours from childhood to adulthood: a systematic review. Maturitas. 2011;70 doi: 10.1016/j.maturitas.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Viner RM, Ross D, Hardy R, Kuh D, Power C, Johnson A, et al. Life course epidemiology: recognising the importance of adolescence. Journal of epidemiology and community health. 2015 doi: 10.1136/jech-2014-205300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vansteenkiste M, Simons J, Lens W, Sheldon KM, Deci EL. Motivating learning, performance, and persistence: the synergistic effects of intrinsic goal contents and autonomy-supportive contexts. J Pers Soc Psychol. 2004;87(2):246–60. doi: 10.1037/0022-3514.87.2.246. [DOI] [PubMed] [Google Scholar]
- 46.Blakemore SJ, Mills KL. Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- 47.Grace M, Woods-Townsend K, Griffiths J, Godfrey K, Hanson MA, Galloway I, et al. A science-based approach to developing teenagers’ views on their health and the health of their future children. Heath Education. 2012;112(6):543–59. [Google Scholar]
- 48.Woods-Townsend K, Bagust L, Barker M, Christodoulou A, Davey H, Godfrey K, et al. Engaging teenagers in improving their health behaviours and increasing their interest in science (Evaluation of LifeLab Southampton): study protocol for a cluster randomized controlled trial. Trials. 2015;16(1):372. doi: 10.1186/s13063-015-0890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michie S, West R. Behaviour change theory and evidence: a presentation to Government. Health Psychology Review. 2013;7(1):1–22. [Google Scholar]
- 50.Draper CE, Micklesfield LK, Kahn K, Tollman SM, Pettifor JM, Dunger DB, et al. Application of Intervention Mapping to develop a community-based health promotion pre-pregnancy intervention for adolescent girls in rural South Africa: Project Ntshembo (Hope) BMC Public Health. 2014;14(Suppl 2):S5. doi: 10.1186/1471-2458-14-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhutta ZA, Lassi ZS, Bergeron G, Koletzko B, Salam R, Diaz A, et al. Delivering an action agenda for nutrition interventions addressing adolescent girls and young women: priorities for implementation and research. Annals of the New York Academy of Sciences. 2017;1393(1):61–71. doi: 10.1111/nyas.13352. [DOI] [PubMed] [Google Scholar]
- 52.Bachrach CA, Morgan SP. A Cognitive-Social Model of Fertility Intentions. Population and development review. 2013;39(3):459–85. doi: 10.1111/j.1728-4457.2013.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller WB. Childbearing motivations, desires, and intentions: a theoretical framework. Genetic, Social, and General Psychology Monographs. 1994 [PubMed] [Google Scholar]
- 54.Nettle D. Flexibility in reproductive timing in human females: integrating ultimate and proximate explanations. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366(1563):357–65. doi: 10.1098/rstb.2010.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norris SA, Ho JCC, Rashed AA, Vinding V, Skau JKH, Biesma R, et al. Pre-pregnancy community-based intervention for couples in Malaysia: application of intervention mapping. BMC Public Health. 2016;16:1167. doi: 10.1186/s12889-016-3827-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuomainen H, Cross-Bardell L, Bhoday M, Qureshi N, Kai J. Opportunities and challenges for enhancing preconception health in primary care: qualitative study with women from ethnically diverse communities. BMJ Open. 2013;3(7) doi: 10.1136/bmjopen-2013-002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Centre for Disease Control. My Reproductive Life Plan Atlanta, GA2014. Available from: https://www.cdc.gov/preconception/reproductiveplan.html.
- 58.Black C, Lawrence W, Cradock S, Ntani G, Tinati T, Jarman M, et al. Healthy Conversation Skills: increasing competence and confidence in front-line staff. Pub Health Nutr. 2014;17(3):700–7. doi: 10.1017/S1368980012004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baird J, Jarman M, Lawrence W, Black C, Davies J, Tinati T, et al. The effect of a behaviour change intervention on the diets and physical activity levels of women attending Sure Start Children's Centres: results from a complex public health intervention. BMJ Open. 2014;4(7):e005290. doi: 10.1136/bmjopen-2014-005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawrence W, Black C, Tinati T, Cradock S, Begum R, Jarman M, et al. 'Making every contact count': longitudinal evaluation of the impact of training in behaviour change on the work of health and social care practitioners. Journal of health psychology. 2016;21(2):138–51. doi: 10.1177/1359105314523304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaziano TA, Abrahams-Gessel S, Denman CA, Montano CM, Khanam M, Puoane T, et al. An assessment of community health workers' ability to screen for cardiovascular disease risk with a simple, non-invasive risk assessment instrument in Bangladesh, Guatemala, Mexico, and South Africa: an observational study. The Lancet Global health. 2015;3(9):e556–63. doi: 10.1016/S2214-109X(15)00143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel V, Weobong B, Weiss HA, Anand A, Bhat B, Katti B, et al. The Healthy Activity Program (HAP), a lay counsellor-delivered brief psychological treatment for severe depression, in primary care in India: a randomised controlled trial. The Lancet. 2016 doi: 10.1016/S0140-6736(16)31589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pechey R, Monsivais P. Supermarket Choice, Shopping Behavior, Socioeconomic Status, and Food Purchases. American journal of preventive medicine. 2015;49(6):868–77. doi: 10.1016/j.amepre.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogel C, Ntani G, Inskip H, Barker M, Cummins S, Cooper C, et al. Education and the Relationship Between Supermarket Environment and Diet. American journal of preventive medicine. 2016;51(2):e27–34. doi: 10.1016/j.amepre.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Development Initiatives. Global Nutrition Report 2017: Nourishing the SDGs. Bristol, UK: Development Initiatives; 2017. [Google Scholar]
- 66.Hawkes C. Dietary Implications of Supermarket Development: A Global Perspective. Development Policy Review. 2008;26(6):657–92. [Google Scholar]
- 67.Hammiche F, Laven JS, van Mil N, de Cock M, de Vries JH, Lindemans J, et al. Tailored preconceptional dietary and lifestyle counselling in a tertiary outpatient clinic in The Netherlands. Human reproduction (Oxford, England) 2011;26(9):2432–41. doi: 10.1093/humrep/der225. [DOI] [PubMed] [Google Scholar]
- 68.Twigt JM, Bolhuis ME, Steegers EA, Hammiche F, van Inzen WG, Laven JS, et al. The preconception diet is associated with the chance of ongoing pregnancy in women undergoing IVF/ICSI treatment. Human reproduction (Oxford, England) 2012;27(8):2526–31. doi: 10.1093/humrep/des157. [DOI] [PubMed] [Google Scholar]
- 69.van Dijk MR, Koster MPH, Willemsen SP, Huijgen NA, Laven JSE, Steegers-Theunissen RPM. Healthy preconception nutrition and lifestyle using personalized mobile health coaching is associated with enhanced pregnancy chance. Reproductive biomedicine online. 2017;35(4):453–60. doi: 10.1016/j.rbmo.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 70.Van Dijk MR, Huijgen NA, Willemsen SP, Laven JSE, Steegers EAP, Steegers-Theunissen RPM. Impact of an mHealth Platform for Pregnancy on Nutrition and Lifestyle of the Reproductive Population: A Survey. JMIR mHealth and uHealth. 2016;4(2):e53. doi: 10.2196/mhealth.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee SH, Nurmatov UB, Nwaru BI, Mukherjee M, Grant L, Pagliari C. Effectiveness of mHealth interventions for maternal, newborn and child health in low– and middle–income countries: Systematic review and meta–analysis. Journal of Global Health. 2016;6(1):010401. doi: 10.7189/jogh.06.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dennison L, Morrison L, Lloyd S, Phillips D, Stuart B, Williams S, et al. Does brief telephone support improve engagement with a web-based weight management intervention? Randomized controlled trial. Journal of medical Internet research. 2014;16(3):e95. doi: 10.2196/jmir.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. The Cochrane Database Of Systematic Reviews. 2015;6 doi: 10.1002/14651858.CD007145.pub3. CD007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanentsapf I, Heitmann BL, Adegboye ARA. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy and Childbirth. 2011;11(1):81. doi: 10.1186/1471-2393-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gardner B, Wardle J, Poston L, Croker H. Changing diet and physical activity to reduce gestational weight gain: a meta-analysis. Obesity Reviews. 2011;12:e602–e20. doi: 10.1111/j.1467-789X.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 76.Flynn AC, Seed PT, Patel N, Barr S, Bell R, Briley AL, et al. Dietary patterns in obese pregnant women; influence of a behavioral intervention of diet and physical activity in the UPBEAT randomized controlled trial. Int J Behav Nutr Phys Act. 2016;13(1):124. doi: 10.1186/s12966-016-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dodd JM, Cramp C, Sui Z, Yelland LN, Deussen AR, Grivell RM, et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on maternal diet and physical activity: the LIMIT randomised trial. BMC Med. 2014;12:161. doi: 10.1186/s12916-014-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morrison J, Dulal S, Harris-Fry H, Basnet M, Sharma N, Shrestha B, et al. Formative qualitative research to develop community-based interventions addressing low birth weight in the plains of Nepal. Public Health Nutr. 2017:1–8. doi: 10.1017/S1368980017002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chorghade GP, Barker M, Kanade S, Fall CHD. Why are rural Indian women so thin? Findings from a village in Maharashtra. Public Health Nutrition. 2006;9(1):9–18. doi: 10.1079/phn2005762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barker M, Chorghade G, Crozier S, Leary S, Fall C. Gender differences in body mass index in rural India are determined by socio-economic factors and lifestyle. J Nutr. 2006;136:3062–8. doi: 10.1093/jn/136.12.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huseinovic E, Bertz F, Leu Agelii M, Hellebo Johansson E, Winkvist A, Brekke HK. Effectiveness of a weight loss intervention in postpartum women: results from a randomized controlled trial in primary health care. Am J Clin Nutr. 2016;104(2):362–70. doi: 10.3945/ajcn.116.135673. [DOI] [PubMed] [Google Scholar]
- 82.Peacock AS, Bogossian FE, Wilkinson SA, Gibbons KS, Kim C, McIntyre HD. A Randomised Controlled Trial to Delay or Prevent Type 2 Diabetes after Gestational Diabetes: Walking for Exercise and Nutrition to Prevent Diabetes for You. International Journal of Endocrinology. 2015;2015:8. doi: 10.1155/2015/423717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Pligt P, Willcox J, Hesketh KD, Ball K, Wilkinson S, Crawford D, et al. Systematic review of lifestyle interventions to limit postpartum weight retention: implications for future opportunities to prevent maternal overweight and obesity following childbirth. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14(10):792–805. doi: 10.1111/obr.12053. [DOI] [PubMed] [Google Scholar]
- 84.Lim S, O'Reilly S, Behrens H, Skinner T, Ellis I, Dunbar JA. Effective strategies for weight loss in post-partum women: a systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2015;16(11):972–87. doi: 10.1111/obr.12312. [DOI] [PubMed] [Google Scholar]
- 85.Neville CE, McKinley MC, Holmes VA, Spence D, Woodside JV. The effectiveness of weight management interventions in breastfeeding women--a systematic review and critical evaluation. Birth (Berkeley, Calif) 2014;41(3):223–36. doi: 10.1111/birt.12111. [DOI] [PubMed] [Google Scholar]
- 86.Phelan S, Hagobian T, Brannen A, Hatley KE, Schaffner A, Munoz-Christian K, et al. Effect of an Internet-Based Program on Weight Loss for Low-Income Postpartum Women: A Randomized Clinical Trial. Jama. 2017;317(23):2381–91. doi: 10.1001/jama.2017.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.World Health Organization. Global Nutrition Targets 2025: Stunting policy brief. Geneva: World Health Organisation; 2014. Contract No.: WHO/NMH/NHD/14.3. [Google Scholar]
- 88.Monteiro CA, Benicio MH, Conde WL, Konno S, Lovadino AL, Barros AJ, et al. Narrowing socioeconomic inequality in child stunting: the Brazilian experience, 1974-2007. Bulletin of the World Health Organization. 2010;88(4):305–11. doi: 10.2471/BLT.09.069195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adams J, Mytton O, White M, Monsivais P. Why Are Some Population Interventions for Diet and Obesity More Equitable and Effective Than Others? The Role of Individual Agency. PLOS Medicine. 2016;13(4):e1001990. doi: 10.1371/journal.pmed.1001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lorenc T, Petticrew M, Welch V, Tugwell P. What types of interventions generate inequalities? Evidence from systematic reviews. Journal of epidemiology and community health. 2013;67(2):190–3. doi: 10.1136/jech-2012-201257. [DOI] [PubMed] [Google Scholar]
- 91.Kelly MP, Barker M. Why is changing health-related behaviour so difficult? Public Health. 2016 doi: 10.1016/j.puhe.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shove E, Pantzar M, Watson M. The Dynamics of Social Practice: Everyday Life and how it Changes. London: Sage; 2012. [Google Scholar]
- 93.Birdwell L. A study of the influence of image congruence on consumer choice. The Journal of Business. 1968;41(1):76. [Google Scholar]
- 94.Last A. Business on a Mission: How to Build a Sustainable Brand. Saltaire, Bradford UK: Greenleaf Publishing; 2016. [Google Scholar]
- 95.Unilever. Knorr’s Green Food Steps to improve health and livelihoods. 2015 Available from: https://www.unilever.com/news/news-and-features/Feature-article/2015/knorrs-green-food-steps-to-improve-health-and-livelihoods.html.
- 96.Swinburn B, Kraak V, Rutter H, Vandevijvere S, Lobstein T, Sacks G, et al. Strengthening of accountability systems to create healthy food environments and reduce global obesity. Lancet. 2015;385(9986):2534–45. doi: 10.1016/S0140-6736(14)61747-5. [DOI] [PubMed] [Google Scholar]
- 97.Weible C, Sabatier P. A Guide to the Advocacy Coalition Framework. In: F F, M G, S M, editors. Handbook of Public Policy Analysis: theory, political and methods. Florida, United States: Taylor and Francis Group; 2007. pp. 123–36. [Google Scholar]
- 98.World Health Organisation. Meeting to develop a global consensus on preconception care to reduce maternal and childhood mortality and morbidity; 6-7 February 2012; Geneva: World Health Organization; 2012. [Google Scholar]
- 99.Acosta AM. Analysing nutrition governance: Brazil country report. Brighton: 2011. Examining the poiltical, institutional and governance aspects of delivering a national multi-sectoral response to reduce maternal and child malnutrition. [Google Scholar]
- 100.Kirkpatrick S, Maynard M, Raffoul A, Stapleton J. Population health interventions to curb intake of sugars: Gaps in the evidence. The FASEB Journal. 2017;31(1 Supplement) 640.26. [Google Scholar]