This population-based cohort study uses 2 large electronic primary care data sets linked to hospitalization and mortality records in England and Wales to estimate cause-specific unnatural mortality risks in people with epilepsy and to identify the medication types involved in poisoning deaths.
Key Points
Question
What is the risk and medication contribution to cause-specific unnatural mortality in people with epilepsy?
Findings
In this population-based cohort study, more than 50 000 people with epilepsy and 1 million matched individuals without epilepsy were identified in 2 data sets from the general populations of England and Wales. People with epilepsy had a 3-fold increased risk of any unnatural mortality and a 5-fold increased risk of unintentional medication poisoning; psychotropic and opioid, but not antiepileptic, drugs were most commonly used in poisoning.
Meaning
Clinicians should provide advice on unintentional injury and poisoning and suicide prevention and consider the toxicity of concomitant medication when prescribing drugs for people with epilepsy.
Abstract
Importance
People with epilepsy are at increased risk of mortality, but, to date, the cause-specific risks of all unnatural causes have not been reported.
Objective
To estimate cause-specific unnatural mortality risks in people with epilepsy and to identify the medication types involved in poisoning deaths.
Design, Setting, and Participants
This population-based cohort study used 2 electronic primary care data sets linked to hospitalization and mortality records, the Clinical Practice Research Datalink (CPRD) in England (from January 1, 1998, to March 31, 2014) and the Secure Anonymised Information Linkage (SAIL) Databank in Wales (from January 1, 2001, to December 31, 2014). Each person with epilepsy was matched on age (within 2 years), sex, and general practice with up to 20 individuals without epilepsy. Unnatural mortality was determined using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes V01 through Y98 in the Office for National Statistics mortality records. Hazard ratios (HRs) were estimated in each data set using a stratified Cox proportional hazards model, and meta-analyses were conducted using DerSimonian and Laird random-effects models. The analysis was performed from January 5, 2016, to November 16, 2017.
Exposures
People with epilepsy were identified using primary care epilepsy diagnoses and associated antiepileptic drug prescriptions.
Main Outcomes and Measures
Hazard ratios (HRs) for unnatural mortality and the frequency of each involved medication type estimated as a percentage of all medication poisoning deaths.
Results
In total, 44 678 individuals in the CPRD and 14 051 individuals in the SAIL Databank were identified in the prevalent epilepsy cohorts, and 891 429 (CPRD) and 279 365 (SAIL) individuals were identified in the comparison cohorts. In both data sets, 51% of the epilepsy and comparison cohorts were male, and the median age at entry was 40 years (interquartile range, 25-60 years) in the CPRD cohorts and 43 years (interquartile range, 24-64 years) in the SAIL cohorts. People with epilepsy were significantly more likely to die of any unnatural cause (HR, 2.77; 95% CI, 2.43-3.16), unintentional injury or poisoning (HR, 2.97; 95% CI, 2.54-3.48) or suicide (HR, 2.15; 95% CI, 1.51-3.07) than people in the comparison cohort. Particularly large risk increases were observed in the epilepsy cohorts for unintentional medication poisoning (HR, 4.99; 95% CI, 3.22-7.74) and intentional self-poisoning with medication (HR, 3.55; 95% CI, 1.01-12.53). Opioids (56.5% [95% CI, 43.3%-69.0%]) and psychotropic medication (32.3% [95% CI, 20.9%-45.3%)] were more commonly involved than antiepileptic drugs (9.7% [95% CI, 3.6%-19.9%]) in poisoning deaths in people with epilepsy.
Conclusions and Relevance
Compared with people without epilepsy, people with epilepsy are at increased risk of unnatural death and thus should be adequately advised about unintentional injury prevention and monitored for suicidal ideation, thoughts, and behaviors. The suitability and toxicity of concomitant medication should be considered when prescribing for comorbid conditions.
Introduction
People who receive a diagnosis of epilepsy are 2 to 3 times more likely than the rest of the population to die prematurely. The degree to which this relative risk is elevated varies by cause of death.1,2 Earlier research has focused on sudden unexpected death in epilepsy, with less attention paid to other causes. A recent call for action3 identified the need to better understand cause-specific mortality risk, particularly for unnatural causes (ie, unintentional injury, suicide, homicide, and iatrogenesis), in people with epilepsy. Furthermore, the need for accurate identification of epilepsy and cause of death has been emphasized in epidemiologic studies.4
Several secondary care–based observational studies have reported 2- to 5-fold increased risks of unnatural mortality.1,5,6 Some studies examined the risk by type of unintentional injury,5,6 one reported the method of suicide in people with epilepsy,7 but none comprehensively reported risks across the full range of suicide and unintentional injury or poisoning types. A previous study of suicide that used the US National Violent Death Reporting System reported that antiepileptic drugs (AEDs) were used in just 6% of intentional poisoning deaths in people with epilepsy.7 This contrasts with a study of nonfatal self-poisoning in which AEDs were the most commonly used medication type (27%) in people with epilepsy.8
We estimated the relative risks of the specific causes of unnatural mortality in people with epilepsy vs a matched comparison cohort without epilepsy. We used 2 linked primary care–mortality databases to accurately estimate risks of cause-specific unnatural mortality. We also examined how frequently specific types of medication were involved in medication poisoning deaths.
Methods
Design, Setting, and Participants
We conducted 2 population-based cohort studies using data from the Clinical Practice Research Datalink (CPRD) and the Secure Anonymised Information Linkage (SAIL) Databank. The CPRD, a general practice database that covers approximately 7% of the population in the United Kingdom, contains anonymized patient-level data, including demographic characteristics, diagnoses, test results, and prescribed treatments.9 We used the July 2015 data release, which included 13 979 404 patients whose data were deemed to be of acceptable research quality standards from 689 practices. This data set is considered representative of the age, sex, and ethnicity distributions of the UK population.9 We identified epilepsy and comparison cohorts from a subset of practices whose anonymized patient data were linked to the following additional data sources: Office for National Statistics (ONS) mortality records, Index of Multiple Deprivation 2010, and Hospital Episode Statistics (HES). Those sources provide information on the cause of death (ONS), quintiles of deprivation based on patient postcodes (Index of Multiple Deprivation 2010), and inpatient hospitalizations (HES).9 All of the linked practices were in England and comprised 74% of the English practices in the CPRD. Data from the CPRD were obtained under a license from the UK Medicines and Healthcare Products Regulatory Agency. The Independent Scientific Advisory Committee of the Medicine and Healthcare Regulatory Agency approved access to the CPRD and linked data (protocol No. 15_046RA2R). Approval was granted to access the SAIL Databank from the Information Governance Review panel (approval No. 0204), an independent body consisting of a range of government, regulatory, and professional agencies, which oversees study approvals in line with ethical permissions already granted for conducting data analysis in the SAIL Databank.10,11 Informed consent was not required for use of these anonymized data sets.
The SAIL Databank contains data from 13 health and social care databases in Wales. Data from individuals are anonymously linked between databases though a unique identifier.10 The general practice data set contains diagnostic, treatment, and test data from 360 general practices (76%) in Wales, including 4 052 388 patients.12 Hospital admission diagnoses and discharge dates are detailed in the Patient Episode Database for Wales. The Annual District Death Extract provides ONS data on date and cause of death.13 There was no overlap between the practices included in the CPRD cohorts and the practices included in the SAIL Databank cohorts because none of the Welsh practices in the CPRD were in the subset eligible for data linkage.
The International League Against Epilepsy recommends the presence of both a diagnostic code for epilepsy and an AED treatment code for reliable ascertainment of epilepsy in epidemiologic studies.14 We adopted this recommendation. Epilepsy diagnoses are recorded in the CPRD and the general practice data set using the Read code system, version 2.15 Code lists were generated by searching for epilepsy-associated terms in the CPRD medical dictionary. This list was refined following discussion with a neurologist and in comparison with studies previously conducted using data from the CPRD16,17 and the SAIL Databank.18 A list of AEDs licensed in the United Kingdom was produced by a pharmacist (H.C.G.) based on drugs listed in the British National Formulary (BNF 68)19 and checked by another pharmacist (D.M.A.).
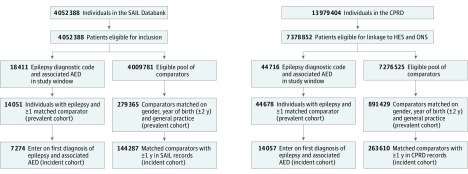
We defined the epilepsy index date as the latest date at which a person had received both an epilepsy diagnostic code and an associated AED prescription. The AED prescription could have been issued in the month before or up to 6 months after the date of the diagnostic code. We required the index date to be within the study periods, which we defined based on corresponding linkage availability as January 1, 1998, to March 31, 2014, for the CPRD and January 1, 2001, to December 31, 2014, for the SAIL Databank. Follow-up began on the index date, which occurred after registration of the patient in a practice. Separately for the CPRD and the SAIL Databank, we matched people with epilepsy with up to 20 individuals who had never received a diagnostic code for epilepsy and were alive on the date that follow-up began (eAppendix in the Supplement). We matched these comparison cohorts to their respective epilepsy cohorts on sex, year of birth (within 2 years), and general practice (Figure 1). Members of the comparison cohorts were followed up from the same day as the individuals they were matched to in the epilepsy cohort.
Figure 1. Flow Diagram Showing Delineation of Epilepsy and Comparison Cohorts.
Identification of epilepsy and comparison cohorts from the Secure Anonymised Information Linkage (SAIL) Databank and the Clinical Practice Research Datalink (CPRD) database. Individuals who did not meet the cohort definition criteria were excluded at the identified stages of cohort construction. AED indicates antiepileptic drug; HES, Hospital Episode Statistics; and ONS, Office for National Statistics.
We followed the epilepsy and comparison cohorts until the earliest date of death, transfer out of the practice, end of data collection from the practice, or study end. It was possible using the SAIL Databank to follow the progress of patients who transferred to other practices.
This study is reported in accordance with the Reporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) statement.20 We identified diagnoses and treatments using general practice data. We used published Read code lists to identify patients who had received a diagnosis of a range of mental illnesses.21,22 Our code lists for migraine, neuropathic pain, and substance misuse were verified by 2 general physicians and are available along with the epilepsy and AED codes at the ClinicalCodes.org website.23 Hospital discharge diagnoses were identified using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) coding system.24
Cause-Specific Mortality Outcomes
We determined the cause of death from the ONS-recorded underlying cause of death, as coded by the ICD-10 codes as follows: unnatural death (ICD-10 codes V01-Y98), transport accidents (ICD-10 codes V01-99 or Y85), other accidents (ICD-10 codes W00-99, X00-39, X50-59, or Y86), accidental poisoning with medication (ICD-10 codes X40-44), any accidental poisoning (ICD-10 codes X40-49), intentional self-poisoning with medication (ICD-10 codes X60-64 or Y10-14), any intentional self-poisoning (ICD-10 codes X60-69 or Y10-19), other suicide (ICD-10 codes X70-84, Y87.0, Y87.2, or Y20-34, excluding code Y33.9), homicide (ICD-10 codes X85-Y09, Y33.9, or Y87.1), and iatrogenesis (ICD-10 codes Y40-84 or Y88). We included events of undetermined intent in suicide estimates as per convention in the United Kingdom because most of these deaths were likely due to suicide.25 We identified the specific types of medication involved in poisoning deaths according to the presence of ICD-10 codes T36 through T50 in the supplementary causes of death fields (eTable in the Supplement).
Statistical Analysis
We applied a common protocol to conduct separate analyses of data from the 2 databases using Stata, version 13 (StataCorp). Baseline characteristics were reported as numerical and percentage frequencies, medians, or means and compared using unpaired, 2-tailed t tests, Mann-Whitney tests, or χ2 tests, as appropriate. We examined the proportion of individuals per cohort with mental health diagnoses, neuropathic pain, or migraine before or after cohort entry. We calculated mortality rates by dividing the number of events by the sum of person-years at risk. We accounted for the matched design by fitting stratified Cox proportional hazards models to estimate unadjusted and adjusted hazard ratios (HRs) for specific causes of death. Proportionality assumptions were examined using a test for Schoenfeld residuals and visually by graphical inspection. Adjustments were made for area-level deprivation because people with epilepsy are known to reside in more deprived areas than those without epilepsy,18 which may independently increase the risk of death. Deprivation was recorded by quintiles using the Index of Multiple Deprivation 2010 in the CPRD and the Welsh Index of Multiple Deprivation in the SAIL Databank. In the event the level of deprivation was missing, individuals were assigned to a category called unknown.
We conducted a meta-analysis of the HRs estimated from each data set using DerSimonian and Laird random-effects models implemented via the metan command in Stata.26 In addition, we restricted the cohorts to the incident epilepsy and comparison cohorts and estimated risks for the 4 main subgroups (all-cause mortality, unnatural death, unintentional injury, or suicide). The purpose of this sensitivity analysis was to explore whether there was evidence of a survival bias in using the prevalent cohort for our primary analysis. We also generated Kaplan-Meier survival estimates for all unnatural deaths separately for the 2 data sets, accounting for competing risks. The frequency of involvement of each medication type in poisoning deaths was pooled across the CPRD and the SAIL Databank and reported as percentages of all poisoning deaths for the prevalent cohorts. The analysis was conducted from January 5, 2016, to November 16, 2017.
Results
We matched 44 678 people with epilepsy to 891 429 persons without epilepsy in the CPRD, and 14 051 people with epilepsy to 279 365 individuals without epilepsy in the SAIL Databank (Figure 1). The baseline demographic and clinical characteristics are described in Table 1. For both data sets, 51% of the epilepsy and comparison cohorts were male. The median age at entry was 40 years (interquartile range [IQR], 25-60 years) in the CPRD cohort and 43 years (IQR, 24-64 years) in the SAIL cohort. People with epilepsy were significantly more likely to have received a diagnosis of any of the comorbid conditions examined at baseline (with the exception of neuropathic pain), to have been treated with psychotropic medication, and to have higher levels of deprivation than the comparison cohort. People with epilepsy were also significantly more likely to have recorded a diagnosis of a new mental illnesses during follow-up. The comparison cohorts had longer median (IQR) follow-up periods (5.1 [2.1-9.3] years in the CPRD and 7.7 [3.6-10.9] years in the SAIL Databank) than the epilepsy cohort (4.0 [1.4-8.4] years in the CPRD and 6.9 [2.9-10.3] years in the SAIL Databank), and the follow-up period ended more commonly owing to death in the epilepsy cohorts (6599 individuals [14.7%] in the CPRD and 2143 individuals [15.3%] in the SAIL Databank) than in the comparison cohorts (62 903 individuals [7.1%] in the CPRD and 23 540 individuals [8.4%] in the SAIL Databank).
Table 1. Clinical and Demographic Characteristics of Epilepsy and Comparison Cohorts.
| Characteristic | CPRD | SAIL Databank | |||
|---|---|---|---|---|---|
| Epilepsy Cohort (n = 44 678) | Comparison Cohort (n = 891 429) | Epilepsy Cohort (n = 14 051) | Comparison Cohort (n = 279 365) | ||
| Male, No. (%) | 22 961 (51.4) | 458 008 (51.4) | 7716 (51.1) | 142 526 (51.0) | |
| Age at entry, median (IQR), y | 41 (25-60) | 40 (25-60) | 44 (24-62) | 43 (24-62) | |
| Level of deprivation, No. (%)a | |||||
| 1 (Least deprived) | 7621 (17.0) | 178 850 (20.1) | 2202 (15.7) | 52 305 (18.7) | |
| 2 | 8878 (19.9) | 194 644 (21.8) | 2314 (16.4) | 50 635 (18.1) | |
| 3 | 8565 (19.2) | 178 281 (20.0) | 3059 (21.8) | 61 063 (21.9) | |
| 4 | 9723 (21.8) | 179 831 (20.2) | 2997 (21.3) | 56 110 (20.1) | |
| 5 (Most deprived) | 9826 (22.0) | 158 696 (17.8) | 3468 (24.7) | 58 849 (21.1) | |
| Missing | 65 (0.1) | 1127 (0.1) | 11 (0.1) | 403 (0.1) | |
| Previous diagnoses, No. (%) | |||||
| Alcohol misusea | 2682 (6.0) | 12 056 (1.4) | 936 (6.7) | 5823 (2.1) | |
| Anxietya | 7195 (16.1) | 104 532 (11.7) | 2452 (17.5) | 34 486 (12.3) | |
| Bipolar disordera | 465 (1.0) | 3293 (0.4) | 143 (1.0) | 1066 (0.4) | |
| Depressiona | 9096 (20.4) | 123 989 (13.9) | 2930 (20.9) | 39 953 (14.3) | |
| Eating disordera | 548 (1.2) | 5849 (0.7) | 183 (1.3) | 2025 (0.7) | |
| Migrainea | 3081 (6.9) | 46 150 (5.2) | 1058 (7.5) | 14 299 (5.1) | |
| Neuropathic pain | 1578 (3.5) | 30 763 (3.5) | 961 (6.8) | 18 253 (6.5) | |
| Personality disordera | 836 (1.9) | 3675 (0.4) | 307 (2.2) | 1996 (0.7) | |
| Schizophreniaa | 1273 (2.9) | 6711 (0.8) | 377 (2.7) | 2584 (0.9) | |
| Self-harma | 3563 (8.0) | 23 248 (2.6) | 1050 (7.5) | 9140 (3.3) | |
| Substance misusea | 2972 (6.7) | 11 923 (1.3) | 1122 (8.0) | 6661 (2.4) | |
| Prior prescription at baseline, No. (%) | |||||
| Antidepressanta | 11 175 (25.0) | 171 866 (19.3) | 4043 (28.8) | 27 104 (20.4) | |
| Antipsychotica | 6823 (15.3) | 88 433 (9.9) | 1843 (13.1) | 19 655 (7.0) | |
| Anxiolytic or hypnotica | 14 562 (32.6) | 124 898 (14.0) | 4938 (35.1) | 42 137 (15.1) | |
| Lithium carbonate or lithium citratea | 162 (0.4) | 1982 (0.2) | 61 (0.4) | 642 (0.2) | |
| Opioida,b | 14 355 (32.1) | 252 308 (28.3) | 6603 (47.0) | 100 331 (35.9) | |
| Follow-up time, median (IQR), ya | 4.0 (1.4-8.4) | 5.1 (2.1-9.3) | 6.9 (2.9-10.3) | 7.7 (3.6-10.9) | |
| New diagnoses during follow-up, No. (%) | |||||
| Alcohol misusea | 784 (1.8) | 9321 (1.1) | 296 (2.1) | 3887 (1.4) | |
| Anxietya | 2901 (6.5) | 45 429 (5.1) | 1069 (7.6) | 19 495 (7.0) | |
| Bipolar disordera | 204 (0.5) | 1595 (0.2) | 69 (0.5) | 535 (0.2) | |
| Depressiona | 2858 (6.4) | 46 189 (5.2) | 1081 (7.7) | 18 892 (6.8) | |
| Eating disordera | 383 (0.9) | 3796 (0.4) | 176 (1.3) | 1667 (0.6) | |
| Migraine | 975 (2.2) | 17 155 (1.9) | 331 (2.4) | 6536 (2.3) | |
| Neuropathic pain | 1217 (2.7) | 23 349 (2.6) | 729 (5.2) | 14 540 (5.2) | |
| Personality disordera | 212 (0.5) | 963 (0.1) | 92 (0.7) | 706 (0.3) | |
| Schizophreniaa | 465 (1.0) | 2861 (0.3) | 213 (1.5) | 1879 (0.7) | |
| Self-harma | 846 (1.9) | 6497 (0.7) | 322 (2.3) | 3382 (1.2) | |
| Substance misusea | 1913 (4.3) | 25 016 (2.8) | 315 (2.2) | 3518 (1.3) | |
| Reason for end of follow-up, No. (%)a | |||||
| Death identified using ONS | 6599 (14.7) | 62 903 (7.1) | 2143 (15.3) | 23 540 (8.4) | |
| Practice stopped contributing | 7175 (16.0) | 170 882 (19.1) | 6330 (45.0) | 60 484 (21.7) | |
| Study ended | 16 623 (37.2) | 403 717 (45.3) | 5578 (39.7) | 195 341 (69.9) | |
| Patient transferred out of practice | 14 281 (32.0) | 253 927 (28.5) | NA | NA | |
Abbreviations: CPRD, Clinical Practice Research Datalink; IQR, interquartile range; NA, not applicable; ONS, Office for National Statistics; SAIL, Secure Anonymised Information Linkage.
Significant difference between epilepsy and comparison cohorts (P < .05).
Includes single prescriptions of an opioid for short-term pain relief (eg, tablets containing a combination of codeine phosphate [8 mg] and acetaminophen [500 mg]).
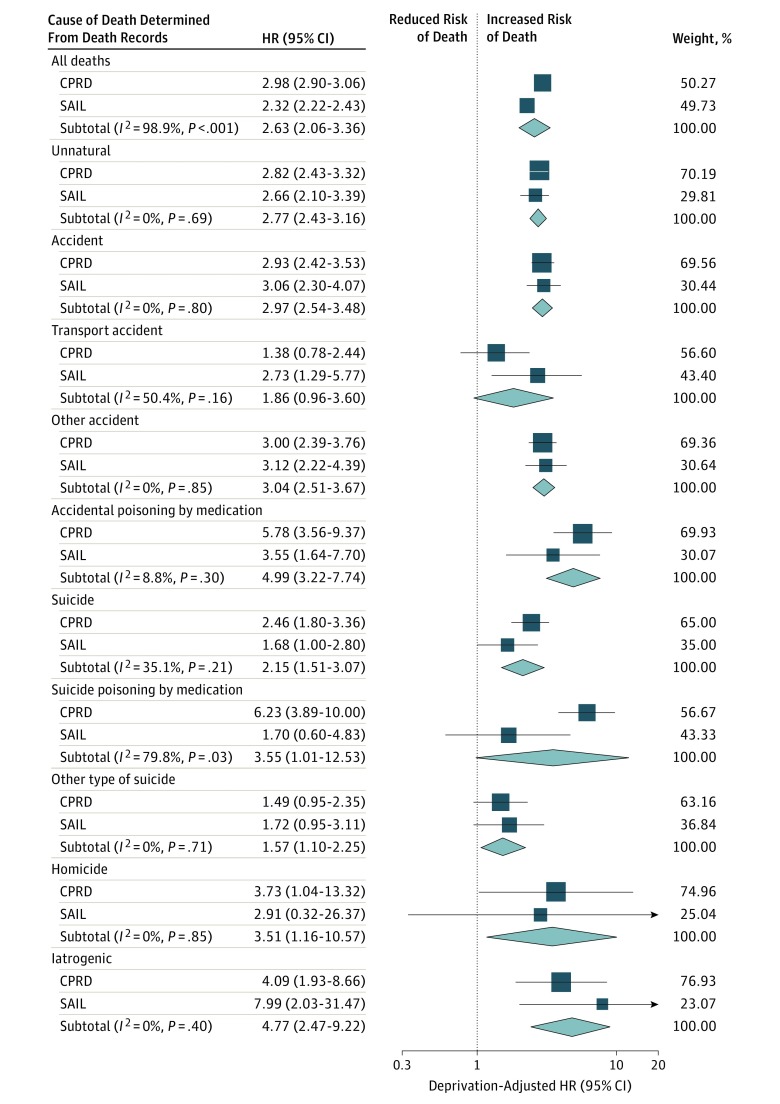
Event counts and mortality rates for each unnatural cause of death are presented separately for the 2 data sets in Table 2. The HRs of the meta-analysis for cause-specific unnatural mortality adjusted for area-level deprivation are shown in Figure 2. These estimates were similar to the unadjusted estimates. Compared with persons without the condition, people with epilepsy were at an increased risk of unnatural mortality (deprivation-adjusted HR, 2.77; 95% CI, 2.43-3.16). We observed this elevated risk for both unintentional death (deprivation-adjusted HR, 2.97; 95% CI, 2.54-3.48), which represented the majority of unnatural deaths (Table 2; Figure 2), and suicide (deprivation-adjusted HR, 2.15; 95% CI, 1.51-3.07). The pooled relative risks for homicide (deprivation-adjusted HR, 3.51; 95% CI, 1.16-10.57) and iatrogenic fatalities (deprivation-adjusted HR, 4.77; 95% CI, 2.47-9.22) were also increased, albeit with wide confidence intervals for these exceptionally rare causes of death.
Table 2. Event Counts and Mortality Rates for Cause-Specific Unnatural Mortality in Prevalent Epilepsy and Comparison Cohorts.
| Cause of Deatha | Clinical Practice Research Datalink | Secure Anonymised Information Linkage Databank | ||||||
|---|---|---|---|---|---|---|---|---|
| Epilepsy (n = 44 687; PY = 232 095) | Comparison Cohort (n = 891 429; PY = 5 272 571) | Epilepsy Cohort (n = 14 051; PY = 93 972) | Comparison Cohort (n = 279 365; PY = 2 044 922) | |||||
| No. | Rate/100 000 PY (95% CI) | No. | Rate/100 000 PY (95% CI) | No. | Rate/100 000 PY (95% CI) | No. | Rate/100 000 PY (95% CI) | |
| All deaths | 6599 | 2826.0 (2758.0-2895.2) | 62 903 | 1193.0 (1183.7-1202.3) | 2143 | 2280.5 (2184.9-2379.1) | 23 540 | 1151.1 (1136.5-1165.9) |
| Natural | 6406 | 2760.0 (2692.9-2828.5) | 61 127 | 1159.3 (1151.0-1168.6) | 2065 | 2197.5 (2103.7-2294.3) | 22 821 | 1116.0 (1101.6-1130.6) |
| Unnatural | 193 | 83.1 (71.8-95.8) | 1776 | 33.7 (32.1-35.3) | 78 | 83.0 (65.6-104.0) | 719 | 35.2 (32.6-37.8) |
| Accident | 134 | 57.7 (48.4-68.4) | 1279 | 24.3 (23.0-25.6) | 58 | 61.7 (46.9-79.8) | 493 | 24.1 (22.0-26.3) |
| Transport accident | 13 | 5.6 (3.0-9.6) | 225 | 4.3 (3.7-4.9) | 8 | 8.5 (3.7-16.8) | 69 | 3.4 (2.6-4.3) |
| Other accident | 93 | 40.7 (32.3-49.1) | 936 | 17.8 (16.6-18.9) | 41 | 43.6 (31.3-59.1) | 361 | 17.7 (15.9-19.6) |
| Accidental medicine poisoning | 24 | 10.3 (6.6-15.4) | 90 | 1.7 (1.4-2.1) | 8 | 8.5 (3.7-16.8) | 53 | 2.6 (1.9-3.4) |
| All accidental poisoning | 28 | 12.1 (8.0-17.4) | 118 | 2.2 (1.9-2.7) | 9 | 9.6 (4.4-18.1) | 63 | 3.1 (2.4-3.9) |
| Suicide | 47 | 20.3 (14.9-26.9) | 407 | 7.7 (7.0-8.5) | 16 | 17.0 (9.7-27.7) | 199 | 9.7 (8.4-11.1) |
| Suicide poisoning with medication | 26 | 11.2 (7.3-16.4) | 80 | 1.5 (1.2-1.9) | <5 | ND | 48 | 2.4 (1.7-3.1) |
| All suicide poisoning | 26 | 11.2 (7.3-16.4) | 100 | 1.9 (1.5-2.3) | <5 | ND | 54 | 2.6 (2.0-3.5) |
| Other suicide | 21 | 9.1 (5.6-13.8) | 307 | 5.8 (5.2-6.5) | 12 | 12.8 (6.6-22.3) | 145 | 7.1 (6.0-8.3) |
| Homicide | <5 | ND | 18 | 0.3 (0.2-0.5) | <5 | ND | 9 | 0.4 (0.2-0.8) |
| Iatrogenic | 9 | 3.9 (1.8-7.4) | 72 | 1.4 (1.1-1.7) | <5 | ND | 18 | 0.9 (0.5-13.9) |
Abbreviations: ND, not disclosed (calculated from cell counts <5; therefore could not be reported without compromising anonymity); PY, person-years.
Determined from the Office for National Statistics–recorded underlying cause of death as classified using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes.
Figure 2. Forest Plot Showing Deprivation-Adjusted Hazard Ratios (HRs) for Cause-Specific Unnatural Mortality.
Deprivation-adjusted HRs for cause-specific mortality in the prevalent epilepsy cohort vs comparison cohort. The HRs were estimated separately using data from the Secure Anonymised Information Linkage (SAIL) Databank and the Clinical Practice Research Datalink (CPRD), and the meta-analyses were conducted using DerSimonian and Laird random-effects models. Weights are from random-effects analysis. Cause of death was determined from the Office for National Statistics–recorded underlying cause of death as classified using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes.
Pooled across the 2 databases, 22.8% (95% CI, 18.0%-28.3%) of unnatural deaths were medication poisonings in the epilepsy cohorts compared with 11.2% (95% CI, 10.0%-12.6%) in the comparison cohorts. The medication types most commonly used in poisoning deaths were opioids (epilepsy cohorts, 56.5% [95% CI, 43.3%-69.0%]; comparison cohorts, 47.3% [95% CI, 41.4%-53.3%]) and psychotropic medication (epilepsy cohorts, 32.3% [95% CI, 20.9%-45.3%]; comparison cohorts, 36.7% [95% CI, 31.0%-42.6%]). By contrast, AEDs were used in relatively few medication poisoning deaths (epilepsy cohort, 9.7% [95% CI, 3.6%-19.9%]; comparison cohort, 2.5% [95% CI, 1.0%-5.1%]).
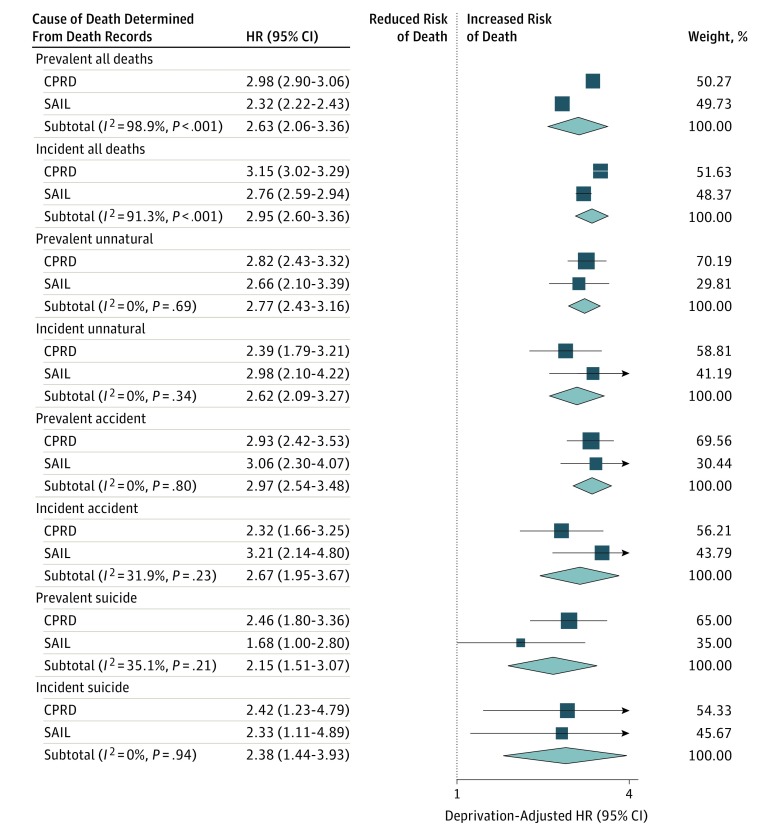
Sensitivity analyses identified 14 057 people with incident epilepsy in the CPRD and 263 610 people without in the comparison cohort and 7274 people with incident epilepsy in the SAIL Databank and 144 287 people without in the comparison cohort. The HRs estimated from a meta-analysis of the incident cohorts were similar to those estimated from the prevalent cohorts (Figure 3). The cause-specific survival rates for unnatural death after 5 years in the CPRD were 99.7% (95% CI, 99.5%-99.8%) for the epilepsy cohort and 99.8% (95% CI, 99.8%-99.8%) for the comparison cohort, and those rates for the SAIL Databank data were 99.5% (95% CI, 99.3%-99.7%) for the epilepsy cohort and 99.8% (95% CI, 99.8%-99.8%) for the comparison cohort (eFigures 1 and 2 in the Supplement).
Figure 3. Forest Plot Showing Deprivation-Adjusted Hazard Ratios (HRs) Estimated From Prevalent and Incident Epilepsy Cohorts.
Deprivation-adjusted HRs for all deaths, unnatural deaths, unintentional deaths, and suicides are shown for prevalent and incident epilepsy cohorts vs comparison cohorts. The HRs were estimated separately using the Secure Anonymised Information Linkage (SAIL) Databank and the Clinical Practice Research Datalink (CPRD) database, and meta-analyses were conducted using DerSimonian and Laird random-effects models. Weights are from random-effects analysis. Cause of death was determined from the Office for National Statistics–recorded underlying cause of death as classified using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes.
Discussion
In 2 primary care– and mortality-linked databases, people with epilepsy had an increased risk of all types of unnatural mortality compared with those without epilepsy. In people with epilepsy and in a matched comparison cohort, psychotropic medication and opioids were the medicine groups most commonly used in poisoning deaths, with AEDs reported much less frequently. To our knowledge, this is the first study to comprehensively examine cause-specific unnatural mortality risks, including both unintentional and intentional medication poisoning separately, in people with epilepsy.
Our relative estimates for all unnatural deaths, unintentional deaths, and suicides are in the same direction, but they are generally lower than those recorded in studies conducted using secondary-care records.5,6,27 Fazel et al5 observed odds ratios of 3.6 (95% CI, 3.3-4.0) for all external injury, 3.5 (95% CI, 3.3-4.2) for suicide, and 3.6 (95% CI, 3.1-4.1) for unintended deaths. Christensen et al27 also reported a slightly higher risk of suicide (relative risk, 3.17; 95% CI, 2.88-3.50). Standardized mortality ratios for injury and poisoning of 5.6 (95% CI, 5.0-6.3) and for suicide of 3.5 (95% CI, 2.6-4.6) were reported by Nilsson et al.6 These studies defined epilepsy populations from hospital records,5,6,27 most of which comprised inpatient episodes with some outpatient coverage.5,6 Therefore, it is possible that only individuals with more severe epilepsy were included. By using 2 primary-care data sets, we showed that increased risks extend to people with epilepsy in the community. Only 1 other study6 has estimated the specific risk of unintentional poisoning; the reported odds ratio of 5.1 (95% CI, 3.9-6.5) was similar to our meta-analyzed HR in the present study.
The reasons for the increased risk of unintended death may include the direct consequences of seizures3 or may be unassociated with epilepsy. The mental illness comorbidities associated with epilepsy are also associated with increased risk of unintentional injury and poisoning28 and suicide.29 Indeed, we detected greater proportions of people in the epilepsy cohort than in the comparison cohort who had received diagnoses involving mental health at baseline and during follow-up. There have been suggestions that suicidality and epilepsy share common neurological pathways,30 which could explain the increased risk of suicide. Clinicians should explore any symptoms of mental illness in people with epilepsy and ask about suicidal thoughts. In addition, the psychosocial impact and stigma surrounding epilepsy may contribute to the increased risk of unnatural death.31 It is possible that the stigma or presence of comorbid mental illness explains much of the 3-fold increase in homicide risk that we observed, an estimate consistent with that reported from a Swedish registry study (adjusted odds ratio, 2.8; 95% CI, 1.6-4.8).5
The increased risk of poisoning death identified in the present study might be attributable to the accessibility of medication in people with epilepsy, both AEDs and medication prescribed to treat comorbidities. The ease of access to a means to take one’s own life strongly influences suicide risk.32 However, AEDs were relatively infrequently recorded as being used in unintentional or intentional poisoning deaths in people with epilepsy. Our estimate that AEDs were used in one-tenth of poisonings is similar to that shown by Tian et al,7 who reported AED involvement in 6% of suicides in people with epilepsy. Many of the AEDs used may have a lower relative toxicity than psychotropic drugs and opioids. This may have changed over time owing to a reduction in the use of phenobarbital,33 which historically contributed to more than half of AED poisonings.34,35 However, AEDs are frequently involved in nonfatal self-harm.8 Ongoing vigilance is required to monitor nonfatal self-harm and poisoning deaths as trends in AED prescribing change. The ONS records indicate increasing involvement of gabapentin and pregabalin in poisoning deaths.36 This has been largely attributed to the diversion of pregabalin and gabapentin for recreational use.37 Because these AEDs are commonly used for treating conditions other than epilepsy, their involvement could extend beyond people with epilepsy.
Strengths and Limitations
The present study meets the International League Against Epilepsy recommendations for defining epilepsy14 and classifying mortality by cause4 in epidemiologic studies. A major strength of our investigation is that we examined 2 large, linked, and nationally representative primary-care data sets. Our outcomes were identified using ONS mortality statistics, which is the most accurate method of ascertaining cause of death in the United Kingdom. These primary-care data sets encompassed the whole spectrum of epilepsy, without restriction to more severe cases, which occurs in hospital-based cohorts. We applied a common study protocol across both data sets to enable a meta-analysis of the relative risks even when observed event counts were small. The increased risk of intentional self-poisoning that was observed in the meta-analysis would not have been detected using only the SAIL Databank. In addition, we verified our main findings by comparison with an incident epilepsy cohort.
We are aware of the potential limitations common to research using routinely collected data to investigate outcomes such as suicide. First, the data sets are not generated primarily for the purpose of conducting research, and residual confounding may therefore be present. Second, some suicides may have been misclassified. We accounted for this by including unnatural deaths of undetermined intent in our suicide definition. However, coroners in England and Wales have in recent years more frequently used narrative verdicts to describe likely suicides, rather than assigning a specific verdict.38 Some of the estimates in the present meta-analysis were of low precision owing to low event counts for the rarest cause-specific mortality outcomes. For this reason, we could not estimate risks for specific subgroups of unnatural death in the incident epilepsy cohort. We were unable to report the medication involvement for each data set independently without compromising anonymity. In addition, we could not subdivide the medications beyond that deducible from ICD-10 “T” codes.
Conclusions
In summary, the results of the present study showed that people with epilepsy have increased risks for most specific causes of unnatural death, including unintentional and intentional poisoning by medication. Further research is needed to identify suitable measures to mitigate these risks. Meanwhile, clinicians should be aware of the increased unnatural mortality risks and should carefully monitor patients accordingly. Antiepileptic drugs seemingly played a minor role in poisoning deaths, with psychotropic drugs and opioids more often involved. Therefore, when prescribing drugs for people with epilepsy, the potential for poisoning and associated relative toxicity of concomitantly prescribed medication should be considered in light of the increased risk of fatal poisoning in this population.
eAppendix. Explanation of the Matching Process Applied to Delineate the Matched-Cohort Study
eTable. ICD-10 Codes Used to Classify Medication Taken in Fatal Poisonings
eFigure 1. Kaplan-Meier Plot Depicting Probability of Cause-Specific Survival in Relation to Unnatural Mortality in the CPRD
eFigure 2. Kaplan-Meier Plot Depicting Probability of Cause-Specific Survival in Relation to Unnatural Mortality in the SAIL Databank
References
- 1.Trinka E, Bauer G, Oberaigner W, Ndayisaba J-P, Seppi K, Granbichler CA. Cause-specific mortality among patients with epilepsy: results from a 30-year cohort study. Epilepsia. 2013;54(3):495-501. [DOI] [PubMed] [Google Scholar]
- 2.Nevalainen O, Raitanen J, Ansakorpi H, Artama M, Isojärvi J, Auvinen A. Long-term mortality risk by cause of death in newly diagnosed patients with epilepsy in Finland: a nationwide register-based study. Eur J Epidemiol. 2013;28(12):981-990. [DOI] [PubMed] [Google Scholar]
- 3.Devinsky O, Spruill T, Thurman D, Friedman D. Recognizing and preventing epilepsy-related mortality: a call for action. Neurology. 2016;86(8):779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thurman DJ, Logroscino G, Beghi E, et al. ; Epidemiology Commission of the International League Against Epilepsy . The burden of premature mortality of epilepsy in high-income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. 2017;58(1):17-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazel S, Wolf A, Långström N, Newton CR, Lichtenstein P. Premature mortality in epilepsy and the role of psychiatric comorbidity: a total population study. Lancet. 2013;382(9905):1646-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsson L, Tomson T, Farahmand BY, Diwan V, Persson PG. Cause-specific mortality in epilepsy: a cohort study of more than 9,000 patients once hospitalized for epilepsy. Epilepsia. 1997;38(10):1062-1068. [DOI] [PubMed] [Google Scholar]
- 7.Tian N, Cui W, Zack M, Kobau R, Fowler KA, Hesdorffer DC. Suicide among people with epilepsy: a population-based analysis of data from the U.S. National Violent Death Reporting System, 17 states, 2003-2011. Epilepsy Behav. 2016;61:210-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer N, Voysey M, Holmes J, Casey D, Hawton K. Self-harm in people with epilepsy: a retrospective cohort study. Epilepsia. 2014;55(9):1355-1365. [DOI] [PubMed] [Google Scholar]
- 9.Herrett E, Gallagher AM, Bhaskaran K, et al. . Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyons RA, Jones KH, John G, et al. . The SAIL Databank: linking multiple health and social care datasets [published online January 16, 2009]. BMC Med Inform Decis Mak. 2009;9:3. doi: 10.1186/1472-6947-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford DV, Jones KH, Verplancke J-P, et al. . The SAIL Databank: building a national architecture for e-health research and evaluation. BMC Health Serv Res. 2009;9:157-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Administrative Data Research Centre Wales Data Brief: An Overview Of Administrative Health Data Within the SAIL Databank. Swansea: Administrative Data Research Centre Wales; 2015. [Google Scholar]
- 13.John A, Dennis M, Kosnes L, et al. . Suicide Information Database-Cymru: a protocol for a population-based, routinely collected data linkage study to explore risks and patterns of healthcare contact prior to suicide to identify opportunities for intervention. BMJ Open. 2014;4(11):e006780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurman DJ, Beghi E, Begley CE, et al. ; ILAE Commission on Epidemiology . Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52(suppl 7):2-26. [DOI] [PubMed] [Google Scholar]
- 15.Chisholm J. The Read clinical classification. BMJ. 1990;300(6732):1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer KT, D’Angelo S, Harris EC, Linaker C, Coggon D. Epilepsy, diabetes mellitus and accidental injury at work. Occup Med (Lond). 2014;64(6):448-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridsdale L, Charlton J, Ashworth M, Richardson MP, Gulliford MC. Epilepsy mortality and risk factors for death in epilepsy: a population-based study. Br J Gen Pract. 2011;61(586):e271-e278. doi: 10.3399/bjgp11X572463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickrell WO, Lacey AS, Bodger OG, et al. . Epilepsy and deprivation, a data linkage study. Epilepsia. 2015;56(4):585-591. [DOI] [PubMed] [Google Scholar]
- 19.Joint Formulary Committee; British Medical Association; Royal Pharmaceutical Society . British National Formulary. 68 ed London, England: BMJ Group and Pharmaceutical Press; 2014. [Google Scholar]
- 20.Benchimol EI, Smeeth L, Guttmann A, et al. ; RECORD Working Committee . The Reporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carr MJ, Ashcroft DM, Kontopantelis E, et al. . The epidemiology of self-harm in a UK-wide primary care patient cohort, 2001-2013. BMC Psychiatry. 2016;16:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle M, While D, Mok PLH, et al. . Suicide risk in primary care patients diagnosed with a personality disorder: a nested case control study. BMC Fam Pract. 2016;17:106-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springate DA, Kontopantelis E, Ashcroft DM, et al. . ClinicalCodes: an online clinical codes repository to improve the validity and reproducibility of research using electronic medical records. PLoS One. 2014;9(6):e99825. doi: 10.1371/journal.pone.0099825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization ICD-10 Version:2016 http://www.who.int/classifications/icd/en/. Updated November 29, 2016. Accessed September 1, 2016.
- 25.Office for National Statistics Suicides in the UK: 2014 registrations. Newport: Office for National Statistics, 2016. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/suicidesintheunitedkingdom/2014registrations. Accessed December 12, 2016.
- 26.Oxford University Research Archive Harris RJ, Bradburn MJ, Deeks JJ, et al. metan: fixed- and random-effects meta-analysis. Stata J. 2008;8(1):3-28. https://ora.ox.ac.uk/objects/uuid:e9b64f65-f3d3-4301-97a6-633ef6f15481. Accessed December 5, 2016.
- 27.Christensen J, Vestergaard M, Mortensen PB, Sidenius P, Agerbo E. Epilepsy and risk of suicide: a population-based case-control study. Lancet Neurol. 2007;6(8):693-698. [DOI] [PubMed] [Google Scholar]
- 28.Crump C, Sundquist K, Winkleby MA, Sundquist J. Mental disorders and risk of accidental death. Br J Psychiatry. 2013;203(3):297-302. [DOI] [PubMed] [Google Scholar]
- 29.Singhal A, Ross J, Seminog O, Hawton K, Goldacre MJ. Risk of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012;72(2):184-191. [DOI] [PubMed] [Google Scholar]
- 31.Nimmo-Smith V, Brugha TS, Kerr MP, McManus S, Rai D. Discrimination, domestic violence, abuse, and other adverse life events in people with epilepsy: population-based study to assess the burden of these events and their contribution to psychopathology. Epilepsia. 2016;57(11):1870-1878. [DOI] [PubMed] [Google Scholar]
- 32.Hawton K, van Heeringen K. Suicide. Lancet. 2009;373(9672):1372-1381. [DOI] [PubMed] [Google Scholar]
- 33.Nicholas JM, Ridsdale L, Richardson MP, Ashworth M, Gulliford MC. Trends in antiepileptic drug utilisation in UK primary care 1993-2008: cohort study using the General Practice Research Database. Seizure. 2012;21(6):466-470. [DOI] [PubMed] [Google Scholar]
- 34.Mackay A. Self-poisoning: a complication of epilepsy. Br J Psychiatry. 1979;134:277-282. [DOI] [PubMed] [Google Scholar]
- 35.Hawton K, Fagg J, Marsack P. Association between epilepsy and attempted suicide. J Neurol Neurosurg Psychiatry. 1980;43(2):168-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Office for National Statistics Deaths related to drug poisoning in England and Wales: 2015 registrations. Newport: Office for National Statistics, 2016. https://www.ons.gov.uk/releases/deathsrelatedtodrugpoisoninginenglandandwales2015registrations. Accessed December 12, 2016.
- 37.DrugScope Down a stony road: The 2014 DrugScope Street Drug Survey. http://www.sfad.org.uk/userfiles/files/DownAStonyRoadDrugTrendsSurvey2014.pdf. Accessed January 15, 2015.
- 38.Gunnell D, Hawton K, Kapur N. Coroners’ verdicts and suicide statistics in England and Wales. BMJ. 2011;343:d6030-d6031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Explanation of the Matching Process Applied to Delineate the Matched-Cohort Study
eTable. ICD-10 Codes Used to Classify Medication Taken in Fatal Poisonings
eFigure 1. Kaplan-Meier Plot Depicting Probability of Cause-Specific Survival in Relation to Unnatural Mortality in the CPRD
eFigure 2. Kaplan-Meier Plot Depicting Probability of Cause-Specific Survival in Relation to Unnatural Mortality in the SAIL Databank