Abstract
The commercialization of engineered nanomaterials (ENMs) began in the early 2000’s. Since then the number of commercial products and the number of workers potentially exposed to ENMs is growing, as is the need to evaluate and manage the potential health risks. Occupational exposure limits (OELs) have been developed for some of the first generation of ENMs. These OELs have been based on risk assessments that progressed from qualitative to quantitative as nanotoxicology data became available. In this paper, that progression is characterized. It traces OEL development through the qualitative approach of general groups of ENMs based primarily on read-across with other materials to quantitative risk assessments for nanoscale particles including titanium dioxide, carbon nanotubes and nanofibers, silver nanoparticles, and cellulose nanocrystals. These represent prototypic approaches to risk assessment and OEL development for ENMs. Such substance-by-substance efforts are not practical given the insufficient data for many ENMs that are currently being used or potentially entering commerce. Consequently, categorical approaches are emerging to group and rank ENMs by hazard and potential health risk. The strengths and limitations of these approaches are described, and future derivations and research needs are discussed. Critical needs in moving forward with understanding the health effects of the numerous EMNs include more standardized and accessible quantitative data on the toxicity and physicochemical properties of ENMs.
Keywords: Nanoparticles, Nanomaterials, Quantitative risk assessment, Occupational exposure limits, Particle overload, Biomarkers, Respiratory effects, Lung disease
1. Introduction
Risk assessments are conducted to estimate the risk following exposure to hazardous substances. Few risk assessments have been performed to date on engineered nanomaterials (ENMs) due to limited data. However, there is a growing body of data that raises concerns about potential adverse health effects from exposure to ENMs (Hristozov et al., 2012; Kreyling et al., 2004; Kuempel et al., 2012; Ma-Hock et al., 2009; Nel et al., 2013; Oberdörster et al., 1995, Sargent et al., 2009; Savolainen and Vartio, 2017; Schmid and Stoeger, 2016). The commercialization of nanotechnology generally began in the early 2000s and precautionary guidance followed soon after (Hett, 2004; HSE, 2004; NIOSH, 2005; The Royal Society and The Royal Academy of Engineering, 2004). By 2005, 54 consumer products were reported to contain nanomaterials, while today that number is over 1800 products (Vance et al., 2015). Workers are involved in all aspects of ENM production from research to production, use, and disposal, and are potentially exposed to nanomaterials. Employers, workers, insurers, government decision-makers, and other stakeholders all need information on the hazard of nanomaterials and the health risk to workers. In response, there has been a concerted effort to identify the hazards of nanomaterials and the underlying mechanisms of action, determine exposures, assess risks, and provide guidance on managing those risks.
Quantitative risk assessment (QRA) methods for ENMs generally have been consistent with those in the standard risk assessment paradigm (NAS, 1983, 2009_; OECD, 2012). When quantitative dose-response data are available, risk assessment for ENMs and other substances involves the following five steps: 1) evaluating available data; 2) selecting an appropriate adverse response; 3) determining the critical dose; 4) calculating the human equivalent dose; and 5) determining the working lifetime exposure concentration that would result in that dose (Jarabek et al., 2005; Kuempel et al., 2006; Oberdörster, 1989; Schulte et al., 2010; U.S. EPA, 1994). QRA involves estimation of a point of departure (POD), which is a point on the dose-response curve that identifies the dose associated with an adverse response at a low level or a level that is not biologically or statistically different from background. A POD based on animal data is extrapolated to humans by estimating an equivalent dose (e.g., using interspecies adjustments) to lower risk levels based on quantitative modeling and/or uncertainty factors. OELs, critical tools in risk management, then are derived from estimates of the airborne exposure concentrations associated with no or low risk of adverse health effects in workers. Additionally, consideration is given to specific factors pertaining to the nanoscale, such as potential differences in the uptake and distribution of nanoscale and microscale particles in the body, and potential differences in the hazard potency of nanoscale vs. microscale particles of the same composition on a mass basis. When quantitative dose-response data are not available, other methods are needed, including read-across methods based on knowledge about the underlying biological mechanism of action, and grouping based on similar physicochemical properties, or comparative potency using shorter-term data in animals or cell systems (Arts et al., 2014, 2015; Gordon et al., 2014; Kuempel et al., 2012; Maier, 2011; NAS, 2017; Nel et al., 2013; Schoeny and Margosches, 1989; Sobels, 1977, 1993; Stone et al., 2014).
It is possible to characterize the trajectory of risk assessments of ENMs according to approaches that have been used in the past. This characterization requires seeing the trajectory in the context of the natural history of the development of commercial nanotechnology. The risk assessment of ENMs builds on earlier work with ultrafine particles and fine dusts (Dankovic et al., 2007; Donaldson et al., 1990; Driscoll et al., 1990; Kreyling et al., 2013; Oberdörster et al., 1992; Stone et al., 2016b; Tran et al., 1999; Tran and Buchanan, 2000; Wichmann and Peters, 2000). Fig. 1 shows the trajectory for risk assessment of ENMs in terms of the approaches used. In the early 2000s, concern about the potential hazards of ENMs was great. While there were preliminary data (air pollution epidemiology, health effects of welding fumes, and some studies of nanoparticle translocation from nose to brain), generally there was a major lack of information about hazards, risks, and exposures of ENMs. Consequently, the initial approach to risk assessment was based on precautionary appraisal to fill the pressing need for any kind of guidance to anchor risk management decisions (The Royal Society and The Royal Academy of Engineering, 2004; BSI, 2007; IFA, 2009). For ENMs with sufficient data, quantitative risk assessment methods have been used to develop OELs (e.g., NIOSH, 2011; NIOSH, 2013). Given the challenges in developing individual OELs for all ENMs - many of which have limited data - methods have been developed to prioritize or group ENMs based on the available subchronic or chronic dose-response data for benchmark materials and the utilization of shorter-term in vivo data for many ENMs (e.g., Arts et al., 2016; Hristozov et al., 2016; Drew et al., 2017). No OELs have been developed based on these methods to date, and efforts are underway to further develop quantitative methods to categorize ENMs by hazard potency, as well as to evaluate the use of data from alternative test systems including in vitro models.
Fig. 1.
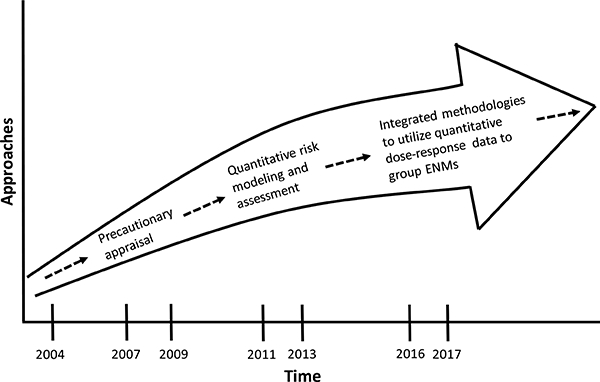
The eras of risk assessment and development of occupational exposure limits for engineered nanomaterials.
Fig. 2 shows the trajectory of risk assessments for selected ENMs related to the development of OELs. While there are thousands of ENMs in commerce, only a minute fraction of those has an OEL. A recent systematic review study cited 56 OELs that have been developed for ENMs, although many of these are for the same set of ENMs, and this number includes both individual and categorical OELs (Mihalache et al., 2017). The first two examples, the British Standards Institute (BSI) and the German Occupational Safety and Health authority (IFA), utilized professional judgement to describe broad categories of ENMs, called benchmark exposure levels (BSI, 2007; IFA, 2009). The categories were selected to utilize size, density, shape, and biopersistence and the exposure levels were derived as fractions of the OEL for benchmark bulk material of the same composition or physical chemical characteristics as the ENM. For fibrous materials, such as carbon nanotubes (CNTs), the benchmark exposure level was one-tenth of the asbestos or 0.01 fibers/ml (BSI, 2007; IFA, 2009). OELs based on quantitative risk assessments have been developed for titanium dioxide (TiO2), carbon nanotubes and nanofibers, and silver, as discussed in Section 2. No OELs have been developed to date for nanoscale cellulose given the limited dose-response data, and methods to develop categorical OELs for ENMs are under development, as discussed in Section 3.
Fig. 2.
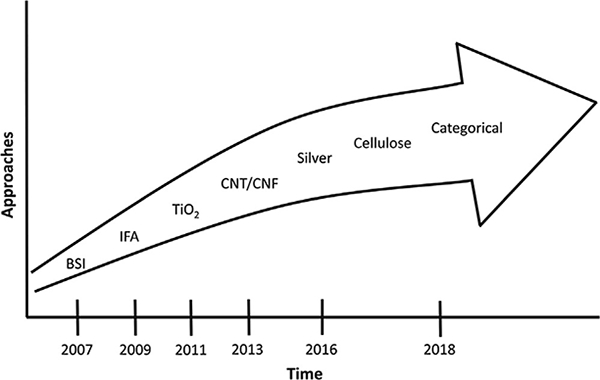
Trajectory of risk assessments and development of occupational exposure limits for engineered nanomaterials.
Abbreviations:
BSI: British Standards Institute
IFA: Institute for Occupational Safety and Health of the German Social Accident Insurance
TiO2: Titanium dioxide
CNT/CNF: Carbon nanotubes and carbon nanofibers
2. Protoypic nanomaterial risk assessment
2.1. Titanium dioxide
One of the first QRAs of a nanomaterial was on titanium dioxide (TiO2). (Dankovic et al., 2007). A QRA is a systematic process to assess risks, in this case from chemical substances. The assessment procedure involves the four main steps of hazard identification, dose-response assessment, exposure assessment and risk characterization (NAS, 1983; NAS, 2009). Ultimately, it is the process of extrapolating from a range of direct observation to a lower potentially safer range for which there are few or no data (NRC, 1987; Schulte et al., 2002). While TiO2 has been used in commerce for decades, it has been increasingly formulated with a greater proportion of primary particle sizes in the sub-100 nm range. The dose-response data available for the TiO2 risk assessment included subchronic (13-week) and chronic (104-week) inhalation studies. Benchmark dose (BMD) and BMD lower confidence limit (BMDL) estimates (Crump, 1984) were derived from the dose-response data of pulmonary neutrophilic inflammation or lung tumors in rats, using the total particle surface area retained dose in the lungs to normalize across particle sizes. The BMDL estimate was used as the POD in this risk assessment. Extrapolation of the animal doses to humans utilized data and models to account for the inter-species differences in breathing rates, particle deposition fraction and clearance kinetics, and lung surface areas (Kuempel et al., 2006).
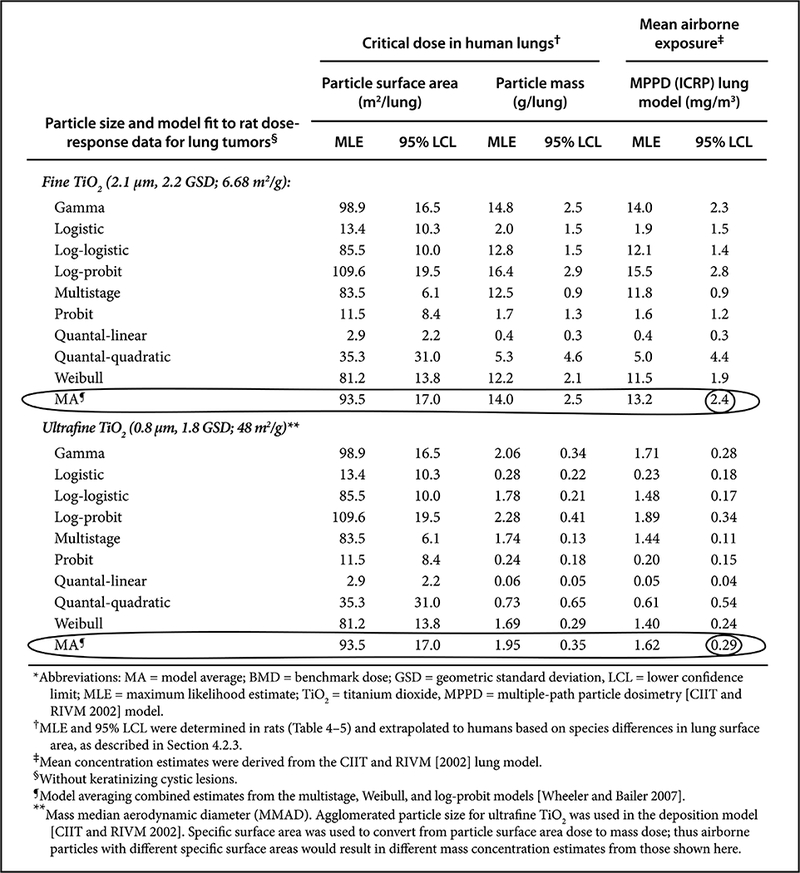
Dankovic et al. (2007) and NIOSH (2011) used these QRA methods to derive recommended exposure limits (RELs) for nanoscale and microscale TiO2. This was the first REL to address two size ranges of respirable particles (≤100 nm ultrafine and > 100 nm fine). The primary data used were lung tumor data from a chronic inhalation study of rats (with one dose point for nanoscale TiO2) exposed to ultrafine TiO2 (Heinrich et al., 1995) and from studies of fine TiO2 (Lee et al., 1985; Muhle et al., 1991). These data were pooled, and BMD and BMDL estimates of the particle surface area retained dose in the lungs were determined at target risk levels from 1:10–1:1000 excess lifetime risk. These estimates were extrapolated to human-equivalent working life-time exposures using a human lung dosimetry model (Multiple-Path Particle Dosimetry, MPPD, v. 1.0) (CIIT and RIVM, 2002). This procedure illustrated that various dose-response models could be fit to the same data (Table 1), and that the best approach was to use model averaging (Wheeler and Bailer, 2007) using three models (NIOSH, 2011). This approach incorporates statistical variability and model uncertainty into the BMD and BMDL estimation. It uses all the information from various dose-response models and weighs each model by how well it fits the data. The weighted average of the models for ultrafine and fine TiO2 at a target risk level of a lifetime excess risk of 1 per 1000 for lung tumors were selected as the OELs 0.3 mg/m3 and 2.4 mg/m3 (10hr TWA), respectively. This risk level has been considered to be a significant risk (U.S. Supreme Court, 1980). More recently, NIOSH has updated its policy on RELs and risk levels for chemical carcinogens (NIOSH, 2017b).
Table 1.
Various dose-response models for TiO2 – Lung dose and airborne exposure concentration estimates are associated with 1/1000 excess risk of lung cancer after a 45-year working lifetime (Table 4–6 from NIOSH, 2011).
 |
It is known that there are many different types of TiO2 ENMs based on crystal structure and coatings. Available data at the time indicated that TiO2 crystal structure did not significantly affect the pulmonary inflammation or tumor responses, and some particle surface coatings increased the inflammation response. NIOSH (2011) concluded that the TiO2 risk assessment could be used as a reasonable baseline for potential toxicity because other particle treatments or formulations could potentially affect toxicity. Another approach that focused on inflammatory effects was utilized in Japan (Morimoto et al., 2010). This approach, referred to as the “biaxial” approach, compared results from inhalation studies of one type of TiO2 ENM with the results of intratracheal instillation studies of other types of TiO2 ENMs and the results extrapolated to humans. An acceptable concentration was estimated to be 1.2mg/m3 (8-hr TWA and 40-hr work week) (Morimoto et al., 2010). The route of exposure and dose rate can influence the pulmonary responses to TiO2 (Baisch et al., 2014; Shi et al., 2013), which Morimoto et al. (2010) evaluated in their comparisons of EMN toxicity across doses and routes of exposure in rats.
2.2. Carbon nanotubes and nanofibers
In the early 2000’s, a new ENM came into commerce, which was CNT with single and multiwall typologies. Early on in the appearance of CNTs was the concern that the fiber toxicity paradigm that pertained to fibrous materials like asbestos might pertain to CNTs. CNTs are thin, long and biopersistent and these are characteristics of toxic fibers (Donaldson et al., 2006). Several animal studies published beginning in 2004 showed the development of pulmonary fibrosis (early onset and persistent granulomatous inflammation) from CNT exposure in rats and mice. These effects occurred at relatively low mass dose and occurred regardless of whether CNTs were purified or unpurified regarding metal contamination. There were also concerns that some CNTs could persist in the lungs and migrate to the pleura. Other studies showed the CNT exposure resulted in genotoxic effects including aneuploidy (Sargent et al., 2009). Various risk assessments of CNTs were conducted using data in rats and mice. Central to the risk assessments by NIOSH (2013) and others (Nanocyl, 2009; Pauluhn, 2010b) were two published sub-chronic (13-week) inhalation studies of two types of multi-walled CNTs (MWCNTs) in rats (Ma-Hock et al., 2009; Pauluhn, 2010a). A follow-on study with additional evaluation of the lung tissues from the Ma-Hock et al. (2009) study by Treumann et al. (2013) provided more in-depth information regarding the nature of the granulomatous, inflammogenic, and fibrotic tissue responses in rats (discussed in IARC, 2017); while providing more in-depth information about the observed responses, these findings would not likely change the quantitative dose-response data or POD estimates based on the Ma-Hock et al. (2009) study.
Several shorter-term studies of other types of CNTs or carbon nanofibers (CNFs) in rodents provided additional data (NIOSH, 2013). The pulmonary responses, which included various measures of inflammation and fibrosis, were qualitatively similar across the various CNTs and CNFs, whether purified or unpurified with differing metal content, and of different dimensions. The fibrotic lung effects in the rodents subchronic studies (25/54) developed early (within a few weeks) after exposure to CNT or CNF, at relatively low-mass lung doses, and persisted or progressed during the post-exposure follow-up (~ 1–6 months). Pulmonary fibrosis was the primary endpoint used in the NIOSH (2013) risk assessment. The NIOSH REL of 1 μg/m3 (8-hr time-weighted average) was set at the analytical limit of quantification for respirable elemental carbon (NIOSH Method 5040), and was associated with risk estimates of approximately 0.5%−16% (upper confidence limit estimates) of developing early-stage (slight or mild) lung effects (i.e. fibrosis) over a working lifetime (NIOSH, 2013). The NIOSH REL was meant to pertain to all types of CNTs and CNFs based on the available data, but it was recognized that there could be variability in toxicity due to physical-chemical characteristics and that the guidance may be reevaluated as new data becomes available.
Fig. 3 shows the OELs from several different risk assessments of various CNTs. The differences in these proposed OELs are due to the differences in the types of CNTs, rodent studies and endpoints, methods to estimate human-equivalent concentration, and uncertainty factors used. At the time the OELs were developed, there were no regulatory OELs for CNTs (as remains the case today), and so regulatory OELs for other carbonaceous materials, such as carbon black (3500 μg/m3) or graphite (5000), may have been the closest OELs available. Had a precautionary approach not been taken, worker exposures to CNTs could have been roughly 3500–5000 times what had been estimated to be the human-equivalent concentrations associated with adverse lung effects in animal studies. Moreover, even though the four OELs that were derived ranged from 1 to 50 μg/m3, these are all relatively low mass concentrations compared to the exposure limits for other carbonaceous particles of 3500 μg/m3 or 5000 μg/m3.
Fig. 3.
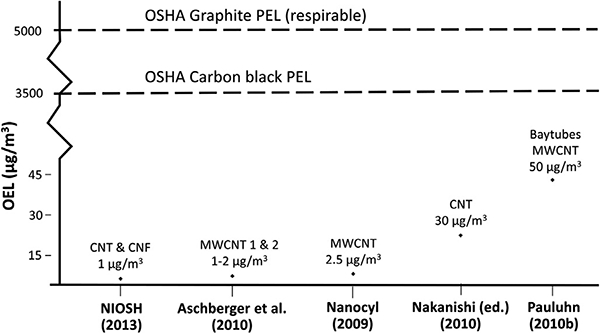
Example of proposed occupational exposure limits (OELs) for carbon nanotubes and carbon nanofibers, with comparison to existing regulatory OELs for microscale carbonaceous particles.
Notes:
OELs shown are for 8-hr time-weighted average concentration.
In Aschberger et al. (2010), OEL for MWCNT1 is 2 μg/m3 based on data from Pauluhn (2010b); OEL for MWCNT2 is 1 μg/m3 based on LOAEL from Ma-Hock et al. (2009).
In Nakanishi (ed.) (2011), the OEL is limited to a period of 15-yr. Information also provided in Nakanishi et al. (2015).
BSI (2007) OEL for CNT (not shown) is 0.01 f/ml (benchmark exposure level) for high aspect ratio nanomaterials, established at 1/10th of asbestos OEL.
Abbreviations:
PEL: Permissible Exposure Limit, U.S. Occupational Safety and Health Administration (OSHA) (29 CFR 1910 CFR 1910.1000, Table Z-1)
CNT: Carbon nanotubes
CNF: Carbon nanofibers
MWCNT: Multi-walled carbon nanotubes
More recently, animal cancer bioassay data have been published for some CNTs. In the IARC (2017) evaluation, based on evidence available at the time of the monograph meeting (October 2014), one type of MWCNT was classified as possibly carcinogenic to humans (Group 2B). After this evaluation, a 2-year inhalation study showing increased lung cancer incidence in rats following exposure to the specific MWCNT was published (Kasai et al., 2016). Overall, most types of MWCNTs and single-walled carbon nanotubes (SWCNT) were not classifiable as to their carcinogenicity to humans (Group 3) (IARC, 2017).
In addition to lung effects, inhalation exposure to CNTs has been shown to elicit pulmonary secretion of acute phase proteins to the blood (Poulsen et al., 2017). The induction of a pulmonary acute phase response following inhalation of particles including nanoparticles has been proposed as a causal link between particle inhalation and risk of cardiovascular disease (Saber et al., 2014). Inhalation exposure of rats to MWCNT has also been shown to decrease responsiveness of coronary arterioles to dilators and to affect heart rate variability (Stapleton et al., 2012; Zheng et al., 2016).
2.3. Silver nanoparticles
Another illustration of risk assessment and OEL development involves silver nanoparticles. Increased production and wide spread use of silver nanoparticles were reasons to consider what would be safe levels for workers. Prior to the initiation of commercial nanotechnology, silver dusts were controlled by an OEL of 10 μg/m3 (NIOSH, 1988; OSHA, 1988). However, that OEL did not explicitly address silver nanoparticles. Rat subchronic inhalation studies of silver nanoparticles (Song et al., 2013; Sung et al., 2008, 2009) were determined to be relevant for risk assessment for silver nanoparticles. These studies showed early-stage adverse lung and liver effects in male and female rats, including lung function deficits and histopathological findings of lung inflammation and liver bile duct hyperplasia and neoplasia.
Christensen et al. (2010) used a LOAEL of 49 μg/m3 for lung function deficits in female rats and a NOAEL of 133 μg/m3 for liver bile duct hyperplasia in male and female rats (Sung et al., 2008, 2009). They followed the E.U. risk assessment methods (ECHA, 2010) to estimate the human indicative no-effect levels (INELs), which appear to be equivalent to the ECHA DNELS (derived no effect levels) at occupational exposure conditions (note that Christensen et al., 2010 cite the 2008 version of ECHA guidelines). The factors applied to the animal critical effect levels included adjustments for the duration of rat vs. worker exposure day, worker vs. resting ventilation rate, LOAEL to NOAEL estimation, subchronic to chronic extrapolation, and worker inter-individual variability. Three INELs were derived, ranging from 0.098 to 0.67 μg/m3 (Christensen et al., 2010).
Weldon et al. (2016) estimated BMDs and BMDLs for lung and liver effects reported in Sung et al. (2008, 2009). They selected liver bile duct hyperplasia as the critical effect in rats because it was the lowest BMDL of a specific quantitative endpoint. They adjusted the rat critical effect level to estimate a human-equivalent concentration (using dosi-metric adjustment factors for ventilation rate, pulmonary deposition fractions, pulmonary particle clearance rates, and interspecies dose normalization based on lung surface area). Uncertainty factors were applied for animal to human toxicodynamic differences, subchronic to chronic extrapolation, and worker individual variability. Their derived OEL was 0.19 μg/m3. Dissolution and clearance of silver nanoparticles was not explicitly considered in either of these assessments, but this likely explained in part the effects that were observed in the liver. This raises the question of how in vivo dissolution rates might be incorporated in risk models, for example, using a physiologically based pharmacokinetic model (Bachler et al., 2013).
NIOSH (2016) evaluated several risk assessment methods and assumptions in developing a draft document to examine the adequacy of the existing NIOSH REL of 10 μg/m3 (8-hr time-weighted average concentration, total mass sample, silver metal dust and soluble compounds, as Ag) (NIOSH, 2007) for silver nanoparticles. This REL, and the equivalent regulatory limit in the U.S., were derived to protect workers from developing argyria and argyrosis, a bluish-gray coloring of the skin and eyes. In its evaluation, NIOSH (2016) used a published physiologically-based pharmacokinetic (PBPK) model (Bachler et al., 2013) to estimate the worker-equivalent exposure concentrations to those associated with the rat early-stage, adverse lung and liver effects (Song et al., 2013; Sung et al., 2008, 2009). The human-equivalent 45-year working lifetime concentrations estimates varied from 0.19 to 3.8 μg/m3 for total silver, and from 6.2 to 195 μg/m3 for soluble/active tissue doses (estimates also depended on particle diameter of 15-nm- to 100-nm-diameter) (NIOSH, 2016). In the draft document, NIOSH found that the available scientific evidence was insufficient to estimate a REL that was specific to particle size. NIOSH is currently evaluating the public and peer review comments and updating the literature searches to further evaluate the scientific evidence on the potential health risk of occupational exposure to silver nanoparticles.
2.4. Cellulose nanocrystals
An illustration of the dilemma investigators face in conducting risk assessments and deriving OELs when data are sparse and contradictory involves cellulose nanocrystals. These materials are rod-shaped with diameters less than 100 nm and lengths from 100 nm to 1000 nm. Cellulose nanocrystals have many of the commercially useful properties as CNTs but at a lower cost for production. Consequently, they could be produced in high volumes, and the potential for worker exposure could be great. Cellulose nanocrystals are entering commerce and already there may be worker exposures, but there is no OEL for nanoscale cellulose against which to assess exposure.
The size and shape of cellulose nanocrystals raises the concern for potential fiber toxicity, and studies have shown pulmonary effects in animals. Acute phase pulmonary responses (elevated neutrophils and other inflammatory cells) were more prominent in mice exposed to cellulose nanocrystals than in mice exposed to an equivalent mass dose of crocidolite asbestos at 24-hr after pharyngeal aspiration exposure (Yanamala et al., 2014). Studies of male and female mice (C57BL/6 mice by pharyngeal aspiration) resulted in pulmonary inflammation (elevated leukocytes and eosinophils in bronchoalveolar lavage fluid), oxidative stress, impaired pulmonary function, and elevated TGF-β (Shvedova et al., 2016; Yanamala et al., 2014). Toxicity was more pronounced in female mice (Shvedova et al., 2016). Lung collagen was measured as an indicator of fibrosis by Shvedova et al. (2016); at a total dose of 240 μg/mouse of cellulose nanocrystals, collagen was significantly increased in male and female mice relative to controls. This dose is 12 times greater than the dose of SWCNT (20 μg/mouse) associated with fibrosis measured as the average thickness of alveolar connective tissue in an earlier study (Shvedova et al., 2005). However, estimates of the relative potency of SWCNT and cellulose nanocrystals based on these data would be uncertain given the one dose group only for cellulose nanocrystals and the different measures of fibrotic response in the two studies.
No risk assessment has been conducted for cellulose nanocrystals, but Shvedova et al. (2016) estimated that if workers were exposed at the current OEL for cellulose (5 mg/m3), then after 42 days of exposure they would achieve a dose equivalent to the 240 μg total dose in mice that caused the observed pathology. Although these data are not sufficient for a QRA, this example illustrates the limited amount of information on inhalation risks of cellulose nanocrystals.
Stockmann-Juvala et al. (2014) suggested an OEL of 0.01 fibers/cm3 for cellulose nanocrystals based on the BSI (2007) benchmark exposure level for fibrous particles derived from the asbestos limit value. To date, there is no strong evidence that cellulose nanocrystals would follow the asbestos fiber paradigm (Greim, 2004), but in the absence of adequate data, Stockmann-Juvala et al. (2014) based their OEL on the precautionary principle. At this time, overall evidence for cellulose nano-crystal toxicity is limited and inconclusive. This situation illustrates that there are times when adequate data are not available and a quantitative risk assessment for a specific substance cannot be conducted. In such cases, alternative approaches such as read-across or occupational hazard banding methods might be used to estimate an occupational exposure band (OEB) to guide risk management decision-making (ISO, 2016; NIOSH, 2017a).
3. Categorical approaches to developing OELs
Categorical approaches explore how categories of ENMs can be treated similarly or how individual ENMs can be put into categories. Some of these various categorical approaches are meant to be used for screening ENM for prioritization for in vivo toxicological testing. Other categorical approaches attempt to consider ways to fill in the steps between an untested ENM and the derivation of an OEL by showing linkages and projections that span the continuum from physical-chemical properties to in vitro results to in vivo results to dose-response models and OEL derivation.
3.1. Generic approach for poorly soluble low toxicity particles
A generic method to estimate OELs for the poorly-soluble low toxicity category of respirable particles has been proposed (Pauluhn, 2011, 2014). This model utilizes the particle volumetric dose (6% of total macrophage cell volume) which has been associated with overloading of pulmonary clearance in rats (Morrow, 1988). The Pauluhn (2011) model also allows for a changing pool size of the alveolar macrophage cell volume and accounts for interspecies differences in particle size-specific lung deposition fractions and first-order clearance kinetics. The use of the rat dose associated with overloading of clearance as the POD for risk assessment is based on the hypothesis that prevention of overloading would prevent deleterious secondary conditions from occurring, as observed in rats. Based on this model, Pauluhn (2011, 2014) proposed a generic OEL for preventing particulate matter (PM)-induced pulmonary overload-like conditions in workers, which is calculated using a volume-based generic exposure of 0.54 μl PMresp × 𝜌/m3alv, where PMresp is the respiratory particulate matter and 𝜌 is the apparent density of the poorly-soluble particles within the total macrophage pool volume, ) (Pauluhn, 2011, 2014). This generic OEL is based on rat data from 13-week inhalation exposure to poorly-soluble particles. An equivalent expression based on two-year rat inhalation data was estimated at 0.36 μl (Pauluhn, 2011). The theoretical model was verified through prediction of the NOAELs in rat inhalation studies of poorly soluble particles from 4 to 104 weeks of exposure (Pauluhn, 2011). Evaluation of the rat overloading dose to humans remains to be evaluated, since the rat first-order lung clearance kinetics model has been shown to under-predict the human long-term retention of particles in the lungs, which requires accounting for particle sequestration doses below those associated with overloading in rats (Gregoratto et al., 2010; ICRP, 2015; Kuempel et al., 2001).
In other rodent studies, the particle surface area dose was the bio-logically most relevant metric for describing the overloading of nano-particles (Tran et al., 2000) and the relationship between particle dose and acute or subchronic pulmonary inflammation across a range of particle sizes (Elder et al., 2005; Monteiller et al., 2007; Oberdörster et al., 1994b; Schmid and Stoeger, 2016). Other particle properties influencing the biologically-effective dose include solubility, shape, and surface reactivity (Donaldson et al., 2013; Duffin et al., 2007). In comparative potency analyses of microscale and nanoscale particles, it would be most useful to have experimental data sufficient to convert between various dose metrics in order to further evaluate the most predictive dose metrics across a range of endpoints (Drew et al., 2017). In developing individual or categorical OELs, the dose used as the POD to estimate human-equivalent exposure could be converted to airborne mass concentration to conform to standard mass-based concentration measurements in the workplace (as was done for TiO2) (NIOSH, 2011}.
3.2. Approaches using predictive toxicology
Early in the commercial history of nanotechnology, it was determined that the vast number of potential ENMs could not all be recommended for toxicology testing in animals. Two other developments converged with this recognition. One was the growing move to minimize or cease animal testing of chemicals, and the other was growth of “21st century toxicology” the use of computational toxicology, mechanistic and biological models, and high throughput technologies to assess chemicals rather than using animal studies. All of these movements were the foundation for new approaches for prioritizing toxicity testing or alternative testing (NAS, 2007; NAS, 2017; Savolainen et al., 2010; Shatkin et al., 2016; Stone et al., 2016a). Categorical approaches have a long history with chemicals. The “parallelogram” approach was utilized to identify genotoxicants in Sobels (1993). For substances with similar structure-activity relationships, a parallelogram approach was used to derive OEBs for pharmaceutical intermediates by comparing in vitro assay results to those for well-studied substances with both in vitro and in vivo data (Maier, 2011). With regard to nanomaterials, the early semi-quantitative examples from BSI (2007) and IFA (2009) were categorical in the sense that ENMs that met the descriptive definitions would be treated as being in one of the prescribed groups and controlled to limits for those groups. After that, the concept of benchmark particles was used (Kuempel et al., 2012; Nel et al., 2013; Oberdörster et al., 2005). Benchmark particles are well-studied materials whose characteristics are known and which have risk-based OELs. Benchmark particles provide points of reference for comparison of dose-response relationships and the derivation of OELs. ENMs that are similar in chemical and physical characteristics to those benchmarks would be assigned the same OELs.
Risk assessments are now at the frontier of categorical research (OECD, 2014). This frontier involves predictive toxicological modeling from a large set of characteristics, such as physicochemical, structure-activity, in vitro test results, in vivo test results, or various biological indicators (Fig. 4). These large data sets can be evaluated to indicate characteristics that will predict toxic effects. ENMs that are shown to have these effects can be placed in the same category as a benchmark material that has an OEL. Some approaches use grouping of ENMs by mechanisms of toxicity and hazard potencies and also utilize relevant benchmark materials (Drew et al., 2017; Kuempel et al., 2012). However, the use of in vitro dose-response data to predict in vivo responses involves additional considerations, including the relevance of the in vitro doses to those in vivo. In vitro doses are typically much higher and may involve different biological mechanisms (Nel et al., 2013). In vitro dosimetry models can provide estimates of the dose of particles that reach cells given the particle density and settling rates, as well as dissolution in the cell culture media (Hinderliter et al., 2010; Liu et al., 2015). Gangwal et al. (2011) proposed methods for quantitative comparison of in vitro doses to equivalent total doses in human exposure, although differences in dose rate were not considered. Currently, use of in vitro data to predict of acute in vivo responses is most promising (see Fig. 4).
Fig. 4.
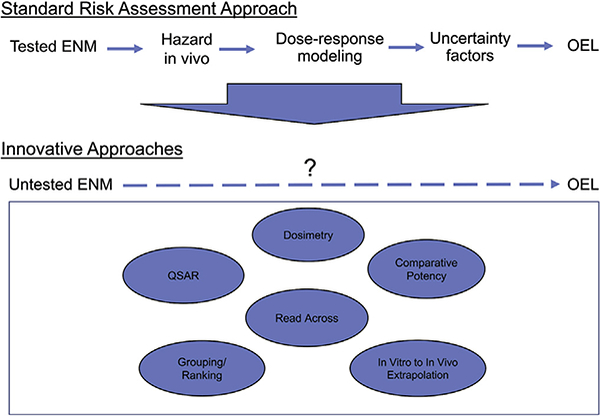
Frontier of risk assessment for developing occupational exposure limits for engineered nanomaterials.
Abbreviations: ENM: engineered nanomaterial; OEL: Occupational exposure limit; QSAR: quantitative structure-activity relationship
A limiting question is how these various toxicity indications can be linked to PODs and used to develop OELs or OEBs. Fig. 4 shows the various options for such linkage. There is concern that the approach to using quantitative structure-activity relationship (QSAR) modeling, read across techniques, and various grouping approaches underestimate or misrepresent risk, as these alternative models require making certain assumptions given the limited information, and may not be sufficient to establish the robust dose-response relationships used for traditional QRA. Therefore, these approaches may be useful initially for prioritizing nanomaterials for further testing, but may be insufficient for risk assessment and development of OELs. If there are only limited relevant data, there will not be sufficient data for characterizing a dose-response relationship. Further evaluation and validation of these methods will be needed before these methods can be implemented for OEL derivation. Quantitative evaluations of dose-response relationships for key end-points across a range of nanoscale and microscale benchmark materials (including in vitro data and acute and chronic in vivo data) would provide useful information to support that process. Needed in such an evaluation are quantification of the doses and responses in the context of adverse outcome pathways (AOPs), including consideration of kinetic processes that influence the internal dose of particles.
Whether and how these hazard test results can be subject to dose-response analysis and extrapolated to humans to develop the human equivalent dose are major ongoing questions. This step, as well as the preceding steps of linkage of physical-chemical characteristics to in vitro and to in vivo toxicities are impeded by huge data gaps. These gaps are due to heterogeneity of the data, for example, methodologic differences in tests and assays; uncertainty about relevance of early response endpoints to human health risk assessment; limited chronic exposure data; lack of minimum data reporting requirements; and lack of criteria for in vitro to in vivo extrapolation. Filling the gaps and pursing the use of these data in risk assessment requires enhanced conceptual and technical understanding. Two major issues arise: how can equivalent doses be determined in in vitro or in vivo studies, and how can toxicity of ENMs be classified based on those data? One approach to safety classification involved testing or gathering data on 31 different ENMs. Out of 8 million data points involving in vivo and in vitro models, 11 bio-markers were identified that indicated that ENMs were toxic. These biomarkers become emblematic of toxicity and suggest that further untested ENMs can be assessed for these 11 biomarkers to predict the toxicity of the ENM (Savolainen and Vartio, 2017).
In another study, a quantitative framework for assessing the hazard potency of ENMs was developed as a proof of concept using a data set consisting of in vivo rodent dose-response data of pulmonary neutrophilic inflammation from published studies including from two separate nanotoxicology research consortia (Drew et al., 2017). Doses were normalized across rodent species, strain and sex as the total particle mass concentration in the lungs. Doses associated with specific measures of pulmonary neutrophilic inflammation were estimated by modeling the continuous dose-response relationships using benchmark dose (BMD) modeling (U.S. EPA, 2012; Wang et al., 2014). One set of various types of ENMs was grouped by BMD estimates, and the group assignments of a separate set of ENMs were predicted based on physicochemical properties only. Following further evaluation with a more comprehensive dataset, this framework could be used to estimate categorical OELs for ENMs with limited dose-response data. The lower confidence estimates of the BMDs in a potency group could be used as points of departure (PODs) in risk assessment for extrapolation to estimate human-equivalent concentrations and OELs (Drew et al., 2017).
3.3. Systems approach to nanotoxicology
These methods to assess categories of ENMs are amenable to a systems approach to nanotoxicology, utilizing data on how nanomaterials cause biological perturbations and focusing on underlying bio-logical mechanisms. These approaches will enable a gradual shift from using solely apical end-points toward understanding the biological pathways perturbed (Costa and Fadeel, 2016; DeBord et al., 2015; Sturla et al., 2014). One manifestation of this effort is to identify adverse outcome pathways (AOPs) (Ankley et al., 2010; Villeneuve et al., 2014a; b). Development of AOPs allows for integration of all types of information at different levels of biological organization. An AOP is a biological map from the molecular-initiating event through the resulting adverse outcome that describes both the overall mode of action and the specific mechanisms or key events. However, the determination of a molecular-initiating event requires extensive evidence to construct an AOP and to determine how the characteristics of ENM affect these events.
The AOP has been widely promoted as a powerful tool for linking predictive toxicology to ENM risk assessment however a number of concerns have been raised. These include: whether it is premature to use AOP in risk assessment; whether AOP use may restrict needed toxicological research; that AOPs are difficult to validate; that they may falsely present the illusion of safety; and that they need to be based on robust data when they are used as a predictive tool (Pesticide Action Network Europe, 2016). The use of alternative testing methods can also help rank ENMs for further testing (Nel et al., 2013). This approach is illustrated by ITS-Nano research prioritization tool (Stone et al., 2014).
These approaches may involve strategies that incorporate systems biology approaches (Costa and Fadeel, 2016). An example of this is the study by Pisani et al. (2015) that used a microarray-based approach combined with secretomics (a subset of proteomics that analyzes the secreted proteins of a cell, tissue, or organism) to assess cellular responses to fumed silica in a human lung carcinoma cell line. The investigators derived what Lobenhofer et al., 2004 termed a “no observed transcriptomic effect level” (NOTEL). The NOTEL was lower than conventional NOAEL. The NOTEL could be used as a POD for deriving a reference value after application of uncertainty or safety factors for benchmark dose modeling of gene expression or pathway activity (Schulte et al., 2015). This kind of approach needs further study to determine the extent of its utility.
The study of the global transcriptional profiling of ENM-exposed mice has also led to identification of new mechanisms-of-action for nanomaterials. Inhalation of nano-TiO2 was shown to induce pulmonary acute phase response in mice (Halappanavar et al., 2011). The acute phase response is dose-and time dependent, proportional to the deposited particle surface area dose and closely associated with neutrophil influx (Saber et al., 2014). Since acute phase response is a well-known risk factor for cardiovascular disease in humans, this finding suggests a possible causal link between ENM inhalation and cardio-vascular disease (Saber et al., 2014). For example, inhalation exposure to nanoscale TiO2 has been shown to decrease the responsiveness of peripheral and coronary arterioles to vasodilation (LeBlanc et al., 2010; Nurkiewicz et al., 2009).
4. Development of OEBs
Occupational exposure bands (OEBs) are a type of categorical OELs and an approach to developing occupational exposure guidance when data are limited or minimal. Some OEBs are “order-of-magnitude” categories of hazard of substances and can be applied to ENMs (ISO, 2016; Kuempel et al., 2012; NIOSH, 2017a). The basis for assignment to such hazard categories are weight of evidence approaches utilizing standard data quality criteria (NIOSH, 2017a; OECD, 2014; Stone et al., 2014). Because of the pragmatic focus and immediate need in certain situations, occupational exposure banding utilizes available but often limited toxicological data to determine the potential range of chemical exposure levels that can be used as targets for ENM exposure controls. OEBs are not meant to replace OELs, rather they are risk management tools that can be used to control exposures.
5. In vivo and in vitro model systems in toxicology and risk assessment of ENMs
To put categorical approaches to risk assessment in perspective there is need to review some of the underlying issues pertaining to in vivo and in vitro models. This of particular importance with regard to extrapolation of in vitro and in vivo data to humans.
5.1. In vivo models
Generally, data in humans are not available for risk assessment of ENMs. When adequate exposure or dose and response data are available in animal models, QRA may be feasible. An important question is the extent to which data are available to evaluate the relevance of animal models to humans. This is of particular importance for nanomaterials because animal data are currently the primary basis for OEL development. There is a rich and long history supporting the use of animal models to make recommendations in the form of OELs to protect workers (Phalen et al., 2008; Rall, 1979). However, differences between humans and animals need to be considered in risk assessment, either through science-based extrapolations or the use of uncertainty factors. The deposition and clearance rates of inhaled particles are species-dependent, and there are differences in gross, sub-gross, and respiratory tract biology and anatomy (Phalen et al., 2008). Allometric relationships for respiratory physiologic parameters based on body weight and metabolism have been developed and evaluated from empirical data across species (U.S. EPA, 1994). These differences can result in differences in the internal dose of particles in the respiratory tracts in animals and humans.
Dose estimation of inhaled nanomaterials involves many of the same principles and concepts as for inhaled microscale particles, but also may involve differences in the distribution within tissues and clearance rates. For example, at 24 hours after 4–6 hour inhalation exposures to metal oxide nanoparticles in rats, nanoparticles were observed (via enhanced darkfield microscopy) in the lungs, lymphatics, pulmonary blood vessels, liver, spleen, and kidney (Guttenberg et al., 2016). Particle size has been shown to influence the biodistribution and biokinetics of particles (Balasubramanian et al., 2013; Kreyling et al., 2013). Other factors may include agglomeration state, shape, surface properties, and solubility. In recent years, several dosimetry models focusing on nanomaterials deposition, translocation, retention, and/or clearance have been published (Asgharian and Price, 2007; Asgharian et al., 2014; Bachler et al., 2013 Sturm et al., 2015; 2017; Sweeney et al., 2015). In general, prediction of the deposited dose of inhaled nanoparticles based on airborne particle size may be better understood than the fate of the nanoparticles following deposition.
The long-term clearance kinetics of respirable particles in rodents and humans is an important consideration in the QRA of inhaled particles including ENMs. Lung particle overloading in rats was described 30 years ago by Morrow (1988) as the continuous prolongation of particle lung clearance after reaching a retained mass burden-threshold. Overloading thresholds have also been described for particle surface area dose (Oberdörster et al., 1994a; b; Tran et al., 2000) and particle volumetric dose (Pauluhn, 2011, 2014), and these dose metrics help to explain particle-size dependent differences in particle clearance. For nearly as many years, the interpretation and use of rat overload dose and response data of inhaled particles in human hazard and risk assessment and OEL development has been discussed and debated (Borm et al., 2015; Cherrie et al., 2013; ECETOC, 2013; IARC, 2010; ILSI , 2000; Kuempel et al., 2014; Morfeld et al., 2015; Oberdörster, 1995; Pauluhn, 2014; Warheit et al., 2016; Yu, 1996).
The effect of overload kinetics on dose can be taken into account in QRA by using science-based dosimetry models to estimate the human-equivalent respiratory tract doses to the rodent effect levels (e.g. ARA, 2017; ICRP, 2015). However, the role of particle characteristics (such as size, shape, and solubility) on the clearance and retention of the deposited particle dose has not been as thoroughly studied. The human and rat biological responses to equivalent mass, surface area, or volumetric particle lung doses are also not fully understood. With regard to lung cancer, the rat chronic bioassay data have been shown to give fewer false negatives than have the mouse and hamster data by comparison to particles classified by IARC as human carcinogens (Mauderly, 1997). ILSI (2000) concluded that the rat is a useful model for human non-neoplastic lung responses to PSLT, and in the absence of mechanistic data to the contrary, the rat model is also relevant to identifying potential carcinogenic hazards in humans. Overloading doses in rats have been shown to be equivalent on a mass basis to the retained particle doses measured in the lung tissues of workers in dusty jobs such as coal mining (IARC, 2010; Kuempel et al., 2014; NIOSH, 2011). IARC (2010) included rat bioassay data in its evaluation of the carcinogenicity of inhaled PSLT (carbon black and TiO2), and NIOSH (2011) used rat data in its hazard classification and REL development for nanoscale and microscale TiO2.
A less favorable view of rat models and overload by PSLT is that resultant lung tumors are unique to rats and overload particle exposures to PSLTs do not produce neoplastic responses to mice or hamsters or larger mammals such as humans or nonhuman primates, hence the rat data would not be relevant to workers. However, as noted in an editorial by Borm et al. (2015), the question about relevance for humans of both neoplastic and non-neoplastic effects observed in rats chronically exposed to PSLTs is still a subject for debate. Borm et al. (2015) identified a number of scientific questions that still need to be resolved, and they cited two papers to further contribute to the debate (Morfeld et al., 2015; Pauluhn, 2014). To date there is no clear resolution of this issue in the scientific community. Therefore, interpretations of the rat dose-response data for risk assessment have differed widely for inhaled PSLT including for nanoscale TiO2, using the same basic data (NIOSH, 2011; Relier et al., 2017; Warheit et al., 2016). While the scientific debate continues, dosimetric adjustments to account for differences in PSLT aerosol particle size and respiratory tract disposition and/or clearance between rodents and workers can be used to adjust for toxicokinetic differences, and uncertainty factors can be considered for toxicodynamic differences (ICRP, 2015; Jarabek et al., 2005; Kuempel et al., 2015; Oller and Oberdörster, 2016).
The utility of in vivo data for risk assessment will be predicated on the quality of input data, and animal toxicology studies should conform to good laboratory practice and international guidelines such as provided by the Organization for Economic Cooperation and Development (OECD, 2005; OECD, 2009). Factors to be considered in evaluating study quality include the adequacy of the study hypothesis, experimental design, sample size, assay methods, execution of experiments, statistical analysis, and interpretation of results (NTP, 2015). The relevance the animal model to humans and evaluation of dose metrics and kinetics are also important, as discussed above. In the design of toxicology studies of particles, sufficient doses should be included to characterize the dose-response relationship from low doses to over-loading doses (ILSI , 2000; Oberdörster, 1997; Kuempel et al., 2014; Pauluhn, 2011). Mice are another commonly used species in nanotoxicology studies, and further evaluations are needed to compare the dose-response relationships in mice to humans, and in other animal species, strains, and sexes (Teeguarden et al., 2014).
5.2. In vitro models
Many of the anticipated approaches to identify hazards of ENMs will involve the use of in vitro models, which are a key component of alternative test systems (Maier, 2011; Nel et al., 2013; Oberdörster et al., 2005; Stone et al., 2014; Savolainen and Vartio, 2017). The use of in vitro testing for ENMs has increased dramatically in the last decade and the number of possible tests is large. In vitro tests may be useful in comparative potency analyses and categorical frameworks. In vitro assays should assess key events in the biological mechanism of action to ensure that appropriate endpoints are addressed (Stone et al., 2014). Since in vitro assays target specific processes, a combination of several in vitro assays are likely required to assess different aspects of hazard. Lai (2017) has identified the limitations of in vitro tests for ENMs (Table 2). Clearly, in vitro testing of ENMs is a critical part of hazard and ultimately risk assessment according to 21st century toxicology (NAS, 2007; NAS, 2017). Approaches suggested for using in vitro toxicology data of ENMs in risk assessment involve a tiered approach. The first tiers include physicochemical particle characterization and in vitro toxicology testing, followed by the selection of a subset of nanoparticles for a limited number of in vivo tests in rodents and comparison of dose-response relationships to those for reference materials of each class/subclass of nanoparticles (Kuempel et al., 2012; Lai and Warheit, 2015; Lai, 2017; Nel et al., 2013; Oberdörster et al., 2005; Stone et al., 2014). Biological mode of action data may be used in defining the categories by the performance of in vitro or in vivo high throughput genomics and or proteomics to investigate underlying mechanisms which can be tested further. As discussed in Section 3.2, a key issue in the use of in vitro data in hazard and risk assessment is to determine the in vitro doses that are equivalent to realistic in vivo exposures Oberdörster et al., 2005).
Table 2.
Limitations of in vitro test data for ENMs. Adapted from (Lai, 2017).
| • No single short-term test can be used to predict all health effects of ENMs |
| • A large number of false positive and false negative results occur |
| • Effects at high dose levels may not extrapolate to low-dose levels |
| • Endpoints identified in short-term tests may not be predictive of long-term exposure effects |
| • Different cell lines may yield different responses |
| • Some in vitro tests involve release of protein, but various types of ENMs can absorb protein, thus confounding results (Dutta et ah, 2007) |
| • Various physico-chemical characteristics of ENMs can interfere with some in vitro tests (e.g. fluorescent quantum dots in a fluorescent assay) (Monteiro-Riviere and Inman, 2006) |
| • Particle kinetics of ENMs in culture media often not considered, resulting in erroneous interpretations of dose-response relationship (Mecke et ah, 2005) |
6. Future directions and research needs
Much of the activity in generating categorical approaches to developing OELs for ENM is currently occurring with physical-chemical and in vitro data (Arts et al., 2014; Lai, 2017; Nel et al., 2013; Stone et al., 2014). However, various approaches need to be developed to optimize in vitro testing strategies. For example, one framework to evaluate ENM exposure characterization data for designing in vitro studies would provide useful information for risk assessment (Sharma et al., 2016). This work concluded that “... effective risk assessment of ENM depends on focusing in vitro testing on relevant exposure pathway with ENM in dose forms and at dose levels that reflect environmental transformation.” More of this type of thinking would help to improve the realism of risk assessment by including sufficient doses in in vitro and in vivo studies to characterize the dose-response relationship, including doses that reflect workplace exposure levels. Duration of exposure is another important consideration, and models are currently underdeveloped to quantitatively link and predict acute and chronic endpoints for inhaled particles.
More broadly, there are three research needs to enable risk assessment of ENMs for development of OELs. These are to: 1) determine the characteristics of categories that encompass these ENMs, with regard to physicochemical properties and biological mode of action; 2) apply the proposed priority schemes for standardized in vivo testing to develop comprehensive databases for qualitative and quantitative data analysis; and 3) identify the means to utilize physicochemical characteristics and in vitro data to incorporate into predictive modeling of exposure-response relationships and risk determinations. Efforts are occurring in each of these areas as discussed in this paper. However, in most instances they are pilot efforts and comprehensive data for validation are still needed.
One of the issues that arise in using a biomarker-based approach is that the earlier endpoints in an AOP may result in increased sensitivity compared with conventional approaches, which could result in OELs that are much lower than might be determined using in vivo data of apical endpoints if there is not a good understanding of the relationship between the biomarker and the apical endpoint. PODs based on these earlier biological responses might lead to OELs that are overly protective with regard to the risk of developing adverse health effects. This issue needs to be addressed. Nonetheless, the power of a systems biology approach is something that could be harnessed to support risk assessment and the development of OELs for ENMs.
7. Conclusions
The history of risk assessment for engineered nanomaterials generally spans less than 20 years but during that time various approaches have been utilized. The scientific evidence basis for these approaches began with investigations of the differences in the dose-response relationships of respirable particles by size, i.e., ultrafine (nanoscale) and fine (microscale) particles. Generally, dosimetry models and methods are available to estimate equivalent deposited doses of inhaled nanoparticles in animals and humans, although data are much more limited to evaluate the long-term doses and the dose-response relationships across species. The realization that there are and could be many more ENMs than could be effectively tested in animal models leads to thinking about the need for ways to look at categories of ENM or to group ENM in homogenous categories for hazard assessments and ultimately risk assessment. Generally, it is likely that risk assessments will rely increasingly on data on how ENMs can cause biological perturbations and focus more on underlying mechanisms. Many approaches have been tried and a path forward appears likely to emerge from these efforts.
Acknowledgments
The authors thank the following for comments on earlier drafts: Drs. Ulla Vogl, Terry Gordon, and Alison Elder; and Nikki Romero, Amanda Keenan, and Vanessa Williams for graphics and processing the manuscript.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Transparency document
Transparency document related to this article can be found online at http://dx.doi.Org/10.1016/j.yrtph.2018.03.018.
References
- Ankley GT, Bennett RS, Erickson RJ, Hofl DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmeider RK, 2010. Conceptual framework to support exotoxicology research and risk assessment. Environ. Toxicol. Chem 29, 730–741. 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- ARA, 2017. Multiple-path Particle Dosimetry Model (MPPD V. 3.02). Applied Research Associates, Inc., Raleigh, NC: https://www.ara.com/products/multiple-path- particle-dosimetry-model-mppd-v-304 (freely available). [Google Scholar]
- Arts JH, Hadi M, Keene AM, Kreiling R, Lyon D, Maier M, Michel K, Petry T, Sauer UG, Warheit D, Wiench K, Landsiedel R, 2014. A critical appraisal of existing concepts for the grouping of nanomaterials. Regul. Toxicol. Pharmacol 70 (2), 492–506. https://doi.Org/10.1016/j.yrtph.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Arts JH, Hadi M, Irfan MA, Keene AM, Kreiling R, Lyon D, Maier M, Michel K, Petry T, Sauer UG, Warheit D, Wiench K, Wohlleben W, Landsiedel R, 2015. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul. Toxicol. Pharmacol 71 (2 Suppl. I), S1–S27. 10.1016/j.yrtph.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Arts JH, Irfan MA, Keene AM, Kreiling R, Lyon D, Maier M, Michel K, Neubauer N, Petry T, Sauer UG, Warheit D, Wiench K, Wohlleben W, Landsiedel R, 2016. Case studies putting the decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping) into practice. Regul. Toxicol. Pharmacol 76, 234–261. http://dx.doi.Org/10.1016/j.yrtph.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Asgharian B, Price OT, 2007. Deposition of ultrafine (nano) particles in the human lung. Inhal. Toxicol 19 (13), 1045–1054. 10.1080/08958370701626501. [DOI] [PubMed] [Google Scholar]
- Asgharian B, Price OT, Oldham M, Chen LC, Saunders EL, Gordon T, Mikheev VB, Minard KR, Teeguarden JG, 2014. Computational modeling of nanoscale and microscale particle deposition, retention and dosimetry in the mouse respiratory tract. Inhal. Toxicol 26 (14), 829–842. 10.3109/08958378.2014.935535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschberger K, Johnston HJ, Stone V, Aitken RJ, Hankin SM, Peters SA, Tran CL, Christensen FM, 2010. Review of carbon nanotubes toxicity and exposure—appraisal of human health risk assessment based on open literature. Crit. Rev. Toxicol 40 (9), 759–790. [DOI] [PubMed] [Google Scholar]
- Bachler G, von Goetz N, Hungerbühler K, 2013. A physiologically based pharmaco-kinetic model for ionic silver and silver nanoparticles Int. J. Nanomedicine 8, 3365–3382. 10.3109/17435390.2014.940404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baisch BL, Corson NM, Wade-Mercer P, Gelein R, Kennell AJ, Oberdörster G, Elder A, 2014. Equivalent titanium dioxide nanoparticle deposition by intratracheal instillation and whole body inhalation: the effect of dose rate on acute respiratory tract inflammation. Part. Fibre Toxicol 11 (5). 10.1186/1743-8977-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian SK, Poh KW, Ong CN, Kreyling WG, Ong WY, Yu LE, 2013. The effect of primary particle size on biodistribution of inhaled gold nano-agglom- erates. Biomaterials 34 (22), 5439–5452. [DOI] [PubMed] [Google Scholar]
- Borm P, Cassee FR, Oberdörster G, 2015. Lung particle overload: old school-new insights? Part. Fibre Toxicol 26 12–10. 10.1186/s12989-015-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BSI, 2007. Guide to Safe Handling and Disposal of Manufactured Nanomaterials. BSI PD6699–2.
- Cherrie JW, Brosseau LM, Hay A, Donaldson K, 2013. Low-toxicity dusts: current exposure guidelines are not sufficiently protective. Ann. Occup. Hyg 57, 685–691. https://DOI:10.1093/annhyg/met038. [DOI] [PubMed] [Google Scholar]
- Christensen FM, Johnston HJ, Stone V, Aitken RJ, Hankin S, Peters S, Aschberger K, 2010. Nano-silver - feasibility and challenges for human health risk assessment based on open literature. Nanotoxicology 4 (3), 284–295. dx.doi.org/%2010.3109/17435391003690549. [DOI] [PubMed] [Google Scholar]
- CIIT and RIVM, 2002. Multiple-path Particle Dosimetry (MPPD V 1.0): a Model for Human and Rat Airway Particle Dosimetry Chemical Industry Institute of Toxicology, Centers for Health Research. Bilthoven, The Netherlands: National Institute for Public Health and the Environment (RIVM) in the Netherlands, Research Triangle Park, NC. [Google Scholar]
- Costa PM, Fadeel B, 2016. Emerging systems biology approaches to nanotoxicology: towards a mechanism-based understanding of nanomaterial hazard and risk. Toxicol. Appl. Pharmacol 299, 101–111. https://doi.Org/10.1016/j.taap.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Crump KS, 1984. A new method for determining daily allowable intakes. Fund. Appl. Toxicol 4, 854–871. 10.1016/0272-0590(84)90107-6. [DOI] [PubMed] [Google Scholar]
- Dankovic D, Kuempel E, Wheeler M, 2007. An approach to risk assessment for TiO. Inhal. Toxicol 19 (Suppl. 1), 205–212. 10.1080/08958370701497754. [DOI] [PubMed] [Google Scholar]
- DeBord DG, Burgoon L, Edwards SW, Haber LT, Kanitz MH, Kuempel E, Thomas RS, Yucesoy B, 2015. Systems biology and biomarkers of early effects for occupational exposure limit setting. J. Occup. Environ. Hyg 12 (Suppl. 1), S41–S54. 10.1080/15459624.2015.1060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, Alexander A, 2006. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci 92 (1), 5–22. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Brown GM, Brown DM, Robertson MD, Slight J, Cowie H, Jones AD, Bolton AE, Davis JMG, 1990. Contrasting bronchoalveolar leukocyte responses in rats inhaling coalmine dust, quartz, or titanium dioxide: effects of coal rank, airborne mass concentration, and cessation of exposure. Environ. Res 52, 62–76. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Schinwald A, Murphy F, Cho WS, Duffin R, Tran L, Poland C, 2013. The biologically effective dose in inhalation nanotoxicology. Acc. Chem. Res 46 (3), 723–732. 10.1021/ar300092y. [DOI] [PubMed] [Google Scholar]
- Drew NM, Kuempel ED, Pei Y, Yang F, 2017. A quantitative framework to group nanoscale and microscale particles by hazard potency to derive occupational exposure limits: proof of concept evaluation. Regul. Toxicol. Pharmacol 89, 253–267. https://doi.Org/10.1016/j.yrtph.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll KE, Lindenschmidt RC, Maurer JK, Higgins JM, Ridder G, 1990. Pulmonary response to silica or titanium dioxide: inflammatory cells, alveolar macrophage-derived cytokines, and histopathology. Am. J. Respir. Cell Mol. Biol 2 (4), 381–390. 10.1016/0041-008X(91)90024-9. [DOI] [PubMed] [Google Scholar]
- Duffin R, Tran L, Brown D, Stone V, Donaldson K, 2007. Proinflammogenic effects of low toxicity and metal nanoparticles in vivo and in vitro: highlighting the role of particle surface area and surface reactivity. Inhal. Toxicol 19 (10), 849–856. [DOI] [PubMed] [Google Scholar]
- Dutta D, Sundaram SK, Teeguarden JG, Riley BJ, Fifield LS, Jacobs JM, Addleman SR, Kaysen GA, Moudgil BM, Weber TJ, 2007. Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials. Toxiocol. Sci 100 (1), 303–315. [DOI] [PubMed] [Google Scholar]
- ECETOC, 2013. Poorly Soluble Particles/lung Overload. European Centre for Exotoxicology and Toxicology of Chemicals, Brussels Technical Report No 122.
- ECHA, 2010. Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.8: Characterization of Dose [concentration]-response for Human Health. Version 2. Reference No. ECHA-2010-G-19-EN.
- Elder A, Gelein R, Finkelstein JN, Driscoll KE, Harkema J, Oberdörster G, 2005. Effects of subchronically inhaled carbon black in three species. I. Retention kinetics, lung inflammation, and histopathology. Toxicol. Sci 88 (2), 614–629. [DOI] [PubMed] [Google Scholar]
- Gangwal S, Brown JS, Wang A, Houck KA, Dix DJ, Kavlock RJ, Hubal EA, 2011. Informing selection of nanomaterial concentrations for ToxCast in vitro testing based on occupational exposure potential. Environ. Health Perspect. 119, 1539–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SC, Butala JH, Carter JM, Elder A, Gordon T, Gray G, Sayre PG, Schulte PA, Tsai CS, West J, 2014. Workshop report: strategies for setting occupational exposure limits for engineered nanomaterials. Regul. Toxicol. Pharmacol 68 (3), 305–311. [DOI] [PubMed] [Google Scholar]
- Gregoratto D, Bailey MR, Marsh JW, 2010. Modelling particle retention in the alveolar-interstitial region of the human lungs. J. Radiol. Prot 30 (3), 491–512. [DOI] [PubMed] [Google Scholar]
- Greim H, 2004. Research needs to improve risk assessment of fiber toxicity. Mut. Res/ Fund. Mole. Mech. Mutagenesis 553, 11–22. [DOI] [PubMed] [Google Scholar]
- Guttenberg M, Bezerra L, Neu-Baker NM, Del Pilar Sosa, Idelchik M, Elder A, Oberdörster G, Brenner SA, 2016. Biodistribution of inhaled metal oxide nano-particles mimicking occupational exposure: a preliminary investigation using enhanced darkfield microscopy. J. Biophot 9 (10), 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halappanavar S, Jackson P, Williams A, Jensen KA, Hougaard KS, Vogel U, Yauk CL, Wallin H, 2011. Pulmonary response to surface-coated nanotitanium dioxide particles includes induction of acute phase response genes, inflammatory cascades, and changes in microRNAs: a toxicogenomic study. Environ. Mol. Mutagen 52 (6), 425–439. https://DOI:10.1002/em.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich U, Fuhst R, Rittinghausen S, Creutzenberg O, Bellmann B, Koch W, Levsen K, 1995. Chronic inhalation exposure of Wistar rats and 2 different strains of mice to diesel engine exhaust, carbon-black, and titanium-dioxide. Lnhal. Toxicol 7 (4), 466–533. [Google Scholar]
- Hett A, 2004. Nanotechnology: Small Matter, Many Unknowns. Zurich-Swiss Reinsurance Company, Zurich, https://www.nanowerk.com/nanotechnology/reports/reportpdf/report93.pdf. [Google Scholar]
- Hinderliter PM, Minard KR, Orr G, Chrisler WB, Thrall BD, Pounds JG, Teeguarden JG, 2010. ISDD: a computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part. Fibre Toxicol. 7 (1), 36 10.1186/1743-8977-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristozov DR, Gottardo S, Critto A, Marcomini A, 2012. Risk assessment of engineered nanomaterials: a review of available data and approaches from a regulatory perspective. Nanotoxicology 6 (8), 880–898. 10.3109/17435390.2011.626534. [DOI] [PubMed] [Google Scholar]
- Hristozov D, Zabeo A, Alstrup Jensen K, Gottardo S, Isigonis P, Maccalman L, Critto A, Marcomini A, 2016. Demonstration of a modelling-based multi-criteria decision analysis procedure for prioritisation of occupational risks from manufactured nanomaterials. Nanotoxicology 10 (9), 1215–1228. 10.3109/17435390.2016.1144827. [DOI] [PubMed] [Google Scholar]
- HSE, 2004. Nanotechnology. Horizons Scanning Information Note No. HSIN1 Health and Safety Executive, London, http://www.dguv.de/ifa/fachinfos/nanopartikel-am-arbeitsplatz/beurteilung-von-schutzmassnahmen/index-2.jsp, Accessed date: 9 February 2018. [Google Scholar]
- IARC, 2010. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Carbon Black, Titanium Dioxide, and Talc, vol. 93 International Agency for Research on Cancer, Lyon, France: http://monographs.iarc.fr. [PMC free article] [PubMed] [Google Scholar]
- IARC, 2017. Some Nanomaterials and Some Fibres. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 111 International Agency for Research on Cancer, Lyon, France. [Google Scholar]
- ICRP, 2015. Occupational Intakes of Radionuclides: Part 1, vol. 130 The International Commission on Radiological Protection (ICRP) Publication Ann ICRP 44(2). [Google Scholar]
- IFA, 2009. Criteria for Assessment of the Effectiveness of Protective Measures.
- ILSI (International Life Sciences Institute), 2000. Risk Science Institute. The relevance of the rat lung to particle overload for human risk assessment: a workshop consensus report. Inhal. Toxicol 12, 1–17. https://DOI:10.1080/08958370050029725. [DOI] [PubMed] [Google Scholar]
- ISO, 2016. Nanotechnologies — Overview of Available Frameworks for the Development of Occupational Exposure Limits and Bands for Nano-objects and Their Aggregates and Agglomerates (NOAAs). International Organization for Standardization (ISO) Technical Report. ISO, Geneva, Switzerland: ISO/TR 18637, published Nov. 21. [Google Scholar]
- Jarabek AM, Asgharian B, Miller FJ, 2005. Dosimetric adjustments for interspecies extrapolation of inhaled poorly soluble particles (PSP). Inhal. Toxicol 17, 317–334. 10.1080/08958370590929394. [DOI] [PubMed] [Google Scholar]
- Kasai T, Umeda Y, Ohnishi M, Mine T, Kondo H, Takeuchi T, Matsumoto M, Fukushima S, 2016. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part. Fibre Toxicol 13 (1), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyling WG, Semmler M, Möller, 2004. Dosimetry and toxicology of ultrafine particles. J. Aerosol Med 17, 140–152. https://DOI:10.1089/0894268041457147. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Semmler-Behnke M, Takenaka S, Möller W, 2013. Differences in the biokinetics of inhaled nano- versus micrometer-sized particles. Acc. Chem. Res 46(3), 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel ED, Attfield MD, Stayner LT, Castranova V, 2014. Human and animal evidence supports lower occupational exposure limits for poorly-soluble respirable particles: letter to the Editor re: ‘Low-toxicity dusts: current exposure guidelines are not sufficiently protective’ by Cherrie, Brosseau, Hay and Donaldson. Ann. Occup. Hyg 58 (9), 1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel ED, Castranova V, Geraci CL, Schulte PA, 2012. Development of risk-based nanomaterial groups for occupational exposure control. J. Nanoparticle Res 14 (1029). 10.1007/s11051-012-1029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel ED, O’Flaherty EJ, Stayner LT, Smith RJ, Green FHY, Vallyathan V, 2001. A biomathematical model of particle clearance and retention in the lungs of coal miners. I. Model development. Regul. Toxicol. Pharmacol 34 (1), 69–87. 10.1006/rtph.2001.1479. [DOI] [PubMed] [Google Scholar]
- Kuempel ED, Sweeney LM, Morris JB, Jarabek AM, 2015. Advances in inhalation dosimetry models and methods for occupational risk assessment and exposure limit derivation. J. Occup. Environ. Hyg 12 (Suppl. 1), S18–S40. 10.1080/15459624.2015.1060328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel ED, Tran CL, Castranova V, Bailer AJ, 2006. Lung dosimetry and risk assessment of nanoparticles: evaluation and extending current models in rats and humans. Inhal. Toxicol 18, 717–724. 10.1080/08958370600747887. [DOI] [PubMed] [Google Scholar]
- Lai DY, Warheit DB, 2015. Nanotoxicology: the case for in vivo studies Handbook of Safety Assessment of Nanomaterials: from toxicological testing to personalized medicine. In: Fadeel B (Ed.), pp. 153–219 (Chapter 6). [Google Scholar]
- Lai DY, 2017. Limited usefulness of in vitro toxicity data in hazard identification of nanomaterials. Open Acc. J. of Toxicol. 1 (2) OA-JT.MS. ID. 555559. [Google Scholar]
- LeBlanc AJ, Mosley ALM, Chen BT, Frazier D, Castranova V, Nurkiewiez TR, 2010. Nanoparticle inhalation repairs coronary microvascular via local reactive oxygen species—dependent mechanism. Cardiovasc. Toxicol 10, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Trochimowiez HJ, Reinhardt CF, 1985. Pulmonary response of rates exposed to titanium dioxide (TiO 2) by inhalation for two years. Toxicol. Appl. Pharmacol 79, 179–192. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu HH, Ji Z, Chang CH, Xia T, Nel AE, Cohen Y, 2015. Evaluation of toxicity ranking for metal oxide nanoparticles via an in vitro dosimetry model. ACS Nano 9 (9), 9303–9313. 10.1021/acsnano.5b04420. [DOI] [PubMed] [Google Scholar]
- Lobenhofer EK, Cui X, Bennet L, Cable PL, Merrick BA, Churchhill GA, et al. , 2004. Exploration of low-dose estrogen effects: identification of no observed transcriptional effect level (NOTEL). Toxicol. Pathol 32, 482–492. [DOI] [PubMed] [Google Scholar]
- Ma-Hock I, Treumann S, Strauss V, Brill S, Luizi I, Martiee M, Wiench K, et al. , 2009. Inhalation of multiwall carbon nanotubes in rats exposed for 3 months. Toxicol. Sci 112 (2), 468–481. 10.1093/toxsci/kfp146. [DOI] [PubMed] [Google Scholar]
- Maier MS, 2011. Setting occupational exposure limits for unstudied pharmaceutical intermediates using an in vitro parallelogram approach. Toxicol. Mech. Meth 21 (2), 76–85. [DOI] [PubMed] [Google Scholar]
- Mauderly JL, 1997. Relevance of particle-induced rat lung tumors for assessing lung carcinogenic hazard and human lung cancer risk. Environ. Health Perspect. 105 (Suppl. 5), 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecke A, Orr BG, Banuszak Holl MM, Bake r J.R., 2005. Lipid bilayer discruption by polyamido amine dendrimers: the role of generation and capping. Langmnir 21, 10348–10354. [DOI] [PubMed] [Google Scholar]
- Mihalache R, Verbeek J, Graczyk H, Murashov V, van Broekhuizen P, 2017. Occupational exposure limits for manufactured nanomaterials: a systematic review. Nanotoxicology 11 (1), 7–19. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere NA, Inman AO, 2006. Challenges for assessing carbon nanomaterial toxicity to the skin. Carbon 44 (6), 1070–1078. [Google Scholar]
- Monteiller C, Tran L, MacNee W, Faux S, Jones A, Miller B, Donaldson K, 2007. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup. Environ. Med 64 (9), 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfeld P, Bruch J, Levy L, Ngiewih Y, Chadhuri I, Muranho HJ, Myersen R, McCunney RJ, 2015. Translational toxicology in setting occupational exposure limits for dusts and hazard classification—a critical evaluation of a recent approach to translate dust overload findings from rats to humans. Part. Fibre Toxicol. 12, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto Y, Kobayashi N, Shinohara N, Myojo T, Tanaka I, Nakanishi J, 2010. Hazard assessments of manufactured nanomaterials. J. Occup. Health 12, 325–334. [DOI] [PubMed] [Google Scholar]
- Morrow PE, 1988. Possible mechanisms to explain dust overloading of the lungs. Fund. Appl. Toxicol 10, 369–384. [DOI] [PubMed] [Google Scholar]
- Muhle H, Bellmann B, Creutzenberg O, Dasenbrock C, Ernst H, Kilpper R, MacKenzie JC, Morrow P, Mohr U, Takenaka S, Mermelstein R, 1991. Pulmonary response to toner upon chronic inhalation exposure in rats. Fund. Appl. Toxicol 17, 280–299. https://doi.Org/10.1093/toxsci/17.2.280. [DOI] [PubMed] [Google Scholar]
- NEDO project (P06041) Nakanishi J (Ed.), 2011. Risk Assessment of Manufactured Nanomaterials: “Approaches”—overview of Approaches and Results Final Report Issued on August 17, 2011. Research and Development of Nanoparticle Characterization Methods. [Google Scholar]
- Nakanishi J, Morimoto Y, Ogura I, Kobayashi N, Naya M, Ema M, Endoh S, Shimada M, Ogami A, Myojyo T, Oyabu T, Gamo M, Kishimoto A, Igarashi T, Hanai S, 2015. Risk assessment of the carbon nanotube group. Risk Anal. 35 (10), 1940–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanocyl, 2009. Responsible care and nanomaterials case study In: Nanocyl. Presentation at European Responsible Care Conference, Prague 21–23rd October 2009, . http://www.cefic.org/Documents/ResponsibleCare/04_Nanocyl.pdf, Accessed date: 23 February 2018. [Google Scholar]
- NAS, 1983. Risk Assessment and the Federal Government: Managing the Process National Academy of Sciences. National Academy Press, Washington, D.C. [PubMed] [Google Scholar]
- NAS, 2007. Toxicity Testing in the 21st Century: a Vision and a Strategy National Academy of Sciences. National Academies Press, Washington, DC. [Google Scholar]
- NAS, 2009. Committee on Improving Risk Analysis Approaches Used by the EPA Science and Decisions: Advancing Risk Assessment. National Academy of Sciences. National Academy Press, Washington, D.C. [Google Scholar]
- NAS, 2017. Using 21st Century Science to Improve Risk-related Evaluation National Academy of Sciences. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- National Research Council, 1987. Pharmacokinetics in Risk Assessment: Drinking Water and Health, vol. 8 National Academy Press, Washington DC. [Google Scholar]
- Nel AE, Nasser E, Godwin H, Avery D, Bahadori T, Bergeson L, Beryt E, Bonner JC, Boverhof D, Carter J, Castranova V, Deshazo JR, Hussain SM, Kane AB, Klaessig F, Kuempel E, Lafranconi M, Landsiedel R, Malloy T, Miller MB, Morris J, Moss K, Oberdorster G, Pinkerton K, Pleus RC, Shatkin JA, Thomas R, Tolaymat T, Wang A, Wong J, 2013. A multi-stakeholder perspective on the use of alternative test strategies for nanomaterial safety assessment. ACS Nano 7 (8), 6422–6433. 10.1021/nn4037927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIOSH, 1988. Silver (Metal Dust and Fume). CDC-NIOSH 1988 OSHA PEL Project Documentation. List by Chemical Name: Silver. http://www.cdc.gov/niosh/pel88/7440-22.html.
- NIOSH, 2005. Approaches to Safe Nanotechnology: an Information Exchange with NIOSH US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Draft report for public review, Cincinnati. [Google Scholar]
- NIOSH, 2011. NIOSH Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH: DHHS (NIOSH) Publication No. 2011–160. [Google Scholar]
- NIOSH, 2013. NIOSH Current Intelligence Bulletin 65. Occupational Exposure to Carbon Nanotubes and Nanofibers. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH: DHHS (NIOSH) Publication No. 2013–2145. [Google Scholar]
- NIOSH, 2016. Draft Current Intelligence Bulletin: Health Effect of Occupational Exposure to Silver Nanoparticles. Department of Health and Human Services (HHS), Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health. [Google Scholar]
- NIOSH, 2017a. Draft Current Intelligence Bulletin: the Occupational Exposure Banding Process: Guidance for the Evaluation of Chemical Hazards. Department of Health and Human Services (HHS), Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health (NIOSH). [Google Scholar]
- NIOSH, 2017b. In: Whittaker C, Rice F, McKernan L, Dankovic D, Lentz TJ, MacMahon K, Kuempel E, Zumwalde R, Schulte P (Eds.), Current Intelligence Bulletin 68: NIOSH Chemical Carcinogen Policy on behalf of the NIOSH Carcinogen and RELs Policy Update Committee. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health DHHS (NIOSH) Publication No. 2017–2100. [Google Scholar]
- NIOSH, 2007. NIOSH Pocket Guide to Chemical Hazards U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH: DHHS (NIOSH) Publication No. 2009–125. [Google Scholar]
- NTP, 2015. Handbook for Conducting a Literature-based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. Office of Health Assessment and Translation (OHAT), Division of the National Toxicology Program, National Institute of Environmental Health Sciences, U.S. Department of Health and Human Services. [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Stone S, Chen BT, Frazer DG, Boegehold MA, Castranova V, 2009. Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicol. Sci 110 (1), 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, 1989. Dosimetric principles for extrapolating results of rat inhalation studies to humans, using an inhaled Ni compound as an example. Health Phys. 57 (Suppl. 1), 213–220. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, 1995. Lung particle overload: implications for occupational exposures to particles. Regul. Toxicol. Pharmacol 21, 123–135. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, 1997. Pulmonary carcinogenicity of inhaled particles and the maximum tolerated dose. Environ. Health Perspect. 105 (Suppl. 5), 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Ferin J, Gelein F, Soderholm SC, Finkelstein J, 1992. Role of the alveolar macrophage in lung injury: studies with ultrafine particles. Environ. Health Perspect. 97, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Ferin J, Lehnert BE, 1994a. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 102 (Suppl. 5), 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Ferin J, Soderholm S, Gelein R, Cox C, Baggs R, Morrow PE, 1994b. Increased pulmonary toxicity of inhaled ultrafine particles: due to lung overload alone? Ann. Occup. Hyg 38, 295–302. [Google Scholar]
- Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, et al. , 2005. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part. Fibre Toxicol. 2, 1–35. 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J, 2005. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 113, 823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD, 2005. Good Laboratory Practice: OECD Principles and Guidance for Compliance Monitoring Vol. Organization for Economic Cooperation and Development, Paris. [Google Scholar]
- OECD, 2009. Subchronic Inhalation Toxicity: 90-Day Study Test Guideline No. 413. Organization for Economic Cooperation and Development, Paris. [Google Scholar]
- OECD, 2012. Important Issues on Risk Assessment of Manufactured Nanomaterials Series on the Safety of Manufactured Nanomaterials. No. 33. ENV/JM/MONO(2012)8. Organization for Economic Cooperation and Development, Environmental Health and Safety Publications, Paris, France. [Google Scholar]
- OECD, 2014. Guidance on Grouping Chemicals, second ed. Organization for Economic Cooperation and Development. Environmental Health and Safety Series on Testing Assessment; No. 194. ENV/JM/MOVO 4. [Google Scholar]
- Oller AR, Oberdörster G, 2016. Incorporation of dosimetry in the derivation of reference concentrations for ambient or workplace air: a conceptual approach. J. Aerosol Sci. 99, 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSHA, 1988. Silver (metal dust and fume). Federal Register 53(109): 21215. [Google Scholar]
- Proposed rules: air Contaminants. Fed. Regist 53, 20960–21393. [Google Scholar]
- Pauluhn J, 2010a. Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol. Sci 113 (1), 226–242. 10.1093/toxsci/kfp247. [DOI] [PubMed] [Google Scholar]
- Pauluhn J, 2010b. Multi-walled carbon nanotubes (Baytubes): approach for derivation of occupational exposure limit. Regul. Toxicol. Pharmacol 57 (1), 78–89. 10.1016/j.yrtph.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Pauluhn J, 2014. Derivation of occupational exposure levels (OELs) of low-toxicity isometric biopersistent particles: how can the kinetic lung overload paradigm be used for improved inhalation toxicity study design and OEL-derivation? Part. Fibre Toxicol. 11, 72 10.1186/sl2989-014-0072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauluhn J, 2011. Poorly soluble particulates: searching for a unifying denominator of nanoparticles and fine particles for DNEL estimation. Toxicology 279 (1–3), 176–188. https://doi.Org/10.1016/j.tox.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Pesticide Action Network Europe, 2016. Two Perspectives on Adverse Outcome Pathways. http://www.foodpackagingforum.org/news/two-perspectives-on-adverse-outcome-pathways. [Google Scholar]
- Phalen RF, Oldham MJ, Wolff RK, 2008. The relevance of animal models for aerosol studies. J. Aerosol Med. Pulm. Drug Deliv. 21, 113–124. [DOI] [PubMed] [Google Scholar]
- Pisani C, Gaillard J-C, Nouvel V, Odorico M, Armengand J, Prat O, 2015. High-throughput, quantitative assessment of the effects of low-dose silica nanoparticles on lung cells: grasping complex toxicity with a great depth of field. BMC Genom. 16, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen SS, Knudsen KB, Jackson P, Weydahl IEK, Saber AT, Wallin H, Vogel UB, 2017. Multi-walled carbon nanotube-physicochemical properties predict the systemic acute phase response following pulmonary exposure in mice. PLoS One 12(4), e0174167 10.1371/journal.pone.0174167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall DP, 1979. Relevance of animal experiments to humans. Environ. Health Perspect. 32, 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relier C, Dubreuil M, Lozano Garcia O, Cordelli E, Mejia J, Eleuteri P, Robidel F, Loret T, Pacchierotti F, Lucas S, Lacroix G, Trouiller B, 2017. Study of TiO2 P25 nanoparticles genotoxicity on lung, blood, and liver cells in lung overload and non-overload conditions after repeated respiratory exposure in rats. Toxicol. Sci 156 (2), 527–537. [DOI] [PubMed] [Google Scholar]
- Saber AT, Jacobsen NR, Jackson P, Poulsen SS, Kyjovska ZO, Halappanavar S, Yauk CL, Wallen H, Vogel U, 2014. Particle-induced pulmonary acute phase response may be the causal link between particle inhalation and cardio vascular disease Wiley; Interdiscp. Rev. Nanobiotechnol 6, 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent LM, Shvedova AA, Hubbs AF, Salisbury JL, Benkovic SA, Kashon ML, Lowry OT, Murray AR, Kisin ER, Friend S, McKinstry KT, Battelli L, Reynolds SH, 2009. Induction of aneuploidy by single-walled carbon nanotubes. Environ. Mol. Mutagen 50 (8), 708–717. https://http://dx.doi.org/10:1002/em.25029. [DOI] [PubMed] [Google Scholar]
- Savolainen K, Alenius H, Norppa H, Pylkkänen L, Tuomi T, Kasper G, 2010. Risk assessment of engineered nanomaterials and nanotechnologies—a review. Toxicology 269, 92–104. [DOI] [PubMed] [Google Scholar]
- Savolainen K, Vartio A (Eds.), 2017. The Biological Foundation for the Safety Classification of Engineered Nanomaterials (ENM): Systems Biology Approaches to Understand Interactions of ENM with Living Organisms and the Environment Nanosolutions Final Report, 30. [Google Scholar]
- Schmid O, Stoeger T, 2016. Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J. Aerosol Sci. 99, 133–143. 10.1016/j.jaerosci.2015.12.006. [DOI] [Google Scholar]
- Schoeny RS, Margosches E, 1989. Evaluating comparative potencies: developing approaches to risk assessment of chemical mixtures. Toxicol. Ind. Health 5 (5), 825–837. 10.1177/074823378900500518. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Ahlers JD, Dankovic D, 2002. Risk assessment and regulation of carcinogens in the workplace. Clin. Occup. Environ. Med 2, 727–735. [Google Scholar]
- Schulte PA, Murashov V, Zumwalde R, Kuempel ED, Geraci CL, 2010. Occupational exposure limits for nanomaterials: state of the art. J. Nanoparticle Res. 12, 1971–1987. https://DOI.10.1007/s11051-010-0008-l. [Google Scholar]
- Schulte PA, Whittaker C, Curran CP, 2015. Considerations for using genetic and epigenetic information in occupational health risk assessment and standard setting. J. Occup. Environ. Hyg 12, 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Shatkin JA, Cairns C, Canady R, Clippinger AJ, 2016. Framework to evaluate exposure relevance and data needs for risk assessment of nanomaterials using in vitro testing strategies. Risk Anal. 36, 1551–1563. [DOI] [PubMed] [Google Scholar]
- Shatkin JA, Ong KJ, Beaudrie C, Clippinger AJ, et al. , 2016. Advancing risk analysis for nanoscale materials: report from an international workshop on the role of alternative testing strategies for advancement. Risk Anal. 36,1520–1537. 10.1111/risa.12683. [DOI] [PubMed] [Google Scholar]
- Shi H, Magaye R, Castranova V, Zhao J, 2013. Titanium dioxide nanoparticles: a review of current toxicological data. Part. Fibre Toxicol. 10 (15). 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AL, Tyurma YY, Goerlich O, et al. , 2005. Unusual inflammatory and fibrogenic pulmonary response to single-walled carbon nanotubes in mice. Am. J. Lung Cell. Mol. Physiol 289, L698–L708. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisiner, Yanamala N, Farcas MT, Meanas AL, Williams A, Fournier PM, Reynolds JS, Gutkin DW, S A, Reiner R, Halappanavar S, Kagen V, 2016. Gender differences in murine pulmonary response elicited by cellulose nano-crystals. Part. Fibre Toxicol 13, 28 10.1186/21298-016-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobels FH, 1977. Some problems associated with the testing for environmental mutagens and a perspective for studies in “comparative mutagenesis. Mutat. Res 46, 245–260. 10.1016/0165-1161(77)90001-2. [DOI] [PubMed] [Google Scholar]
- Sobels FH, 1993. Approaches to assessing genetic risks from exposure to chemicals. Environ. Health Perspect. 101, 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Sung JH, Ji JH, Lee JH, Lee JS, Ryu HR, Lee JK, Chung YH, Park HM, Shin BS, Chang HK, Kelman B, Yu IJ, 2013. Recovery from silver-nanoparticle-exposure-induced inflammation and lung function changes in Sprague Dawley rats. Nanotoxicology 7 (2), 169–180. [DOI] [PubMed] [Google Scholar]
- Stapleton PA, Minarchick VC, Campston AM, McKinney W, Chen BT, Sager T, Frazier DB, Mercer RR, Scabilloni J, Andrew ME, Castranova V, Nurkwiew CZ, 2012. Impairment of coronary arteriolar endothelium-dependent dilation after multi-walled carbon nanotube inhalation: a time-course study. Bar Int. J. Mol. Sci 13, 13781–13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmann-Juvala H, Taxell P, Santonen T, 2014. Formulating Occupational Exposure Limits Values (OELs) (Inhalation & Dermal). Finnish Institute of Occupational Health, Helsinki Scaffold Public Documents - SPD7. [Google Scholar]
- Stone V, Johnston HJ, Balharry D, Gernand JM, Gulumian M, 2016a. Approaches to developing alternative testing strategies to inform human health risk assessment of nanomaterials. Risk Anal. 36, 1538–1550. [DOI] [PubMed] [Google Scholar]
- Stone V, Miller MR, Clift MJ, Elder A, Mills NL, Moller P, Schins RP, Vogel U, Kreyling WG, Jensen KA, Kuhlbusch TA, Schwarze PE, Hoet P, Pietroiusti A, De Vizcaya-Ruiz A, Baeza-Squiban A, Tran CL, Cassee FR, 2016b. Nanomaterials vs ambient ultrafine particles: an opportunity to exchange toxicology knowledge. Environ. Health Perspect (Epub ahead of print. Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone V, Pozzi-Mucelli S, Tran L, Aschberger K, et al. , 2014. ITS-NANO-prioritsing nanosafety research to develop stakeholder driven intelligent testing strategy. Part. Fibre Toxicol 13 (11), 9 https://Doi:10.1186/1743-8977-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturla SJ, Boobis AR, Fitzgerald RE, Hoeng J, Kavlock RJ, Schirmer K, Whelan M, Wilks MF, Peitsch MC, 2014. Systems toxicology: from basic research to risk assessment. Chem. Res. Toxicol 2, 314–329. https://DOI:10.1021/tx400410s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R, 2015. A computer model for the simulation of nanoparticle deposition in the alveolar structures of the human lungs. Ann. Transl. Med 3 (19), 281 10.3978/j.issn.2305-5839.2015.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R, 2017. Computer-aided generation and lung deposition modeling of nano-scale particle aggregates. Inhal. Toxicol 29 (4), 160–168. 10.1080/08958378.2017.1329362. [DOI] [PubMed] [Google Scholar]
- Sung JH, Ji JH, Park JD, Yoor JU, Kim DS, Jeong S, et al. , 2009. Subchronic inhalation toxicity of silver nanoparticles. Toxicol. Sci 108, 452–461. [DOI] [PubMed] [Google Scholar]
- Sung JH, Ji JH, Yoon JU, Kim DS, Song MY, Jeong J, et al. , 2008. Lung function changes in sprague-Dauley rats after prolonged inhalation exposure to silver nanoparticles. Inhal. Toxicol 20, 567–574. [DOI] [PubMed] [Google Scholar]
- Sweeney LM, MacCalman L, Haber LT, Kuempel ED, Tran CL, 2015. Bayesian evaluation of a physiologically-based pharmacokinetic (PBPK) model of long-term kinetics of metal nanoparticles in rats. Regul. Toxicol. Pharmacol 73 (1), 151–163. https://doi.Org/10.1016/j.yrtph.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeguarden JG, Mikheev VB, Minard KR, Forsythe WC, Wang W, Sharma G, Karin N, Tilton SC, Waters KM, Asgharian B, Price OR, Pounds JG, Thrall BD, 2014. Comparative iron oxide nanoparticle cellular dosimetry and response mice by the inhalation and liquid cell culture exposure routes. Part. Fibre Toxicol 11 (46). 10.1186/s12989-014-0046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Royal Society and The Royal Academy of Engineering, 2004. Nanoscience and Nanotechnologies: Opportunities and Uncertainties The Royal Society and The Royal Academy of Engineering, London, U.K. [Google Scholar]
- Tran CL, Buchanan D, 2000. Development of a Biomathematical Lung Model to Describe the Exposure-dose Relationship for Inhaled Dust Among U.K. Coal Miners Institute of Occupational Medicine, IOM; Research Report TM/00/02, Edinburgh, UK. [Google Scholar]
- Tran CL, Buchanan D, Cullen RT, Searl A, Jones AD, Donaldson K, 2000. Inhalation of poorly soluble particles. II. Influence of particle surface area on inflammation and clearance. Inhal. Toxicol 12 (12), 1113–1126. [DOI] [PubMed] [Google Scholar]
- Tran CL, Cullen RT, Buchanan D, Jones AD, Miller BG, Searl A, Davis JMG, Donaldson K, 1999. Investigation and prediction of pulmonary responses to dust. Part II In: Investigations into the Pulmonary Effects of Low Toxicity Dusts. Parts I and II. Health and Safety Executive, Suffolk, UK: Contract Research Report 216/1999. [Google Scholar]
- Treumann S, Ma-Hock L, Gröters S, Landsiedel R, van Ravenzwaay B, 2013. Additional histopathologic examination of the lungs from a 4-month inhalation toxicity study with multiwall carbon nanotubes in rats. Toxicol. Sci 134 (1), 103–110. https://doi.0rg/l0.1093/toxsci/kft089. [DOI] [PubMed] [Google Scholar]
- U.S. EPA, 1994. Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry U.S. Environmental Protection Agency, Office of Research and Development, Washington, DC: EPA/600/8e90/066F. http://nepis.epa.gov/EPA/html/Pubs/pubtitleORD.html. [Google Scholar]
- U.S. EPA, 2012. Benchmark Dose Technical Guidance. U.S. Environmental Protection Agency, Washington, DC: EPA/100/R-12/001. [Google Scholar]
- U.S. Supreme Court, 1980. Industrial Union Department, AFL-CIO v. American Petroleum Institute et al. , Case Nos. 78-911, 78–1036. Supreme Court Reporter 100:2844-2905. [Google Scholar]
- Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF Jr., Rejeski D, Hull MS, 2015. Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol 6, 1769–1780. 10.3762/bjnano.6.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M, 2014a. Adverse outcome pathway development II: best practices. Toxicol. Sci 142 (2), 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M, 2014b. Adverse outcome pathway (AOP) development I: strategies and principles. Toxicol. Sci 142 (2), 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Chen X, Yang F, Porter DW, Wu N, 2014. A new stochastic kriging method for modeling multi-source exposure-response data in toxicology studies. ACS Sustain. Chem. Eng 2, 1581–1591. 10.1021/sc500102h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit DB, Kreiling R, Levy LS, 2016. Relevance of the rat lung tumor response to particle overload for human risk assessment—update of new data since ILSI 2000. Toxicology 374, 42–59. https://doi.Org/10.1016/j.tox.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Weldon BA, Faustman EM, Oberdorster G, Workman T, Griffith WC, Kneuer C, Yu IJ, 2016. Occupational exposure limit for silver nanoparticles: considerations on the derivation of a general health-based value. Nanotoxicology 10 (7), 945–956. 10.3109/17435390.2016.1148793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MW, Bailer AJ, 2007. Properties of model-averaged BMDLs: a study of model averaging in dichotomous response risk estimation. Risk Anal 27 (3), 659–670. https://DOI:10.1111/j.1539-6924.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- Wichmann HE, Peters A, 2000. Epidemiological evidence of the effects of ultrafine particle exposure. Philos. T. Roy. Soc 358 (1775), 2757–2769. https://DOI:l0.1098/rsta.2000.0682. [Google Scholar]
- Yanamala N, Farcas MT, Hatfield MK, Kisin ER, Kagan VE, Geraci CL, Shvedova AA, 2014. In vivo evaluation of the pulmonary toxicity of cellulose nanocrystals: a renewable and sustainable nano material of the future. ACS Sustain. Chem. Eng 2, 1691–1698. 10.1021/sc500153k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CP, 1996. Extrapolation modeling of particle deposition and retention from rats to humans. Part. Sei. Technol 14 (1), 1–13. 10.1080/02726359608906682. [DOI] [Google Scholar]
- Zheng W, McKinney W, Kashon M, Salmen R, Castranova V, Kan H, 2016. The influence of inhaled multi-walled carbon nanotubes on the antonomic nervous system. Part. Fibre Toxicol. 13, 8 https://doi.10.0186/sl2989-016-01-19-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


