Abstract
Objectives
To describe patterns and factors associated with mode of delivery among HIV-infected women in the United States in relation to evolving HIV-in-pregnancy guidelines.
Methods
We conducted an analysis of two observational studies, PACTG 367 and IMPAACT P1025, which enrolled HIV-infected pregnant women from 1998 to 2013 at >60 U.S. AIDS clinical research sites. Multivariable analyses of factors associated with an HIV-indicated cesarean delivery (i.e., for prevention of mother-to-child transmission) versus other indications were conducted, and compared according to pre-specified time periods of evolving HIV-in-pregnancy guidelines: 1998–1999, 2000–2008, and 2009–2013.
Results
Among 6444 HIV-infected pregnant women, 21% delivered in 1998–1999, 58% in 2000–2008, and 21% in 2009–2013; 3025 (47%) delivered by cesarean. Cesarean delivery increased from 30% in 1998 to 48% in 2013. Of all cesarean deliveries, repeat cesarean deliveries increased from 16% in 1998 to 42% in 2013; HIV-indicated cesarean deliveries peaked at 48% in 2004, then dropped to 12% by 2013. In multivariable analyses, an HIV diagnosis during pregnancy, initiation of antiretroviral therapy in the third trimester, a plasma viral load ≥500 copies/mL, and delivery between 37–40 weeks gestation increased the likelihood of an HIV-indicated cesarean delivery. In analyses by time period, an HIV diagnosis during pregnancy, initiation of antiretroviral therapy in the third trimester, and a plasma viral load ≥500 copies/mL were progressively more likely to be associated with an HIV-indicated cesarean delivery over time.
Conclusion
Almost 50% of HIV-infected women underwent cesarean delivery. Over time, repeat cesarean deliveries increased, while HIV-indicated cesarean deliveries decreased and were more likely in women at high-risk of mother-to-child transmission. These findings reinforce the need for both early diagnosis and treatment of HIV infection in pregnancy and the option of vaginal delivery after cesarean among HIV-infected pregnant women.
INTRODUCTION
The rate of cesarean deliveries has continued to increase in the United States (US), and in parallel, an increasing number of HIV-infected women are having cesarean deliveries1,2. It is estimated that 8,500 HIV-infected US women deliver annually3. In 1999, data indicated a lower risk of mother-to-child transmission of HIV with cesarean delivery4,5, and the rate of cesarean deliveries soon approached 50%1,2,6,7. Since then, well-tolerated and better studied highly active antiretroviral therapy (HAART), which consists of three or more antiretroviral agents to suppress HIV viral load, has become a cornerstone of HIV management in pregnancy8. In concert, the rate of mother-to-child transmission of HIV in the US has decreased to <2%9,10.
HIV-in-pregnancy guidelines have evolved over the past two decades1. In 1998, new data demonstrated a 50–80% lower risk of mother-to-child transmission of HIV with cesarean delivery4,5. These results led the American College of Obstetricians and Gynecologists (ACOG) in 1999 to recommend that HIV-infected women be offered a scheduled cesarean delivery at 38 weeks of gestation11(the time period is hereafter “1998–1999”). Emerging data in the HAART era suggested that effective treatment markedly reduced the risk of mother-to-child transmission via maternal viral suppression, regardless of mode of delivery (hereafter “2000–2008”)12,13. In 2009, the US Public Health Service recommended HAART for all HIV-infected pregnant women (hereafter “2009–2013”)14. The US Department of Health and Human Services and ACOG currently recommend cesarean delivery before labor and before ruptured membranes to prevent mother-to-child transmission for HIV-infected pregnant women with, >1000 copies/mL or unknown HIV plasma viral load15,16.
1,2,17 We examined changing patterns and factors associated with mode of delivery among HIV-infected women in the US in relation to evolving HIV-in-pregnancy guidelines from 1998 to 2013.
MATERIALS AND METHODS
This is a secondary analysis of two large observational studies of HIV-infected pregnant women, Pediatric AIDS Clinical Trials Group (PACTG) 367 (1998–2004) and International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) network Protocol P1025 (late 2002–2013). PACTG 367 was a prospective chart abstraction study of HIV-infected pregnant women at 72 centers in the US and Puerto Rico designed to assess maternal HIV-infection, mother-to-child transmission, ART use, mode of delivery, and plasma viral load18. The subsequent IMPAACT Protocol P1025 was a prospective cohort study of HIV-infected pregnant women at 56 centers in the US and Puerto Rico designed to assess maternal health, as well as safety and efficacy of ART and interventions for the prevention of mother-to-child transmission19. All women with singleton or multiple gestations with a live birth >20 weeks of gestation were eligible for enrollment. Institutional review boards approved the protocols at all clinical sites, and written informed consent was obtained from all enrolled women.
Primary study outcomes included: 1) cesarean delivery versus vaginal delivery; then among women who underwent a cesarean delivery, 2) elective versus non-elective, and 3) indications for cesarean delivery, grouped as HIV-indicated cesarean delivery versus other clinical indications. Consistent with the originally defined modes of delivery, an “elective” cesarean delivery was defined as a cesarean delivery performed before the onset of labor and before ruptured membranes, and a “non-elective” cesarean delivery was defined as a cesarean section performed after onset of labor and/or ruptured membranes20. All indications for a cesarean delivery were reviewed by 2 obstetricians (K.K.V. and R.E.T.) and were classified into the following five categories: 1) HIV infection (i.e., for prevention of mother-to-child transmission), 2) repeat cesarean section, 3) arrest disorder, 4) fetal indication, and 5) maternal indication. In analyses, we dichotomized this outcome as an “HIV-indicated” cesarean delivery (the primary clinical indication for delivery was HIV infection) versus a “non-HIV-indicated” cesarean delivery (i.e., the other four categories of indications). Data were not available to identify the indication for a woman’s first or initial cesarean section.
Maternal demographic and clinical covariates of interest included the following: study (PACTG 367 vs. IMPAACT P1025), estimated date of confinement (EDC) year, maternal age at delivery, timing of HIV diagnosis (prior to or during pregnancy), trimester of prenatal care initiation, initial trimester of ARV exposure, first and last ARV regimen in pregnancy, total number of trimesters of ARV exposure, first and last CD4 cell count in pregnancy, first and last HIV RNA in pregnancy, ruptured membranes while in labor, intrapartum and neonatal ARV use, gestational age at delivery, infant birthweight, and HIV treatment guideline period, namely 1998–1999, 2000–2008, and 2009–2013 (as described above).
Descriptive statistics for maternal demographic and clinical characteristics were calculated, both overall and by mode of delivery. Logistic regression models with odds ratios (ORs) and 95% confidence intervals (95% CIs) were used to estimate the association between predictors and each of the three study outcomes: vaginal delivery, an elective cesarean delivery, and an HIV-indicated cesarean delivery. Multivariable logistic regression models adjusted for the following variables, namely maternal age at delivery, timing of HIV diagnosis, initial trimester of ARV use, first CD4 cell count in pregnancy, last HIV viral load before delivery, gestational age at delivery, and HIV treatment guideline period; and in the initial model, which included women who delivered vaginally, ruptured membranes in labor. Separate multivariable logistic regression models for each of the three pre-defined HIV treatment guideline periods were constructed to assess whether predictors of an HIV-indicated cesarean delivery varied by time between 1998–1999, 2000–2008, and 2009–2013 (i.e., effect modification).
The multivariable models above included only complete cases (i.e., only women who had complete data for all covariates included in the model). Multiple imputation was used to assess the impact of missing data on the above conclusions. For each study outcome, 30 imputations of missing covariate values were generated for the same study population and the same covariates as the above models assuming that variables were missing at random. To obtain a monotone missing data pattern, continuous covariates were first imputed using Markov Chain Monte Carlo methods, and then categorized, and categorical variables were imputed using logistic regression. For each of the 30 imputations, a logistic regression model was fit with the same covariates and same study population as the complete case analysis plus the women who had been excluded from the complete case analysis, using values from the imputation model. The adjusted ORs (AORs) and standard errors from each of the 30 logistic regressions were then pooled, and final estimates of the AORs and 95% CI were calculated using SAS Proc MIANALYZE. All analyses used SAS Version 9.4 software (SAS Institute, Inc, Cary, NC). Statistical significance was defined as a 2-sided p-value<0.05.
RESULTS
A total of 7846 women were enrolled in PACTG 367 (4,758 women) and IMPAACT P1025 (3,088 women). Of these 7846 women, 1402 (17.8%) were excluded from this analysis for the following reasons: ineligible for study enrollment, no prenatal care at a study site, no obstetrical baseline data, no EDC, or incomplete information on mode of delivery and/or ARV use. Therefore, this analysis included 6444 HIV-infected women. In 1998, the overall rate of mother-to-child transmission was 4% and decreased over time: 4% from 1998–1999, 1% in 2000–2008, and 0.3% in 2009–2013 (p<0.001).
Twenty-one percent of women delivered between 1998–1999, 58% between 2000–2008, and 21% between 2009–2013 (Table 1). More than half of the women (56%) had been enrolled in the earlier PACTG 367 cohort. Most (3,419/53%) delivered vaginally.. Over two thirds (68%) had been diagnosed with HIV infection prior to pregnancy, and 57% accessed prenatal care in the first trimester. Overall, only 22% of women had a plasma viral load >1000 copies/mL at delivery. This changed by time period: 41% from 1999–2000, 21% 2001–2008, and 8% 2009–2013 (p<0.001)
Table 1.
Unadjusted and adjusted analysis of characteristics associated with a cesarean delivery compared to a vaginal delivery among HIV-infected women in the PACTG/IMPAACT cohort (N=6,444)
| Characteristic# | Total (N=6,444) n (%) |
Cesarean delivery (N=3,025) n (%) |
Vaginal delivery (N=3,419) n (%) |
Odds ratio, OR (95% CI) |
Adjusted odds ratio, AOR (95% CI) |
|
|---|---|---|---|---|---|---|
| Guideline period | 1998–1999 | 1,356 (21%) | 481 (16%) | 875 (26%) | Reference | Reference |
| 2000–2008 | 3,743 (58%) | 1,858 (61%) | 1,885 (55%) | 1.79 (1.58–2.04) | 1.62 (1.37–1.91)* | |
| 2009–2013 | 1,345 (21%) | 686 (23%) | 659 (19%) | 1.89 (1.62–2.21)* | 1.99 (1.62–2.43)* | |
| HAART period | Early (1998–2008) | 5,099 (79%) | 2,339 (77%) | 2,760 (81%) | Reference | |
| Late (2009–2013) | 1,345 (21%) | 686 (23%) | 659 (19%) | 1.23 (1.09–1.39)* | ||
| Study | PACTG 367 | 3,584 (56%) | 1,540 (51%) | 2,044 (60%) | Reference | |
| IMPAACT P1025 | 2,860 (44%) | 1,485 (49%) | 1,375 (40%) | 1.43 (1.30–1.58)* | ||
| Age at delivery (years) | <25 | 2,125 (33%) | 968 (32%) | 1,157 (34%) | Reference | Reference |
| 25–30 | 2,167 (34%) | 993 (33%) | 1,174 (34%) | 1.01 (0.90–1.14)* | 0.94 (0.81–1.09) | |
| >30 | 2,146 (33%) | 1,059 (35%) | 1,087 (32%) | 1.17 (1.03–1.31)* | 1.19 (1.02–1.38)* | |
| Unknown | 6 (0%) | 5 (0%) | 1 (0%) | |||
| Timing of HIV diagnosis | Prior to pregnancy | 4,315 (67%) | 2,072 (68%) | 2,243 (66%) | Reference | Reference |
| During pregnancy | 2,047 (32%) | 920 (30%) | 1,127 (33%) | 0.88 (0.80–0.98)* | 1.03 (0.89–1.19) | |
| Unknown | 82 (1%) | 33 (1%) | 49 (1%) | |||
| First trimester of prenatal care | First | 3,686 (57%) | 1,780 (59%) | 1,906 (56%) | Reference | |
| Second | 2,251 (35%) | 1,014 (34%) | 1,237 (36%) | 0.88 (0.79–0.98)* | ||
| Third | 483 (8%) | 222 (7%) | 261 (8%) | 0.91 (0.75–1.10) | ||
| Unknown | 24 (0%) | 9 (0%) | 15 (0%) | |||
| First trimester of ARV use | First | 2,742 (43%) | 1,328 (44%) | 1,414 (41%) | Reference | Reference |
| Second | 2,743 (43%) | 1,275 (42%) | 1,468 (43%) | 0.93 (0.83–1.03) | 0.95 (0.82–1.09) | |
| Third | 774 (12%) | 363 (12%) | 411 (12%) | 0.94 (0.80–1.10) | 0.88 (0.71–1.10) | |
| No ARV use | 185 (3%) | 59 (2%) | 126 (4%) | 0.50 (0.36–0.69)* | 0.32 (0.19–0.55)* | |
| Last ARV regimen before delivery | 3 or more ARVs with PI | 3,774 (59%) | 1,906 (63%) | 1,868 (55%) | Reference | |
| 3 or more ARVs with NNRTI | 886 (14%) | 381 (13%) | 505 (15%) | 0.74 (0.64–0.86)* | ||
| 3 or more NRTIs | 598 (9%) | 283 (9%) | 315 (9%) | 0.88 (0.74–1.05) | ||
| 2 NRTIs | 644 (10%) | 241 (8%) | 403 (12%) | 0.59 (0.49–0.70)* | ||
| Single NRTI only | 326 (5%) | 139 (5%) | 187 (5%) | 0.73 (0.58–0.92)* | ||
| None | 216 (3%) | 75 (2%) | 141 (4%) | 0.52 (0.39–0.70)* | ||
| First CD4 cell count in pregnancy (cells/mm3) | ≥500 | 2,317 (36%) | 1,067 (35%) | 1,250 (37%) | Reference | Reference |
| 350–<500 | 1,494 (23%) | 678 (22%) | 816 (24%) | 0.97 (0.85–1.11) | 0.94 (0.80–1.10) | |
| 200–<350 | 1,493 (23%) | 694 (23%) | 799 (23%) | 1.02 (0.89–1.16) | 0.94 (0.81–1.11) | |
| <200 | 905 (14%) | 492 (16%) | 413 (12%) | 1.40 (1.20–1.63)* | 1.13 (0.93–1.38) | |
| Unknown | 235 (4%) | 94 (3%) | 141 (4%) | |||
| Last CD4 cell count before delivery (cells/mm3) | ≥500 | 2,820 (44%) | 1,296 (43%) | 1,524 (44%) | Reference | |
| 350–<500 | 1,491 (23%) | 667 (22%) | 824 (24%) | 0.95 (0.84–1.08) | ||
| 200–<350 | 1,228 (19%) | 595 (20%) | 633 (19%) | 1.11 (0.97–1.26) | ||
| <200 | 670 (10%) | 373 (12%) | 297 (9%) | 1.48 (1.25–1.75)* | ||
| Unknown | 235 (4%) | 94 (3%) | 141 (4%) | |||
| First HIV viral load in pregnancy (copies/ml) | <500 | 2,269 (35%) | 1,039 (34%) | 1,230 (36%) | Reference | |
| 500–<1,000 | 349 (6%) | 156 (5%) | 193 (6%) | 0.96 (0.76–1.20) | ||
| 1,000–<10,000 | 1,679 (26%) | 762 (25%) | 917 (27%) | 0.98 (0.87–1.12) | ||
| ≥10,000 | 1,894 (29%) | 966 (32%) | 928 (27%) | 1.23 (1.09–1.39)* | ||
| Unknown | 253 (4%) | 102 (3%) | 151 (4%) | |||
| Last HIV viral load before delivery (copies/ml) | <500 | 4,495 (70%) | 1,958 (65%) | 2,537 (74%) | Reference | Reference |
| 500–<1,000 | 328 (5%) | 168 (6%) | 160 (5%) | 1.36 (1.09–1.70)* | 1.54 (1.17–2.01)* | |
| 1,000–<10,000 | 814 (13%) | 456 (15%) | 358 (10%) | 1.65 (1.42–1.92)* | 1.92 (1.58–2.33)* | |
| ≥10,000 | 554 (9%) | 341 (11%) | 213 (6%) | 2.07 (1.73–2.49)* | 2.23 (1.75–2.83)* | |
| Unknown | 253 (3%) | 102 (3%) | 151 (4%) | |||
| Ruptured membranes in labor | No | 3,289 (51%) | 2,312 (76%) | 977 (29%) | Reference | Reference |
| Yes | 2,900 (45%) | 627 (21%) | 2,273 (66%) | 0.12 (0.10–0.13)* | 0.12 (0.11–0.14)* | |
| Unknown | 255 (4%) | 86 (3%) | 169 (5%) | |||
| Gestational age at delivery (weeks) | <32 | 244 (4%) | 100 (3%) | 144 (4%) | Reference | Reference |
| 32–<37 | 950 (15%) | 487 (16%) | 463 (14%) | 1.52 (1.14–2.01)* | 1.89 (1.32–2.70)* | |
| 37–<40 | 3,933 (61%) | 2,020 (67%) | 1,913 (56%) | 1.52 (1.17–1.98)* | 1.53 (1.10–2.13)* | |
| ≥40 | 1,314 (20%) | 415 (14%) | 899 (26%) | 0.67 (0.50–0.88)* | 0.94 (0.66–1.34) | |
| Unknown | 3 (0%) | 3 (0%) | 0 |
Multivariable logistic regression models adjusted for the following variables, namely maternal age at delivery, timing of HIV diagnosis, initial trimester of ARV use, first CD4 cell count in pregnancy, last HIV viral load before delivery, gestational age at delivery, HIV treatment guideline period; and ruptured membranes in labor.
The final n in the adjusted regression model was 5,836HIV-infected women, which included complete cases without missing data.
Reflects statistically significant findings with a p-value<0.05.
All percentages shown are column percentages. Variables with missing data were included in frequencies and percentages, but not in the regression models used to calculate the OR, 95% CI.
The following abbreviations are used in the table above: highly active antiretroviral therapy (HAART) and antiretroviral (ARV), protease inhibitor (PI), non-nucleoside reverse transcriptase inhibitor (NNRTI), and nucleoside reverse transcriptase inhibitor (NRTI).
In 1998, 30% of HIV-infected women had a cesarean delivery compared to 21% of US women, but by 2013, 48% of HIV-infected women had a cesarean delivery compared to 30% of US women (Figure 1). Since 2008, approximately 50% of HIV-infected women underwent a cesarean delivery, of whom 62% had an elective cesarean. Among 774 women undergoing a repeat cesarean delivery, 74% (N=574) were elective.
Figure 1.
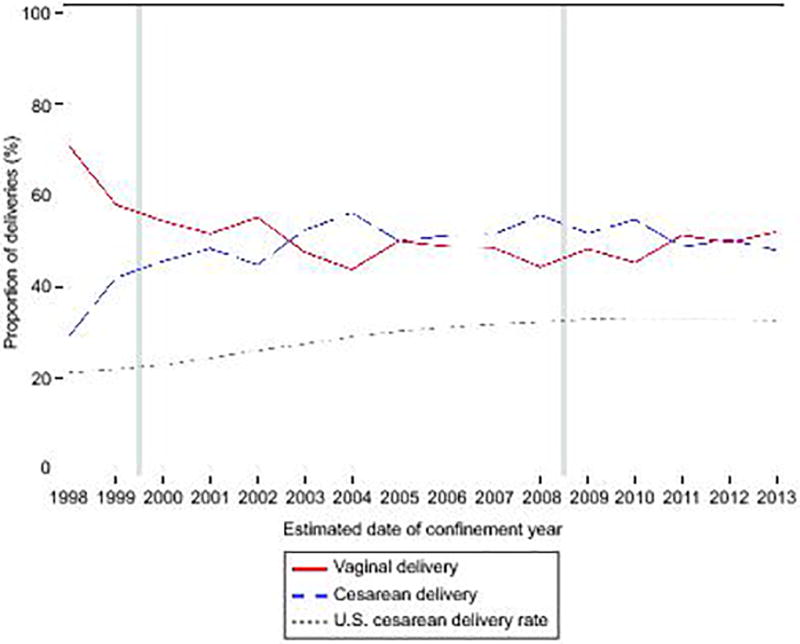
Cesarean delivery rate among women infected with human immunodeficiency virus (HIV) in the Pediatric AIDS Clinical Trials Group (PACTG) or International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) cohorts compared to all U.S. women from 1998–2013. Vertical gray lines indicate guideline change. U.S. cesarean delivery rate obtained from Osterman MJ, Martin JA. Natl Vital Stat Rep 2014;63:1–16. Available at: https://www.cdc.gov/nchs/fastats/delivery.htm. Accessed November 12, 2017.
Of 3025 women who had a cesarean delivery, data on the indication were available for 2886 (95.4%). The 139 women with an unknown indication for cesarean delivery did not differ by time period, viral load before delivery, or gestational age at delivery. Overall, 977 (34%) of cesarean deliveries had an indication of HIV infection as did 774 (27%) repeat cesareans. (Table 2). From 1998 to 2013, the primary indication for cesarean delivery changed from HIV infection to repeat cesarean section (Figure 2). HIV-indicated cesarean deliveries peaked at 48% in 2004, then dropped to 12% by 2013. Between 1998 and 2013, additional changes occurred: increase in repeat cesarean deliveries (16% to 42%), decrease in arrest disorder (25% to 12%), decrease in fetal indications (39% to 28%), and increase in maternal indications (4% to 7%). Among the subset of women in IMPACT 1025 where parity and indication were both collected, 54.5% (263/483) of nulliparous women had a primary cesarean delivery, of which 35.9% (82/228) were HIV-indicated; 80% of these women had a suppressed HIV viral load (i.e. <1000 copies/ml) at delivery.
Table 2.
Primary indications for cesarean delivery among HIV-infected women with documented indication for cesarean delivery (N=2,886)
| Indications for cesarean delivery | ||||||
|---|---|---|---|---|---|---|
| Characteristic | HIV infection (N=977) |
Arrest disorder (N=380) |
Maternal indication (N=94) |
Fetal indication (N=661) |
Repeat cesarean delivery (N=774) |
|
| N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Guideline Period | 1998–1999 | 153 (32%) | 82 (17%) | 17 (4%) | 153 (32%) | 73 (15%)* |
| 2000–2008 | 710 (40%) | 202 (11%) | 56 (3%) | 381 (22%) | 413 (23%) | |
| 2009–2013 | 114 (18%) | 96 (15%) | 21 (3%) | 127 (20%) | 288 (45%) | |
| HAART Period | Early (1998–2008) | 863 (39%) | 284 (13%) | 73 (3%) | 534 (24%) | 486 (22%)* |
| Late (2009–2013) | 114 (18%) | 96 (15%) | 21 (3%) | 127 (20%) | 288 (45%) | |
| Last plasma viral load before delivery (copies/ml) | <1,000 | 493 (25%) | 331 (16%) | 60 (3%) | 503 (25%) | 624 (31%)* |
| ≥1,000 | 453 (58%) | 33 (4%) | 32 (4%) | 127 (16%) | 132 (17%) | |
| Unknown | 31 (32%) | 16 (16%) | 2 (2%) | 31 (32%) | 18 (18%) | |
| Ruptured membranes in labor | No | 866 (39%) | 167 (8%) | 84 (4%) | 424 (19%) | 671 (30%)* |
| Yes | 87 (15%) | 200 (34%) | 7 (1%) | 215 (36%) | 81 (14%) | |
| Unknown | 0 | 13 (22%) | 3 (5%) | 22 (37%) | 22 (37%) | |
| Gestational age at delivery (weeks) | <37 | 111 (20%) | 48 (9%) | 57 (10%) | 218 (39%) | 118 (21%)* |
| ≥37 | 866 (37%) | 332 (14%) | 37 (2%) | 443 (19%) | 653 (28%) | |
| Unknown | 0 | 0 | 0 | 0 | 3 (100%) | |
Reflects statistically significant findings with a p-value<0.001. A Chi-square test was used, which tested the difference in indication for cesarean delivery by strata.
This table is a subset of the 2,886 women with an available indication for cesarean delivery among the women who had a cesarean delivery in this cohort (n=3,025).
Figure 2.

Indications for cesarean delivery among women infected with human immunodeficiency virus (HIV) in the Pediatric AIDS Clinical Trials Group (PACTG) or International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) cohorts from 1998–2013. Vertical gray lines indicate guideline change. The above data is presented in table format in Appendix 4 (available online at http://links.lww.com/xxx).
In multivariable analyses of cesarean delivery versus vaginal delivery (Table 1), women who delivered between 2000–2008 and between 2009–2013 (AORs: 1.62–1.99)) were more likely to have a cesarean delivery compared to women who delivered between 1998–1999. Factors associated with an increased risk of cesarean delivery included: maternal age >30 years (AOR: 1.19; 95% CI: 1.02–1.38), HIV viral load ≥ 500 copies/mL before delivery (AORs: 1.54–2.23), and gestational age at delivery between 32–40 weeks (AORs: 1.53–1.89). Women with ruptured membranes in labor (AOR: 0.12; 95% CI: 0.11–0.14) and who did not use ARVs during pregnancy (AOR: 0.32; 95% CI: 0.19–0.55) were less likely to undergo cesarean delivery. Multiple imputation analyses were used to investigate the effect of missing data, and the results were similar (Appendix 1, available online at http://links.lww.com/xxx).
In multivariable analyses of elective versus non-elective cesarean delivery, women were more likely to have an elective cesarean delivery between 2000–2008 and 2009–2013 (AORs: 1.49–1.59) in comparison to 1998–1999 as were women who delivered between 37–40 weeks of gestation (AOR: 2.96; 95% CI: 1.93–4.54 and who had a HIV viral load >1,000 copies/mL before delivery Among the 774 women who had a repeat cesarean delivery, there were no statistically significant predictors. (not shown).
In multivariable analyses of HIV-indicated versus non HIV-indicated cesarean delivery (Table 3), women who delivered between 2000–2008 were more likely to have a HIV-indicated cesarean delivery compared to those who delivered between 1998–1999. Conversely, those who delivered between 2009–2013 were less likely to have an HIV-indicated cesarean delivery. Women who were diagnosed with HIV infection during pregnancy, who initiated ARVs in the third trimester, who had a last HIV viral load ≥ 500 copies/mL before delivery, and who delivered between 37–40 weeks of gestation had a significantly higher odds of an HIV-indicated cesarean delivery. In multiple imputation analyses, the results were similar (Appendix 2, available online at http://links.lww.com/xxx).
Table 3.
Unadjusted and adjusted analysis of characteristics associated with an HIV-indicated cesarean delivery versus a non-HIV-associated cesarean delivery among HIV-infected women in the PACTG/IMPAACT cohort (N=2,886)
| Characteristic | Total (N=2,886) |
HIV-associated (N=977) |
Not HIV- associated (N=1,909) |
Odds ratio, OR (95% CI) |
Adjusted odds ratio, AOR (95% CI) |
|
|---|---|---|---|---|---|---|
| Guideline period | 1998–1999 | 478 (17%) | 153 (16%) | 325 (17%) | Reference | Reference |
| 2000–2008 | 1,762 (61%) | 710 (73%) | 1,052 (55%) | 1.43 (1.16–1.78)* | 1.69 (1.32–2.16)* | |
| 2009–2013 | 646 (22%) | 114 (12%) | 532 (28%) | 0.46 (0.34–0.60)* | 0.60 (0.44–0.83)* | |
| HAART period | Early (1998–2008) | 2,240 (78%) | 863 (88%) | 1,377 (72%) | Reference | |
| Late (2009–2013) | 646 (22%) | 114 (12%) | 532 (28%) | 0.34 (0.28–0.43)* | ||
| Study | PACTG 367 | 1,515 (52%) | 613 (63%) | 902 (47%) | Reference | |
| IMPAACT P1025 | 1,371 (48%) | 364 (37%) | 1,007 (53%) | 0.53 (0.45–0.62)* | ||
| Age at delivery (years) | <25 | 923 (32%) | 377 (39%) | 546 (29%) | Reference | Reference |
| 25–30 | 948 (33%) | 314 (32%) | 634 (33%) | 0.72 (0.59–0.87)* | 0.86 (0.69–1.06) | |
| >30 | 1,010 (35%) | 283 (29%) | 727 (38%) | 0.56 (0.47–0.68)* | 0.67 (0.54–0.83)* | |
| Unknown | 5 (0%) | 3 (0%) | 2 (0%) | |||
| Timing of HIV diagnosis | Prior to pregnancy | 1,976 (68%) | 619 (63%) | 1,357 (71%) | Reference | Reference |
| During pregnancy | 878 (30%) | 352 (36%) | 526 (28%) | 1.47 (1.24–1.73)* | 1.49 (1.20–1.84)* | |
| Unknown | 32 (1%) | 6 (1%) | 26 (1%) | |||
| First trimester of prenatal care | First | 1,680 (58%) | 500 (51%) | 1,180 (62%) | Reference | |
| Second | 980 (34%) | 371 (38%) | 609 (32%) | 1.44 (1.22–1.70)* | ||
| Third | 217 (8%) | 102 (10%) | 115 (6%) | 2.09 (1.57–2.79)* | ||
| Unknown | 9 (0%) | 4 (0%) | 5 (0%) | |||
| First trimester of ARV use | First | 1,257 (44%) | 368 (38%) | 889 (47%) | Reference | Reference |
| Second | 1,220 (42%) | 413 (42%) | 807 (42%) | 1.24 (1.04–1.47)* | 0.94 (0.76–1.16) | |
| Third | 351 (12%) | 175 (18%) | 176 (9%) | 2.40 (1.89–3.06)* | 1.41 (1.04–1.91)* | |
| No ARV use | 58 (2%) | 21 (2%) | 37 (2%) | 1.37 (0.79–2.37)* | 0.65 (0.28–1.52) | |
| Last ARV regimen in pregnancy | 3 or more ARVs with PI | 1,795 (62%) | 601 (62%) | 1,194 (63%) | Reference | |
| 3 or more ARVs with NNRTI | 376 (13%) | 138 (14%) | 238 (12%) | 1.15 (0.91–1.45) | ||
| 3 or more NRTIs | 268 (9%) | 79 (8%) | 189 (10%) | 0.83 (0.63–1.10) | ||
| 2 NRTIs | 237 (8%) | 88 (9%) | 149 (8%) | 1.17 (0.89–1.55) | ||
| Single NRTI only | 136 (5%) | 46 (5%) | 90 (5%) | 1.02 (0.70–1.47) | ||
| None | 74 (3%) | 25 (3%) | 49 (3%) | 1.01 (0.62–1.66) | ||
| First CD4 cell count in pregnancy (cells/mm3) | ≥500 | 1,011 (35%) | 274 (28%) | 737 (39%) | Reference | Reference |
| 350–<500 | 645 (22%) | 220 (23%) | 425 (22%) | 1.39 (1.12–1.72)* | 1.14 (0.89–1.45) | |
| 200–<350 | 664 (23%) | 262 (27%) | 402 (21%) | 1.75 (1.42–2.16)* | 1.31 (1.03–1.66)* | |
| <200 | 478 (17%) | 187 (19%) | 291 (15%) | 1.73 (1.37–2.18)* | 1.02 (0.78–1.34) | |
| Unknown | 88 (3%) | 34 (3%) | 54 (3%) | |||
| Last CD4 cell count before delivery (cells/mm3) | ≥500 | 1,224 (42%) | 347 (36%) | 877 (46%) | Reference | |
| 350–<500 | 637 (22%) | 232 (24%) | 405 (21%) | 1.45 (1.18–1.78)* | ||
| 200–<350 | 577 (20%) | 217 (22%) | 360 (19%) | 1.52 (1.24–1.88)* | ||
| <200 | 360 (13%) | 147 (15%) | 213 (11%) | 1.74 (1.37–2.23)* | ||
| Unknown | 88 (3%) | 34 (4%) | 54 (3%) | |||
| First HIV viral load in pregnancy (copies/ml) | <500 | 977 (34%) | 226 (23%) | 751 (39%) | Reference | |
| 500–<1,000 | 147 (5%) | 48 (5%) | 99 (5%) | 1.61 (1.11–2.35)* | ||
| 1,000–<10,000 | 738 (26%) | 258 (26%) | 480 (25%) | 1.79 (1.45–2.21)* | ||
| ≥10,000 | 927 (32%) | 414 (42%) | 513 (27%) | 2.68 (2.20–3.27)* | ||
| Unknown | 97 (3%) | 31 (3%) | 66 (4%) | |||
| Last HIV viral load before delivery (copies/ml) | <500 | 1,848 (64%) | 425 (44%) | 1,423 (75%) | Reference | Reference |
| 500–<1,000 | 164 (6%) | 68 (7%) | 96 (5%) | 2.37 (1.71–3.30)* | 2.17 (1.53–3.09)* | |
| 1,000–<10,000 | 445 (15%) | 249 (25%) | 196 (10%) | 4.25 (3.43–5.28)* | 3.91 (3.07–4.97)* | |
| ≥10,000 | 332 (12%) | 204 (21%) | 128 (7%) | 5.34 (4.17–6.83)* | 5.42 (4.08–7.20)* | |
| Unknown | 97 (3%) | 31 (3%) | 66 (3%) | |||
| Gestational age at delivery (weeks) | <37 | 552 (19%) | 111 (11%) | 441 (23%) | Reference | Reference |
| 37–<40 | 1,927 (67%) | 780 (80%) | 1,147 (60%) | 2.70 (2.15–3.39)* | 3.06 (2.38–3.95)* | |
| ≥40 | 404 (14%) | 86 (9%) | 318 (17%) | 1.07 (0.78–1.47) | 1.04 (0.73–1.49) | |
| Unknown | 3 (0%) | 0 | 3 (0%) |
Multivariable logistic regression models adjusted for the following variables, namely maternal age at delivery, timing of HIV diagnosis, initial trimester of ARV use, first CD4 cell count in pregnancy, last HIV viral load before delivery, gestational age at delivery, and HIV treatment guideline period.
The final n in the adjusted regression model was 2,737 HIV-infected women, which included complete cases without missing data.
This table is a subset of the 2,886 women with an available indication for cesarean delivery among the women who had a cesarean delivery in this cohort (n=3,025).
Reflects statistically significant findings with a p-value<0.05.
All percentages shown are column percentages. The "Missing" categories were included in frequencies and percentages, but not the regression models used to calculate the OR, 95% CI, and p-values.
The following abbreviations are used in the table above: highly active antiretroviral therapy (HAART) and antiretroviral (ARV), protease inhibitor (PI), non-nucleoside reverse transcriptase inhibitor (NNRTI), and nucleoside reverse transcriptase inhibitor (NRTI).
We performed subgroup analyses to examine how the predictors of an HIV-indicated versus non-HIV-indicated cesarean delivery changed over time by fitting separate multivariable logistic regression models for each guideline time period (i.e., 1998–1999, 2000–2008, and 2009–2013) (Table 4). Women >30 years were progressively less likely to have an HIV-indicated cesarean delivery in 2000–2008 and 2009–2013 than in 1998–1999. Women who were diagnosed with HIV during pregnancy were more likely to undergo an HIV-indicated cesarean delivery in both 2000–2008 and 2009–2013, but not in 1998–1999. Women who initiated ARVs in the third trimester were more likely to have an HIV-indicated cesarean delivery in 2009–2013, but not in 1998–1999 or 2000–2008. Women with HIV viral load ≥500 copies/mL before delivery were more likely to have an HIV-indicated cesarean delivery in 2000–2008 and 2009–2013, but not in 1998–1999. In multiple imputation analyses for each guideline period, the results were similar (not shown; Appendix 3, available online at http://links.lww.com/xxx).
Table 4.
Adjusted analysis of characteristics associated with an HIV-indicated cesarean delivery versus a non-HIV-indicated cesarean delivery comparing guideline periods 1998–1999, 2000–2008, and 2009–2013 (N=2,886)
| Multivariable analysis comparing guideline period | ||||
|---|---|---|---|---|
| 1998–1999 n=478 |
2000–2008 n=1762 |
2009–2013 n=646 |
||
| Characteristic | Adjusted odds ratio, AOR (95% CI) |
Adjusted odds ratio, AOR (95% CI) |
Adjusted odds ratio, AOR (95% CI) |
|
| Age at delivery (years) | <25 | Reference | Reference | Reference |
| 25–30 | 1.25 (0.72–2.18) | 0.87 (0.67–1.14) | 0.61 (0.36–1.04) | |
| >30 | 1.39 (0.81–2.37) | 0.68 (0.52–0.89)* | 0.25 (0.12–0.52)* | |
| Timing of HIV diagnosis | Prior to pregnancy | Reference | Reference | Reference |
| During pregnancy | 1.06 (0.63–1.78) | 1.58 (1.22–2.06)* | 2.15 (1.17–3.94)* | |
| First trimester of ARV use | First | Reference | Reference | Reference |
| Second | 0.85 (0.49–1.45) | 0.89 (0.68–1.15) | 1.17 (0.67–2.04) | |
| Third | 1.26 (0.65–2.46) | 1.42 (0.98–2.06) | 3.33 (1.23–9.02)* | |
| No ARV use | *** | 1.15 (0.41–3.17) | ** | |
| First CD4 cell count in pregnancy (cells/mm3) | ≥500 | Reference | Reference | Reference |
| 350–<500 | 0.73 (0.40–1.33) | 1.29 (0.96–1.73) | 1.30 (0.67–2.51) | |
| 200–<350 | 0.86 (0.48–1.55) | 1.50 (1.12–2.00)* | 0.84 (0.43–1.63) | |
| <200 | 0.62 (0.31–1.23) | 1.17 (0.84–1.64) | 0.72 (0.34–1.52) | |
| Last HIV viral load before delivery (copies/ml) | <500 | Reference | Reference | Reference |
| 500–<1,000 | 1.52 (0.70–3.29) | 2.02 (1.31–3.11)* | 3.63 (1.33–9.94)* | |
| 1,000–<10,000 | 1.22 (0.69–2.16) | 4.25 (3.17–5.69)* | 13.12 (6.44–26.74)* | |
| ≥10,000 | 1.53 (0.79–2.99) | 6.70 (4.65–9.65)* | 13.87 (6.30–30.58)* | |
| Gestational age at delivery (weeks) | <37 | Reference | Reference | Reference |
| 37–<40 | 6.95 (3.35–14.44)* | 2.73 (2.01–3.69)* | 2.03 (1.01–4.09)* | |
| ≥40 | 1.48 (0.60–3.63) | 1.23 (0.81–1.88) | 0.18 (0.02–1.49) | |
Multivariable logistic regression models adjusted for the following variables, namely maternal age at delivery, timing of HIV diagnosis, initial trimester of ARV use, first CD4 cell count in pregnancy, last HIV viral load before delivery, gestational age at delivery, and HIV treatment guideline period.
The final n in the adjusted regression model was 434 HIV-infected women for 1999–2000, 1,674 for 2001–2008, and 629 for 2009–2013, which included complete cases without missing data.
This table is a subset of the 2,886 women with an available indication for cesarean delivery among the women who had a cesarean delivery in this cohort (n=3,025).
Reflects statistically significant findings with a p-value<0.05.
No participants in this time period received no ARV therapy. Therefore, the AOR (95% CI) was not produced for this group.
Few women received no ARV therapy in this time period. Because of this, the AOR (95% CI) was not stable, and thus is not shown.
DISCUSSION
We analyzed data for over 6000 HIV-infected women across multiple sites in the US and Puerto Rico spanning 1998 to 2013, a time period of evolving obstetric guidelines for HIV infection and access to more effective HAART. Repeat cesarean section replaced HIV infection as the primary indication for cesarean delivery among HIV-infected women. Nevertheless, despite improvements in treatment with mother-to-child transmission at the lowest rate and guideline changes, the prevalence of cesarean delivery remained high among HIV-infected women in the HAART era. It is likely that the prevalence of cesarean delivery is multifactorial, influenced by HIV infection as well as a history of a prior cesarean delivery. Additionally, HIV-infected women having an HIV-indicated cesarean delivery were increasingly more likely over time to be diagnosed with HIV infection during pregnancy, had initiated antiretrovirals later in pregnancy, and had a higher HIV viral load at delivery. These findings highlight that HIV-indicated cesarean deliveries were increasingly reserved for women at high-risk for mother-to-child transmission, suggesting that obstetrical providers and patients at tertiary care sites where these two studies were conducted were changing HIV-in-pregnancy clinical care as new data emerged
HIV infection was less likely to be the primary indication for a cesarean delivery among HIV-infected women in the US over time, similar to recent European data21,22. Though the rate of HIV-indicated cesarean delivery quickly rose from 1998–2000 following guidelines recommending that all HIV-infected women be offered a scheduled cesarean delivery at 38 weeks of gestation, the rate then declined until 2013. This change in indication for cesarean delivery was temporally consistent with guideline changes recommending HAART for all HIV-infected pregnant women, regardless of disease severity, and high rates of viral suppression at the time of delivery12,23.
We found that, since 2008, almost half of HIV-infected women had a cesarean delivery. The main indication for cesarean delivery in HIV-infected women from 2009 onwards was a history of a prior cesarean section, not HIV infection. The rate of elective cesarean delivery was close to 60% of all cesarean deliveries throughout the study. During the current study, increasing numbers of non-HIV infected American women were also undergoing cesarean delivery, though the cesarean rate increased less markedly from 21% to 30%24. A prior analysis from this cohort showed that morbidity was highest with non-elective cesarean delivery and lowest with vaginal delivery, and rates of morbidity in the HAART era were lower than in earlier historical cohorts of HIV-infected women (19% versus 29–49%)20,25. Our findings suggest that, similar to non-HIV infected pregnant women26, HIV-infected women, particularly those on HAART with effective viral suppression, should be offered and should consider a TOLAC when clinically appropriate.
Women who were first diagnosed with HIV infection during pregnancy, who initiated HAART later in pregnancy, and who had an unsuppressed HIV viral load at delivery were more likely to have an HIV-indicated cesarean delivery, and these associations were more pronounced over time. These results highlight the importance of continued screening for HIV prior to pregnancy27,28. Additionally, once HIV-infected women are pregnant, prompt enrollment in prenatal care and early initiation of ART is necessary to maximize the likelihood of viral suppression, decrease the risk of mother-to-child transmission, and increase the chances of vaginal delivery, including a TOLAC29. These evidence-based interventions have the promise to further decrease the high rate of cesarean delivery among HIV-infected women in the US.
This analysis has its limitations. This retrospective observational study across >60 clinical sites does not provide information about how HIV-infected women were counseled about their options for delivery, including TOLAC, compared to HIV-uninfected women. The current study was conducted at tertiary care centers with expertise in HIV and obstetric care and hence these findings may not apply to other clinical settings with fewer resources. The current study did not uniformly collect parity data across the two studies; however, in the subset of women in IMPACT P1025 where parity was collected, the frequency of cesarean delivery, an HIV-associated cesarean delivery, and a suppressed plasma viral load (i.e. <1000 copies/ml) at delivery were similar between nulliparous women and the overall cohort. Among women with a history of a prior cesarean delivery, we do not have data on indications for the first cesarean delivery. It is possible that some repeat cesarean deliveries resulted after an initial cesarean delivery for HIV transmission. It may be difficult to distinguish these cases as distinct from an HIV-indicated cesarean delivery. Some women may prefer a repeat cesarean delivery to decrease perinatal HIV transmission in light of previous counseling in addition to the risks associated with a TOLAC. However, it is likely that TOLAC counseling along with counseling about current recommendations for prevention of perinatal HIV transmission could result in vaginal delivery for many of these women.
Maternal HIV-infection continues to influence mode of delivery, though to a smaller extent than prior to use of HAART. For an HIV-infected pregnant woman and her obstetrical provider, many factors, both HIV-related and patient preference may influence the final choice of mode of delivery.25As in non-HIV infected women, interventions aimed at reducing the cesarean rate among HIV-infected women will need to target obstetric management as well as HIV-specific clinical scenarios.
Supplementary Material
Acknowledgments
Supported by the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Presented at the Infectious Diseases for Obstetrics and Gynecology Annual Meeting. Park City, Utah. August 10–12, 2017.
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
References
- 1.Jamieson D, Read JS, Kourtis AP, Durant TM, Lampe MA, Dominguez KL. Cesarean delivery for HIV-infected women: recommendations and controversies. American Journal of Obstetrics and Gynecology. 2007;197:S96–S100. doi: 10.1016/j.ajog.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 2.Dominguez K, Lindegren ML, D'Almada PJ, Peters VB, Frederick T, Rakusan TA, Ortiz IR, Hsu HW, Melville SK, Sadek R, Fowler MG. Pediatric Spectrum of HIV Disease Consortium. Increasing trend of Cesarean deliveries in HIV-infected women in the United States from 1994 to 2000. Journal of Acquired Immune Deficiency Syndrome. 2003;33:232–8. doi: 10.1097/00126334-200306010-00019. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) HIV Among Pregnant Women, Infants, and Children. Centers for Disease Control and Prevention; 2016. [Accessed November 12, 2017]. (at http://www.cdc.gov/hiv/group/gender/pregnantwomen/.) [Google Scholar]
- 4.International Perinatal HIV Group. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1--a meta-analysis of 15 prospective cohort studies. The International Perinatal HIV Group. New England Journal of Medicine. 1999;340:977–87. doi: 10.1056/NEJM199904013401301. [DOI] [PubMed] [Google Scholar]
- 5.European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035–9. doi: 10.1016/s0140-6736(98)08084-2. [DOI] [PubMed] [Google Scholar]
- 6.Kind C, Rudin C, Siegrist CA, Wyler CA, Biedermann K, Lauper U, Irion O, Schüpbach J, Nadal D. Prevention of vertical HIV transmission: additive protective effect of elective Cesarean section and zidovudine prophylaxis. Swiss Neonatal HIV Study Group. AIDS. 1998;12:205–10. doi: 10.1097/00002030-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn L, Bobat R, Coutsoudis A, Moodley D, Coovadia HM, Tsai WY, Stein ZA. Cesarean deliveries and maternal-infant HIV transmission: results from a prospective study in South Africa. Journal of Acquired Immune Deficiency Syndrome. 1996;11:478–83. doi: 10.1097/00042560-199604150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Anderson B, Cu-Uvin S. Pregnancy and optimal care of HIV-infected patients. Clinical Infectious Disease. 2009;48:449–55. doi: 10.1086/596477. [DOI] [PubMed] [Google Scholar]
- 9.Garcia P, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, Kornegay J, Jackson B, Moye J, Hanson C, Zorrilla C, Lew JF. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. New England Journal of Medicine. 1999;341:394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 10.Chappell C, Cohn SE. Prevention of perinatal transmission of human immunodeficiency virus. Infectious Disease Clinics of North America. 2014;28:529–47. doi: 10.1016/j.idc.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 11.American College of Obstetricians and Gynecologists. Scheduled cesarean delivery and the prevention of vertical transmission of HIV infection: ACOG committee opinion no.: 234 (replaces no.: 219) International Journal of Gynaecology and Obstetrics. 2001;73:279–81. doi: 10.1016/s0020-7292(01)00412-x. [DOI] [PubMed] [Google Scholar]
- 12.Stoszek S, Duarte G, Hance LF, Pinto J, Gouvea MI, Cohen RA, Santos B, Teles E, Succi R, Alarcon JO, Read JS NISDI Perinatal/LILAC Study Group. Trends in the management and outcome of HIV-1-infected women and their infants in the NISDI Perinatal and LILAC cohorts, 2002–2009. International Journal of Gynaecology and Obstetrics. 2013;122:37–43. doi: 10.1016/j.ijgo.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper E, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, Hayani K, Handelsman E, Smeriglio V, Hoff R, Blattner W Women and Infants' Transmission Study Group. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. Journal of Acquired Immune Deficiency Syndrome. 2002;29:484–94. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 14.Perinatal HIV Guidelines Working Group. [Accessed November 12, 2017];Public Health Service Task Force Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. 2009 (at http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf.)
- 15.ACOG Committee on Obstetric Practice. Committee Opinion No. 234. Washington, DC: American College of Obstetricians and Gynecologists; 2015. Scheduled cesarean delivery and the prevention of vertical transmission of HIV infection. [Google Scholar]
- 16.US Department of Health and Human Services. [Accessed November 12, 2017];Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. 2016 (at https://aidsinfo.nih.gov/guidelines/html/3/perinatal-guidelines/0.)
- 17.Read J, Tuomala R, Kpamegan E, Zorrilla C, Landesman S, Brown G, Vajaranant M, Hammill H, Thompson B Women and Infants Transmission Study Group. Mode of delivery and postpartum morbidity among HIV-infected women: the women and infants transmission study. Journal of Acquired Immune Deficiency Syndrome. 2001;26:236–45. doi: 10.1097/00042560-200103010-00005. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro D, Tuomala R, Pollack H, et al. Eleventh Conference on Retroviruses and Opportunistic Infections. San Francisco, CA: 2004. Mother-to-child HIV transmission risk according to antiretroviral therapy, mode of delivery, and viral load in 2,895 US women (PACTG 367) [Google Scholar]
- 19.Katz I, Leister E, Kacanek D, Hughes MD, Bardeguez A, Livingston E, Stek A, Shapiro DE, Tuomala R. Factors associated with lack of viral suppression at delivery among highly active antiretroviral therapy-naive women with HIV: a cohort study. Annals of Internal Medicine. 2015;162:90–9. doi: 10.7326/M13-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livingston E, Huo Y, Patel K, Tuomala RE, Scott GB, Stek A P1025 Team of the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Group. Complications and Route of Delivery in a Large Cohort Study of HIV-1-Infected Women-IMPAACT P1025. Journal of Acquired Immune Deficiency Syndrome. 2016;73:74–82. doi: 10.1097/QAI.0000000000001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aebi-Popp K, Mulcahy F, Glass TR, Rudin C, Martinez de Tejada B, Bertisch B, Fehr J, Grawe C, Scheibner K, Rickenbach M, Hoesli I, Thorne C European Collaborative Study in EuroCoord; Swiss Mother & Child HIV Cohort Study. Missed opportunities among HIV-positive women to control viral replication during pregnancy and to have a vaginal delivery. Journal of Acquired Immune Deficiency Syndrome. 2013;64:58–65. doi: 10.1097/QAI.0b013e3182a334e3. [DOI] [PubMed] [Google Scholar]
- 22.Briand N, Jasseron C, Sibiude J, Azria E, Pollet J, Hammou Y, Warszawski J, Mandelbrot L. Cesarean section for HIV-infected women in the combination antiretroviral therapies era, 2000–2010. American Journal of Obstetrics and Gynecology. 2013;209:e1–e335. doi: 10.1016/j.ajog.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Baroncelli S, Tamburrini E, Ravizza M, Dalzero S, Tibaldi C, Ferrazzi E, Anzidei G, Fiscon M, Alberico S, Martinelli P, Placido G, Guaraldi G, Pinnetti C, Floridia M Italian Group on Surveillance on Antiretroviral Treatment in Pregnancy. Antiretroviral treatment in pregnancy: a six-year perspective on recent trends in prescription patterns, viral load suppression, and pregnancy outcomes. AIDS Patient Care and STDs. 2009;23:513–20. doi: 10.1089/apc.2008.0263. [DOI] [PubMed] [Google Scholar]
- 24.Menacker F, Declercq E, Macdorman MF. Cesarean delivery: background, trends, and epidemiology. Seminars in Perinatology. 2006;30:235–41. doi: 10.1053/j.semperi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Read J, Newell M-L. Efficacy and safety of cesarean delivery for prevention of mother-to-child transmission of HIV-1. Cochrane database of systematic reviews. 2005 doi: 10.1002/14651858.CD005479. CD005479. [DOI] [PubMed] [Google Scholar]
- 26.Tilden E, Cheyney M, Guise JM, Emeis C, Lapidus J, Biel FM, Wiedrick J, Snowden JM. Vaginal birth after cesarean: neonatal outcomes and United States birth setting. American Journal of Obstetrics and Gynecology. 2017;216:e1–e403. doi: 10.1016/j.ajog.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahangdale L, Sarnquist C, Maldonado Y, Cohan D. Patient acceptance of and satisfaction with rapid HIV Testing in a Labor and Delivery Setting. Journal of Womens Health. 2008;17:465–71. doi: 10.1089/jwh.2007.0545. [DOI] [PubMed] [Google Scholar]
- 28.American College of Obstetricians and Gynecologists. Committee opinion no: 635: Prenatal and perinatal human immunodeficiency virus testing: expanded recommendations. Obstetrics and gynecology. 2014;125:1544–7. doi: 10.1097/01.AOG.0000466370.86393.d2. [DOI] [PubMed] [Google Scholar]
- 29.Taylor A, Nesheim SR, Zhang X, Song R, FitzHarris LF, Lampe MA, Weidle PJ, Sweeney P. Estimated Perinatal HIV Infection Among Infants Born in the United States, 2002–2013. JAMA Pediatrics. 2017;171:435–42. doi: 10.1001/jamapediatrics.2016.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


