Abstract
BACKGROUND:
There has been an overall improvement in survival rates for persons with cancer over the past 35 years. However, these gains are less prevalent among adolescents with cancer aged 15 to 19 years, which may be due to lower clinical trial enrollment among adolescents with cancer.
METHODS:
We conducted a literature review to assess current research regarding clinical trial enrollment and subsequent outcomes among adolescents with cancer. The search included English-language publications that reported original data from January 1985 to October 2011.
RESULTS:
The search identified 539 records. Of these 539 records, there were 30 relevant original research articles. Multiple studies reported that adolescents with cancer are enrolled in clinical trials at lower rates compared with younger children and older adults. Treatment setting, physician type, and institution type may all be factors in the low enrollment rate among adolescents. Few data focused solely on adolescents, with many studies combining adolescents with young adults. The number of available studies related to this topic was limited, with significant variability in study design, methods, and outcomes.
CONCLUSIONS:
This literature review suggests that adolescents with cancer are not treated at optimal settings and are enrolled in clinical trials at low rates. This may lead to inferior treatment and poor subsequent medical and psychosocial outcomes. The scarcity in data further validates the need for additional research focusing on this population.
Keywords: clinical trial enrollment, cancer, adolescents, oncology
Over the past 35 years, the number of cancer survivors in the United States has grown from <3 million to nearly 12 million.1 Although there have been improvements in cancer survival, morbidity, and quality of life in the overall population of US cancer survivors, these gains are less prevalent among adolescents aged 15 to 19 years diagnosed with cancer.2–4 This situation is especially true when compared with younger children aged 0 to 14 years and older adults,2–4 for whom improved outcomes are correlated to enrollment in clinical trials.5
Adolescents with cancer are enrolled in clinical trials at much lower rates in the United States compared with younger children and older adults.5–7 One reason adolescents are enrolled in clinical trials at low rates is because of referral patterns.8–10 Unlike younger children or older adults, adolescents with cancer may be referred by pediatricians to either pediatric or adult oncologists.11 Most adolescents are referred to adult oncology centers, and the referral of adolescents to pediatric oncology centers diminishes with age.11–13 Adult cancer centers have lower rates of clinical trial enrollment and less access to clinical trials for adolescents compared with pediatric cancer centers. Additionally, there is evidence that adolescents diagnosed with certain types of cancer who are treated on pediatric protocols have better outcomes compared with those on adult protocols.14–17 To address these and other issues related to clinical trial enrollment and health outcomes for adolescent survivorship, we examined the scientific literature regarding this topic. We performed a literature review to assess current research regarding treatment setting, clinical trial enrollment, and subsequent outcomes among adolescents with cancer.
METHODS
The literature review focused on treatment setting, enrollment in clinical trials, and subsequent medical and psychosocial outcomes among adolescents with cancer. To examine psychosocial outcomes, we examined adolescents who participated in randomized controlled trials targeting behavioral change and emotional symptoms related to cancer trajectory. With the use of the OVID, EBSCOhost, Medline, and Embase search platforms, we performed a keyword search of 5 databases: Medline, Embase, the Psychological Abstracts database (PsychINFO), Cochrane Library, and the Cumulative Index of Nursing and Allied Health Literature database. We reviewed reference lists of eligible articles found through the initial search to identify additional articles. We also identified additional articles not abstracted in the initial search in a cursory review from researchers and practitioners in the fields of oncology and health psychology. Figure 1 shows an example of a search syntax we used. We limited results to peer-reviewed original research studies from the United States, the United Kingdom, Australia, and Canada published in English between January 1, 1985, and October 31, 2011.
FIGURE 1.

Sample search syntax for Medline, Embase, PsychINFO, and Cochrane databases.
A 2-phase classification procedure was used to determine relevance. In phase I, a primary reviewer assessed all titles and abstracts and classified them as either potentially relevant, relevant, or not relevant. In phase II, the primary reviewer reviewed the full text of all potentially relevant articles and classified them as either relevant or not relevant. A quality assurance procedure with 3 reviewers was used to verify the accuracy of relevance classifications. A first reviewer read a 25% random sample of articles and classified them as potentially relevant, relevant, or not relevant. A second reviewer repeated this process independently. Results from the 2 reviewers were sent to a third reviewer who identified and reconciled any discordance in classification.
RESULTS
The combined database and reference list searches resulted in 66 relevant articles (Fig 2). Of these 66 articles, 36 were review articles or commentaries and 30 were original research articles. Of the 30 original research articles, there were 4 randomized controlled trials, 2 prospective cross-sectional studies, and 24 retrospective cross-sectional studies (Table 1). Although there was an emphasis on cancer types most commonly found in the adolescent population, the majority of articles examined all cancer types. Among all articles, there was variation in the definition of the adolescent and adolescent and young adult (AYA) population; age ranges were as low as 13 years for adolescents and up to 40 years for young adults. We present an overview of the relevant articles by the following topic areas: treatment setting, clinical trial enrollment, and subsequent outcomes.
FIGURE 2.
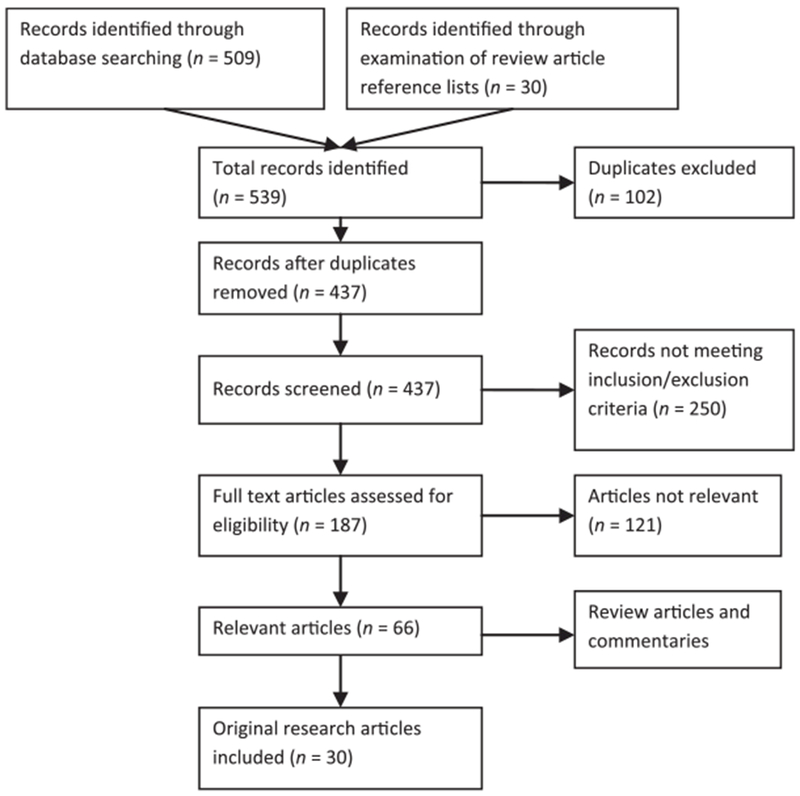
Flowchart of article screening, assessment, and inclusion.
TABLE 1.
Studies on Patterns of Referral, Clinical Trial Enrollment, and Subsequent Outcomes Among Adolescents With Cancer
| First Author | Year | Sample Size | Age of Study Population | Study Design | Type of Cancer | Primary Outcome |
|---|---|---|---|---|---|---|
| Alderfer39 | 2009 | 144 | 11–19 y | Prospective, cross-sectional | All | Family functioning |
| Baider45 | 1989 | 8 | 15–25 y | Prospective, cross-sectional | All | Psychological distress |
| Bleyer5 | 1997 | 29 859 | 0–20 y | Retrospective, cross-sectional | All | Clinical trial enrollment |
| Burkhardt30 | 2011 | 378 | 15–18 y | Retrospective, cross-sectional | Non-Hodgkin’s lymphoma | Survival |
| Cairo29 | 2003 | 470 | 0–21 y | Retrospective, cross-sectional | Burkitt’s lymphoma | Survival |
| Cox44 | 2005 | 272 | 12–18 y | Randomized controlled trial | All | Behavior change |
| Creutzig31 | 2007 | 1181 | 0–30 y | Retrospective, cross-sectional | Acute myeloid leukemia | Survival |
| Downs-Canner24 | 2009 | 91 | 15–22 y | Retrospective, cross-sectional | All | Clinical trial enrollment |
| Hill46 | 2007 | 77 | 7–19 y | Retrospective, cross-sectional | Colorectal | Clinical and pathologic features |
| Joshi32 | 2004 | 2343 | 0–21 y | Retrospective, cross-sectional | Rhabdomyosarcoma | Survival |
| Judge Santacroce41 | 2009 | 21 | 15–25 y | Randomized controlled trial | All | Coping skills |
| Kazak40 | 2004 | 150 | 11–19 y | Randomized controlled trial | All | Posttraumatic stress symptoms |
| Klein-Geltink19 | 2005 | 204 | 15–19 y | Retrospective, cross-sectional | All | Provider type |
| Krailo23 | 1993 | 2788 | 0–19 y | Retrospective, cross-sectional | All | Clinical trial enrollment |
| Millot34 | 2011 | 44 | 10 mo–17 y | Retrospective, cross-sectional | Chronic myelogenous leukemia | Survival |
| Mitchell20 | 2004 | 576 | 10–24 y | Retrospective, cross-sectional | All | Clinical trial enrollment |
| Moreno33 | 2009 | 16 | 14–24 y | Retrospective, cross-sectional | Ependymoma | Survival |
| Pao47 | 2006 | 347 | 1–21 y | Retrospective, cross-sectional | All | Psychotropic medication use |
| Parsons22 | 2011 | 1358 | 15–39 y | Retrospective, cross-sectional | All | Factors associated with clinical trial enrollment |
| Pinkerton36 | 2010 | 11 915 | 0–29 y | Retrospective, cross-sectional | Hematologic malignancies | Survival |
| Polishchuk35 | 2011 | 39 | ≥10 y | Retrospective, cross-sectional | Neuroblastoma | Survival |
| Portteus48 | 2006 | 216 | — | Retrospective, cross-sectional | All | Antidepressant medication use |
| Ramanujachar27 | 2007 | 128 | 15–17 y | Retrospective, cross-sectional | Acute lymphoblastic leukemia | Provider type |
| Ramanujachar28 | 2006 | 48 | 15–21 y | Retrospective, cross-sectional | Acute lymphoblastic leukemia | Provider type |
| Shaw25 | 2007 | 640 | 0–22 y | Retrospective, cross-sectional | All | Clinical trial enrollment |
| Shaw26 | 2010 | 57 | 15–22 y | Retrospective, cross-sectional | All | Clinical trial enrollment |
| Silverman37 | 2010 | 1457 | 0–18 y | Retrospective, cross-sectional | Acute lymphoblastic leukemia | Survival |
| Sultan49 | 2010 | 159 | 4–20 y | Retrospective, cross-sectional | Colorectal | Clinical and pathologic features |
| Tercyak43 | 2006 | 75 | 11–21 y | Randomized controlled trial | All | Behavioral risk factors |
| Yeager18 | 2006 | 169 | 15–19 y | Retrospective, cross-sectional | All | Provider type |
Treatment Setting
Several studies focused primarily on the setting in which adolescents with cancer were treated. One study in Ohio found that of 169 adolescent patients aged 15 to 19 years, 47% were treated at pediatric institutions, 25% were treated at adult academic centers, and 29% were treated at community hospitals.18 Treatment at pediatric centers decreased with increasing age. However, cancer type was found to be an important diagnostic factor independent of age: malignancies traditionally regarded as “pediatric,” such as acute lymphoblastic leukemia, central nervous system tumors, and osteosarcomas, were treated more often at pediatric hospitals regardless of age, whereas malignancies traditionally regarded as “adult,” such as melanoma and germ cell tumors, were more often treated at adult institutions regardless of age.18 Another study in Canada found that ~30% of adolescents aged 15 to 19 years were treated at a pediatric institution.19 Consistent with the study from Ohio, the likelihood of treatment at a pediatric institution decreased with increasing age, and adolescents treated at adult institutions were more likely to have a diagnosis of carcinoma or germ cell tumor and less likely to have lymphoma.19 Another study found that only 14% of adolescents aged 16 to 19 years were treated at a pediatric institution.20
Clinical Trial Enrollment
A number of identified studies assessed clinical trial enrollment among adolescents with cancer. The range of adolescent patients enrolled in clinical trials ranged from 5% to 34%. A study of Children’s Oncology Group enrollment data showed that only 21% of patients aged 15 to 19 years of age were enrolled in clinical trials.5 The Children’s Oncology Group accounted for >97% of all clinical trial participants <20 years of age, whereas adult cooperative groups collectively accounted for <3% of clinical trials for adolescents in the 15- to 19-year range.21 A National Cancer Institute Patterns of Care study showed that 34% of adolescents aged 15 to 19 years were enrolled in a clinical trials.22 The study showed that older patients and those treated by adult oncologists were less likely to be enrolled into clinical trials.22 A decreasing rate of clinical trial enrollment with increasing age was also seen in the Los Angeles County Cancer Surveillance Program.23 Adolescents aged 15 to 19 years, compared with children aged 14 and younger, were less commonly diagnosed at a Children’s Oncology Group institution and were also enrolled in a clinical trial at lower rates.23 In addition to lower clinical trial enrollment rates among adolescents, there were also fewer therapeutic trials available for this age group.23 A comparison of clinical trial enrollment between adolescent and young adult oncology patients aged 15 to 22 years treated at affiliated adult and pediatric centers revealed that clinical trial enrollment was higher with treatment at a pediatric center. Of 91 cases with new cancer diagnoses treated at the pediatric center, 24 (26%) were enrolled, whereas only 5 of 121 (4%) cases with new cancer diagnoses treated at the adult center were enrolled in a clinical trial24 A study in Australia showed that adolescents aged 10 to 19 years were more likely to be enrolled in a clinical trial if treated at a pediatric institution rather than at an adult institution (38% vs 3%).20 Even if adolescents are seen at a pediatric institution, appropriate clinical trials may not be available. In a study in 640 patients with newly diagnosed cancer at a pediatric institution, 38% of patients under the age of 15 years were enrolled in a clinical trial and 27% of patients aged 15 to 22 years were enrolled in a clinical trial25 More than half of the older patients were not enrolled because a trial was not available.25 Clinical trial enrollment may be affected not only by differences in pediatric and adult institutions but by differences in academic compared with community institutions. Because most clinical trials are primarily conducted at academic institutions, most accrual into these trials is from academic centers. By reaching out to community oncologists and practices, physicians and researchers in academic settings may expand access to clinical trials.18 Cooperative group protocols that could be implemented practically at community hospitals and outpatient offices could increase patient accrual.18
There is also evidence that clinical trial enrollment is improved when adolescent patients are seen at a dedicated AYA oncology program.26 In the 3 years before the establishment of an AYA program at the University of Pittsburgh, clinical trial enrollment at the adult center was 4%. In the 4 years after the creation of the AYA program, clinical trial enrollment at the pediatric center and adult center increased to 33%.26 Thus, the development of AYA programs may be a strategy to increase clinical trial enrollment for adolescent patients with cancer.26 Another potential strategy to increase clinical trial enrollment is expanding national clinical trials accessibility to patients treated at both pediatric and medical oncology tertiary care centers through collaboration between pediatric and adult oncologists.24–26 This collaboration could be facilitated through improved communication between pediatric and adult oncologists, such as the establishment of an AYA cancer resource network that provides current information about currently available clinical trials.20,22
Survival
Several studies reported on survival trends among adolescents with cancer.27–37 A number of studies showed that increasing age was a poor prognostic factor for certain cancers, including acute myeloid leukemia, non-Hodgkin’s lymphoma, Burkitt’s and Burkitt-Iike lymphoma, and rhabdomyosarcoma.29–32 Two studies reported that adolescents with acute lymphoblastic leukemia were shown to have superior outcomes when treated on pediatric protocols compared with adult protocols.27,28 Despite limitations in the comparison of clinical trials due to differences in methodology, comparative studies indicate that adolescents with cancer may have a survival advantage when treated on pediatric protocols compared with adult protocols.27,28 However, this finding may not hold true for all patients, cancers, and therapies. Ultimately, improved survival may be best achieved by risk-directed selection of therapies based on the biology and response to therapy.27 The age limits for recruitment into clinical trials may need to be reconsidered.27,28
Psychosocial Outcomes
Several studies have identified that adolescent cancer survivors and their families may struggle with posttraumatic stress disorder, depression, and other clinical emotional and behavioral concerns as a result of their cancer diagnosis, treatment, and long-term trajectory.38 However, the onset, frequency, and duration of adverse emotional or behavioral symptoms and diagnosed psychosocial disorders among adolescent cancer survivors are vastly underexplored. Psychosocial interventions and their effect on psychosocial outcomes of adolescent cancer survivors have been examined in randomized controlled trials.39 Two randomized controlled trials showed that an integrated cognitive-behavioral and family therapy approach as well as a telephone-delivered coping skills training intervention may be effective components in the reduction in posttraumatic stress symptoms and other symptoms among adolescent cancer survivors.40–42 Efforts to decrease posttraumatic stress symptoms are especially important in this population because stress may increase risk behaviors such as physical inactivity, smoking, and nonadherence to sun protection among adolescent cancer survivors.43 One study examined the effect of a multicomponent educational intervention to decrease risk behaviors and health-protective behaviors among adolescent cancer survivors44 Results indicated that age and gender may have a strong influence on the impact of interventions targeting health behaviors in this population, and future trials should consider more factors including an improved understanding of patient-clinician interactions, patient motivations, and more specific outcome measures.44
DISCUSSION
This literature review suggests that adolescents with cancer are not treated at optimal settings and are enrolled in clinical trials at low rates. This situation may lead to inferior treatment and poor subsequent medical and psychosocial outcomes. Barriers that prevent adolescents from enrolling in clinical trials include the treatment setting, treating physician, and institution type. Adolescents with cancer are often referred to nonpediatric centers and community hospitals. Access to clinical trials is more limited for adolescents in these settings compared with pediatric tertiary care institutions, thereby reducing the possibility of clinical trial enrollment for a majority of adolescents with cancer.
Increasing referral to pediatric centers, when appropriate, is needed to increase enrollment of adolescents in clinical trials. Because it may be more appropriate for adolescents with certain types of cancer, such as melanoma and germ cell tumors, to be treated at adult institutions for medical reasons, it is also important to increase enrollment for adolescents with cancer who are seen in adult and community settings. This increase may be achieved through collaboration between pediatric and adult institutions and oncologists. There is also a need for unified clinical trial protocols for adolescents that can be followed by both pediatric and adult oncologists in national cooperative groups. Increased collaboration may also be facilitated through dedicated AYA oncology programs, which have been shown to increase clinical trials enrollment for both adolescents and young adults.
The number of available studies related to this topic was limited. The identified studies had significant variability in study design, methods, populations, and outcomes. Therefore, conclusions based on these studies may not be generalizable to all populations. The lack of a standardized age category may also lead to misclassification and bias among the studies reviewed.
The limited number and scope of articles identified through this literature review is reflective of the small but emerging view of adolescents with cancer as a distinct and unique entity. Results from this literature review indicate that few data focused solely on adolescents are available, with many studies combining adolescents with young adults. This scarcity in data further validates the need for additional research focusing on this population. The experience and needs of adolescent patients with cancer are different from those of other age groups in many respects. Continued focus on the biology of AYA tumors, therapies to improve survival and decrease toxicity, and the long-term impacts of cancer treatment is needed. Increased attention is needed on initial engagement with the health care system after a cancer diagnosis, including referral and choosing optimal treatment settings and physicians. As the cancer survivor population continues to increase, it is of public health importance that adolescents are not excluded from improvements seen in other age groups and receive the highest standard of care.
Acknowledgments
The findings and conclusions in this article are those of the author(s) and do not necessarily represent the official position of U.S. Department of Health and Human Services, the Public Health Service, or the Centers for Disease Control and Prevention. All authors have read and approved the manuscript as well as any additional information that may affect the review process. This manuscript has not been previously accepted or reviewed by any other journal publication.
FUNDING: All funding was provided by the Centers for Disease Control and Prevention.
ABBREVIATION
- AYA
adolescent and young adult
Footnotes
FINANCIAL DISCLOSURE: Dr Bleyer is a consultant for Sigma-Tau Pharmaceuticals and is on its Speakers’ Bureau; and Dr Buchanan, Ms Westervelt, Ms Elimam, and Dr Lawvere have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose
REFERENCES
- 1.Centers for Disease Control and Prevention. Cancer survivors—United States, 2007. MMWR Morb Mortal Wkly Rep. 2011; 60(9):269–272 [PubMed] [Google Scholar]
- 2.Bleyer A Young adult oncology: the patients and their survival challenges. CA Cancer J Clin. 2007:57(4):242–255 [DOI] [PubMed] [Google Scholar]
- 3.Bleyer A, O’Leary M, Barr R, Ries LAG, eds. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. National Cancer Institute, NIH Pub. No. 06-5767 Bethesda, MD:National Institutes of Health; 2006 [Google Scholar]
- 4.Adolescent and Young Adult Oncology Press Review Group; National Cancer Institute; LiveStrong Young Adult Alliance. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults With Cancer. Bethesda, MD: National Institutes of Health; 2006. NIH Publication 06-6067 [Google Scholar]
- 5.Bleyer WA, Tejeda H, Murphy SB, et al. National cancer clinical trials: children have equal access; adolescents do not. J Adolesc Health. 1997;21(6):366–373 [DOI] [PubMed] [Google Scholar]
- 6.Burke ME, Albritton K, Marina N. Challenges in the recruitment of adolescents and young adults to cancer clinical trials. Cancer. 2007;110(11):2385–2393 [DOI] [PubMed] [Google Scholar]
- 7.Freyer DR Transition of care for young adult survivors of childhood and adolescent cancer: rationale and approaches. J Clin Oncol. 2010;28(32):4810–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleyer WA. Cancer in older adolescents and young adults: epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Med Pediatr Oncol. 2002;38(1):1–10 [DOI] [PubMed] [Google Scholar]
- 9.Bleyer A Older adolescents with cancer in North America deficits in outcome and research. Pediatr Clin North Am. 2002;49(5):1027–1042 [DOI] [PubMed] [Google Scholar]
- 10.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006;107(7 suppl):1645–1655 [DOI] [PubMed] [Google Scholar]
- 11.Albritton KH, Wiggins CH, Nelson HE, Weeks JC. Site of oncologic specialty care for older adolescents in Utah. J Clin Oncol. 2007:25(29):4616–4621 [DOI] [PubMed] [Google Scholar]
- 12.Howell DL, Ward KC, Austin HD, Young JL, Woods WG. Access to pediatric cancer care by age, race, and diagnosis, and outcomes of cancer treatment in pediatric and adolescent patients in the state of Georgia. J Clin Oncol. 2007:25(29):4610–4615 [DOI] [PubMed] [Google Scholar]
- 13.Rauck AM, Fremgen AM, Hutchinson CL. Adolescent cancers in the United States: A national Cancer Database report [abstract]. J Pediatr Hematol Oncol. 1999;21(4):310 [Google Scholar]
- 14.Stock W, La M, Sanford B, et al. ; Children’s Cancer Group; Cancer and Leukemia Group B. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112(5):1646–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Testi AM, Valsecchi MG, Confer V, et al. : Difference in outcome of adolescents with acute lymphoblastic leukemia (ALL) enrolled in pediatric (AIEOP) and adult (GIMEMA) protocols. [Abstract] Blood. 2004;104: A–1954 [Google Scholar]
- 16.Boissel N, Auclerc MF, Lhéritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21(5):774–780 [DOI] [PubMed] [Google Scholar]
- 17.Paulussen M, Ahrens S, Juergens HF. Cure rates in Ewing tumor patients aged over 15 years are better in pediatric oncology units: Results of GPOH CESS/EICESS studies [abstract]. Proc Am Soc Clin Oncol. 2003;22(816):3279 [Google Scholar]
- 18.Yeager ND, Hoshaw-Woodard S, Ruymann FB, Termuhlen A. Patterns of care among adolescents with malignancy in Ohio. J Pediatr Hematol Oncol. 2006;28(1):17–22 [PubMed] [Google Scholar]
- 19.Klein-Geltink J, Shaw AK, Morrison HI, Barr RD, Greenberg ML. Use of paediatric versus adult oncology treatment centres by adolescents 15-19 years old: the Canadian Childhood Cancer Surveillance and Control Program. Ear J Cancer. 2005;41(3):404–410 [DOI] [PubMed] [Google Scholar]
- 20.Mitchell AE, Scarcella DL, Rigutto GL, et al. Cancer in adolescents and young adults: treatment and outcome in Victoria. Med J Aust. 2004;180(2):59–62 [DOI] [PubMed] [Google Scholar]
- 21.Albritton K, Bleyer WA The management of cancer in the older adolescent. Eur J Cancer. 2003:39(18):2584–2599 [DOI] [PubMed] [Google Scholar]
- 22.Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29(30):4045–4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krailo MD, Bernstein L, Sullivan-Halley J, Hammond GD. Patterns of enrollment on cooperative group studies: an analysis of trends from the Los Angeles County Cancer Surveillance Program. Cancer. 1993;71(10 suppl):3325–3330 [DOI] [PubMed] [Google Scholar]
- 24.Downs-Canner S, Shaw PH. A comparison of clinical trial enrollment between adolescent and young adult (AYA) oncology patients treated at affiliated adult and pediatric oncology centers. J Pediatr Hematol Oncol. 2009;31(12):927–929 [DOI] [PubMed] [Google Scholar]
- 25.Shaw PH, Ritchey AK Different rates of clinical trial enrollment between adolescents and young adults aged 15 to 22 years old and children under 15 years old with cancer at a children’s hospital. J Pediatr Hematol Oncol. 2007;29(12):811–814 [DOI] [PubMed] [Google Scholar]
- 26.Shaw P, Boyiadzis M, Tawbi H, et al. Improved clinical trial enrollment in adolescent and young adult (AYA) oncology patients after the establishment of an AYA oncology program uniting pediatric and medical oncology divisions. Cancer 2012;118(14):3614–3617 [DOI] [PubMed] [Google Scholar]
- 27.Ramanujachar R, Richards S, Hann I, et al. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr Blood Cancer. 2007;48(3) :254–261 [DOI] [PubMed] [Google Scholar]
- 28.Ramanujachar R, Richards S, Hann I, Webb D. Adolescents with acute lymphoblastic leukaemia: emerging from the shadow of paediatric and adult treatment protocols. Pediatr Blood Cancer. 2006;47(6):748–756 [DOI] [PubMed] [Google Scholar]
- 29.Cairo MS, Sposto R, Perkins SL, et al. Burkitt’s and Burkitt-like lymphoma in children and adolescents: a review of the Children’s Cancer Group experience. Br J Haematol. 2003;120(4) :660–670 [DOI] [PubMed] [Google Scholar]
- 30.Burkhardt B, Oschlies I, Klapper W, et al. Non-Hodgkin’s lymphoma in adolescents: experiences in 378 adolescent NHL patients treated according to pediatric NHL-BFM protocols. Leukemia. 2011;25(1):153–160 [DOI] [PubMed] [Google Scholar]
- 31.Creutzig U, Büchner T, Sauerland MC, et al. Significance of age in acute myeloid leukemia patients younger than 30 years: a common analysis of the pediatric trials AML-BFM 93/98 and the adult trials AMLCG 92/99 and AMLSG HD93/98A. Cancer. 2008;112(3):562–571 [DOI] [PubMed] [Google Scholar]
- 32.Joshi D, Anderson JR, Paidas C, Breneman J, Parham DM, Crist W; Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Age is an independent prognostic factor in rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Pediatr Blood Cancer. 2004;42(1):64–73 [DOI] [PubMed] [Google Scholar]
- 33.Moreno L, Bautista FJ, Zacharoulis S. Outcome of teenagers and young adults with ependymoma: the Royal Marsden experience. Childs Nerv Syst. 2009;25(9):1047–1052 [DOI] [PubMed] [Google Scholar]
- 34.Millot F, Baruchel A, Guilhot J, et al. Imatinib is effective in children with previously untreated chronic myelogenous leukemia in early chronic phase: results of the French national phase IV trial. J Clin Oncol. 2011;29(20):2827–2832 [DOI] [PubMed] [Google Scholar]
- 35.Polishchuk AL, Dubois SG, Flaas-Kogan D, Hawkins R, Matthay KK. Response, survival, and toxicity after iodine-131-metaiodobenzylguanidine therapy for neuroblastoma in preadolescents, adolescents, and adults. Cancer. 2011;117(18):4286–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinkerton R, Wills RA, Coory MD, Fraser CJ. Survival from haematological malignancy in childhood, adolescence and young adulthood in Australia: is the age-related gap narrowing? Med J Aust. 2010;193(4):217–221 [DOI] [PubMed] [Google Scholar]
- 37.Silverman LB, Stevenson KE, O’Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000). Leukemia. 2010;24(2):320–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schultz KA, Ness KK, Whitton J, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2007;25(24):3649–3656 [DOI] [PubMed] [Google Scholar]
- 39.Alderfer MA, Navsaria N, Kazak AE. Family functioning and posttraumatic stress disorder in adolescent survivors of childhood cancer. J Fam Psychol. 2009;23(5):717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazak AE, Alderfer MA, Streisand R, et al. Treatment of posttraumatic stress symptoms in adolescent survivors of childhood cancer and their families: a randomized clinical trial. J Fam Psychol. 2004;18(3):493–504 [DOI] [PubMed] [Google Scholar]
- 41.Judge Santacroce S, Asmus K, Kadan-Lottick N, Grey M. Feasibility and preliminary outcomes from a pilot study of coping skills training for adolescent—young adult survivors of childhood cancer and their parents. J Pediatr Oncol Nurs. 2010;27(1)40–20 [DOI] [PubMed] [Google Scholar]
- 42.Seitz DC, Besier T, Goldbeck L. Psychosocial interventions for adolescent cancer patients: a systematic review of the literature. Psychooncology. 2009;18(7):683–690 [DOI] [PubMed] [Google Scholar]
- 43.Tercyak KP, Donze JR, Prahlad S, Mosher RB, Shad AT. Multiple behavioral risk factors among adolescent survivors of childhood cancer in the Survivor Health and Resilience Education (SHARE) program. Pediatr Blood Cancer. 2006;47(6):825–830 [DOI] [PubMed] [Google Scholar]
- 44.Cox CL, McLaughlin RA, Rai SN, Steen BD, Hudson MM. Adolescent survivors: a secondary analysis of a clinical trial targeting behavior change. Pediatr Blood Cancer. 2005;45(2):144–154 [DOI] [PubMed] [Google Scholar]
- 45.Baider L, De-Nour AK. Group therapy with adolescent cancer patients. J Adolesc Health Care. 1989;10(1):35–38 [DOI] [PubMed] [Google Scholar]
- 46.Hill DA, Furman WL, Billups CA, et al. Colorectal carcinoma in childhood and adolescence: a clinicopathologic review. J Clin Oncol. 2007;25(36):5808–5814 [DOI] [PubMed] [Google Scholar]
- 47.Pao M, Ballard ED, Rosenstein DL, Wiener L, Wayne AS. Psychotropic medication use in pediatric patients with cancer. Arch Pediatr Adolesc Med. 2006;160(8):818–822 [DOI] [PubMed] [Google Scholar]
- 48.Portteus A, Ahmad N, Tobey D, Leavey P. The prevalence and use of antidepressant medication in pediatric cancer patients. J Child Adolesc Psychopharmacol. 2006;16(4):467–473 [DOI] [PubMed] [Google Scholar]
- 49.Sultan I, Rodriguez-Galindo C, El-Taani H, et al. Distinct features of colorectal cancer in children and adolescents: a population-based study of 159 cases. Cancer. 2010;116(3):758–765 [DOI] [PubMed] [Google Scholar]


