Abstract
Purpose:
To assess whether radiation treatment modality with proton beam therapy (PBT) or intensity-modulated radiation therapy (IMRT) is associated with lymphopenia in patients treated with definitive chemoradiation for esophageal cancer.
Methods and Materials:
Patients with esophageal cancer treated with bimodality therapy (n = 448) between 2004 and 2016 were retrospectively reviewed. Patients treated with PBT were matched by propensity score with those treated with IMRT, based on key patient and disease factors, and stratified by clinical disease stage. Patients who developed early, distant metastatic disease within 1 month of completing radiation were excluded. Univariable and multivariable logistic regression were used to identify variables associated with increased risk of grade 4 lymphopenia. Multivariable Cox proportional hazards regression was used to assess factors associated with overall survival, disease-free survival, and locoregional relapse-free survival.
Results:
Patients who had IMRT and PBT matched by propensity score (n = 220) were not different with respect to age, sex, stage, performance status, tumor location, histology, tumor target volume, or induction chemotherapy. Treatment with IMRT, compared with PBT (odds ratio [OR], 2.13; 95% confidence interval [95% CI], 1.19-3.81; P = .01), increased age (OR, 1.039/y increase; 95% CI, 1.003-1.076; P = .03), and greater planning target volume (OR, 3.47 per 1-unit increase in log (planning target volume); 95% CI, 1.67-7.21; P < .001), was associated with increased risk of grade 4 lymphopenia. Radiation modality was associated with lymphocyte reduction in patients with tumors in the lower esophagus (P = .005) but not for those with tumors in the upper or middle esophagus (P = 0.32).
Conclusions:
In patients with esophageal cancer treated with definitive chemoradiation, PBT reduces the risk of severe, treatment-related lymphopenia, particularly in tumors of the lower esophagus.
Keywords: lymphopenia, proton therapy, intensity-modulated radiation therapy, esophageal cancer, chemoradiation
Introduction
Lymphopenia during treatment for esophageal cancer [1–4] and other malignancies [5–9] is an independent predictor of worse clinical outcomes, including survival. Although chemoradiation (CRT) is the standard of care in the treatment of esophageal cancer [10], treatment-associated immunosuppression can occur to varying degree via mechanisms of direct lymphocyte depletion and blunting of lymphocyte responsiveness [11–14]. Recently, we reported that grades 3 and 4 lymphopenia during CRT occur in nearly 90% of patients [15]. In particular, sustaining grade 4 lymphopenia nadir during CRT was prognostic for poorer overall survival (OS) and cancer-specific outcomes. We identified several factors associated with a reduction in risk for grade 4 lymphopenia; one of which was the use of proton beam therapy (PBT) (versus intensity-modulated radiation therapy [IMRT]), and the other was the ability of patients to undergo surgery after completing CRT. It appeared that patients who were not able to complete trimodality therapy not only had worse survival outcomes but also had significantly greater risk of developing grade 4 lymphopenia during CRT. The degree that PBT can protect against grade 4 lymphopenia and affect clinical outcomes in these patients with higher risk is not well understood.
To better understand the lymphocyte-sparing effects of PBT in a cohort of patients treated definitively with CRT for reasons including selective observation after complete clinical response on positron emission tomography and endoscopy [16] or unsuitability for surgery, we conducted a propensity-matched analysis of PBT and IMRT.
Methods and Materials
Patients
We retrospectively evaluated patients treated at our institution with definitive CRT and either PBT or IMRT for esophageal cancer between March 2004 and June 2016. The institutional review board approved this study and waived the requirement for informed consent. To control for potential differences in patient selection for PBT or IMRT treatment, we conducted a propensity-matched analysis to select patients matched for key clinical risk factors. For each patient, we extracted clinical and disease characteristics, including age, gender, race, Karnofsky performance status (KPS), tumor location and histology, and cancer stage as classified by the American Joint Commission on Cancer 6th edition. Treatment characteristics including the use of induction or concurrent chemotherapy, surgery, radiation dose, radiation-treatment modality, and dosimetric parameters, including planning target volume (PTV) size, were obtained. Only patients treated nonsurgically with chemotherapy and radiation were included in this analysis. Patients with cervical tumor location or tumor histology other than squamous cell or adenocarcinoma were excluded. Peripheral blood absolute lymphocyte count (cells per cubic millimeter) was recorded before any therapy, including during induction chemotherapy and radiation therapy (RT), weekly during radiation treatment, and at first follow-up 1 month after completing radiation treatment. The Common Terminology Criteria for Adverse Events, version 4.0, was used to score lymphopenia.
Treatment
Patients were simulated supine in an upper-body cradle, with their arms abducted overhead. Four-dimensional computed tomography (CT) simulation was used to track tumor motion throughout the respiratory cycle because patients were free breathing when treated. The gross tumor volume was contoured based on the maximal-intensity projection from the 4-dimensional–CT scan, prior positron emission tomography imaging, and the results from endoscopy. The clinical target volume expansion was typically 3 cm superiorly and inferiorly and 1 cm radially, without violating anatomic boundaries. The PTV expansion was 0.5 cm concentrically. Daily kilovoltage imaging and weekly cone-beam CT was used to reduce setup errors. The IMRT plans were generated with the Pinnacle treatment planning system (version 9.0, Philips, Andover, Massachusetts). The PBT plans were generated using the Eclipse treatment planning system (Varian Medical Systems, Liverpool, New York). The IMRT plan optimization was achieved with direct machine parameter optimization and 7 to 9 coplanar and noncoplanar 6-MV photon beams, based on target volumes and nearby organs at risk. Patients were most commonly treated to 50.4 Gy (or cobalt gray equivalent) in 28 fractions (92%). A smaller group was treated to 45 Gy in 25 fractions (6.5%).
Propensity Score Matched Analysis
Age, log (PTV), clinical stage, KPS, tumor location, and treatment with induction chemotherapy were included in a multivariable logistic-regression model selected by backward elimination to obtain propensity scores (Supplementary Figure 1 (109.5KB, tif) ). We excluded patients who developed early, distant metastases within 1 month of radiation treatment because it is likely that those patients harbored occult, distant disease at presentation, which would confound any treatment effects. A total of 222 patients (111 in each IMRT and PBT) were selected by propensity score matching, and 220 (110 pairs) were included in the analysis after one pair was excluded for an outlying change in lymphocyte count.
Statistical Analysis
The OS, disease-free survival (DFS), locoregional relapse-free survival (LRRFS), and distant metastasis relapse-free survival were estimated by the Kaplan-Meier method. The time to event was calculated from the radiation treatment end date to the first occurrence of the considered event or censored at last follow-up.
Lymphopenia during radiation therapy was dichotomized to grade 4 lymphopenia versus grades 0 to 3 lymphopenia, and clinical and treatment factors of patients with and without grade 4 lymphopenia were compared by the 2-sample t test or Wilcoxon test for continuous variables and by χ2 test or Fisher exact test for categorical variables. We chose to differentiate between grade 4 and grades 0 to 3 lymphopenia out of interest for identifying factors causing clinically meaningful lymphopenia. Univariable and multivariable logistic-regression models were used to identify variables associated with increased risk of grade 4 lymphopenia. Multivariable Cox proportional hazards regression was used to model the independent effects of patient demographic, disease, and treatment factors associated with OS, DFS, and LRRFS. Patients were stratified by stage I and II versus III and IVA for survival analyses (because stage is known to be highly correlated with survival) to identify treatment-modality effects. To determine whether lymphocyte change during treatment was dependent on tumor location, separate multivariate linear regressions for patients with tumors located in the upper and middle versus lower esophagus, and clinical and treatment factors, including baseline lymphocyte count, radiation modality, PTV volume, and KPS, were performed. The estimated hazard ratios (HRs) and their 95% confidence intervals (95% CIs) were reported. Statistical tests were based on a 2-sided significance level, and a P value of < .05 was considered statistically significant. The SAS 9.4 software (SAS Institute Inc, Cary, North Carolina) was used for data analysis.
Results
Patient Characteristics
A total of 448 patients with esophageal received nonsurgical treatment with CRT and IMRT (n = 283) or PBT (n = 165) during the March 2004 to June 2016 period (Table 1). The median age was 68 years (range, 20-92 years), and the median follow-up was 55 months from the end of RT (95% CI, 48-64 months). The predominant histology was adenocarcinoma (AC; 75% of patients [336 of 448]), followed by squamous cell carcinoma (SCC; 25% of patients [112 of 448]), and most patients (59%; 264 of 448) had stage III disease followed by stage IIA disease (28%; 125 of 448). Ninety-two patients (21%) had early, distant metastases within 1 month of completion of radiation treatment and were excluded from the analysis. Of the remaining patients, 220 (110 with PBT and 110 with IMRT) were included in the analysis and were matched based on age, log (PTV), clinical stage, KPS, and tumor location. One matched pair was excluded because of an outlying change in lymphocyte count. The two matched groups did not show a significant differences in age, PTV, sex, clinical stage, length, KPS, tumor location, treatment with induction chemotherapy, or tumor histology (Table 2).
Table 1.
Patient and disease characteristics among all nonsurgical patients (n = 448).
|
Characteristic |
Patients, No. (%) |
| Median age at diagnosis, y | 68 (range, 20-92) |
| Sex | |
| Male | 371 (83) |
| Female | 77 (17) |
| Histology | |
| Adenocarcinoma | 336 (75) |
| Squamous cell carcinoma | 112 (25) |
| Stage | |
| I | 15 (3) |
| IIA | 124 (28) |
| IIB | 23 (5) |
| III | 264 (59) |
| IVA | 22 (5) |
| Tumor location | |
| Upper thoracic esophagus | 28 (6) |
| Middle thoracic esophagus | 77 (17) |
| Lower thoracic esophagus | 343 (77) |
| Karnofsky performance status | |
| 100 | 19 (4) |
| 90 | 165 (37) |
| 80 | 218 (49) |
| 70 | 46 (10) |
| Induction chemotherapy | |
| Yes | 132 (29) |
| No | 316 (71) |
| Radiation Modality | |
| PBT | 165 (37) |
| IMRT | 283 (63) |
| Median PTV (cm3) | 589 (range, 93-3125) |
Abbreviations: PBT, proton beam therapy; IMRT, intensity-modulated radiation therapy, PTV, planning treatment volume.
Table 2.
Distribution of patients, tumors, and treatment factors for proton beam therapy (PBT) and intensity-modulated radiation therapy (IMRT) in patients matched by propensity score (n = 220).a
|
Characteristic |
IMRT (n = 110) |
PBT (n = 110) |
P
value |
| Age (y), at diagnosis, mean, median (minimum-maximum) | 69, 69 (44-84) | 69, 70 (41-86) | .93 |
| PTV (cm3), mean, median (minimum-maximum) | 546, 508 (96-1570) | 534, 510 (104-1303) | .88 |
| Sex, No. (%) | .23 | ||
| Female | 25 (22.7) | 18 (16.4) | |
| Male | 85 (77.3) | 92 (93.6) | |
| Stage, No. (%) | .98 | ||
| I | 6 (5.5) | 4 (3.6) | |
| IIA | 34 (30.9) | 35 (31.8) | |
| IIB | 4 (3.6) | 4 (3.6) | |
| III | 62 (56.4) | 64 (58.2) | |
| IVA | 4 (3.6) | 3 (2.7) | |
| KPS, No. (%) | .82 | ||
| 70 | 11 (10) | 10 (9.1) | |
| 80-100 | 99 (90) | 100 (90.9) | |
| Tumor location in esophagus, No. (%) | 1.00 | ||
| Upper and middle | 26 (23.6) | 26 (23.6) | |
| Lower | 84 (76.4) | 84 (76.4) | |
| Induction chemotherapy, No. (%) | .88 | ||
| No | 79 (71.8) | 80 (72.7) | |
| Yes | 31 (28.2) | 30 (27.3) | |
| Histology, No. (%) | .44 | ||
| Adenocarcinoma | 84 (76.4) | 79 (71.8) | |
| Squamous cell carcinoma | 26 (23.6) | 31 (28.2) |
Abbreviations: PTV, planning treatment volume; KPS, Karnofsky performance status.
To calculate the propensity score, age, log (PTV), clinical stage, KPS, and tumor location were included.
Rates of Grade 4 Lymphopenia among All Nonsurgical Patients
Among all nonsurgical patients (n 448), 201 patients (45%) experienced grade 4 lymphopenia and 247 patients (55%) had grade 0 to 3 lymphopenia during the course of treatment.
Factors Associated with Grade 4 Lymphopenia among PBT/IMRT Patients Matched by Propensity Score (n = 220)
Among all factors examined in the cohort matched by propensity score (equal numbers of patients receiving IMRT [n = 110; 50%] and PBT [n = 110; 50%] patients), RT modality, PTV, tumor location, and age were significantly different in patients with and without grade 4 lymphopenia (Table 3). Patients experiencing grade 4 lymphopenia tended to have larger PTV treated. More patients treated with IMRT had grade 4 lymphopenia as compared with those treated with PBT (Table 3). On multivariable analysis, greater PTV (odds ratio [OR], 3.47 per 1 unit increase in log (PTV); 95% CI, 1.667-7.212; P < .001) and IMRT modality (OR = 2.13; 95% CI, 1.19-3.82; P = .01) were significant predictors of grade 4 lymphopenia (Table 4). When patients with tumors located in the upper and middle thoracic area versus those located lower were analyzed separately, patients with upper and middle tumors had greater lymphocyte reduction during treatment associated with PTV (P = .035) and baseline lymphocyte count (P < .001), but radiation modality was not significant (P = .32). However, in patients with tumors located lower in the thoracic cavity, greater lymphocyte reduction was associated with radiation modality (P = .005), PTV (P = .005), and baseline lymphocyte count (P < .001).
Table 3.
Comparison of propensity-matched patient, tumor, and treatment factors with and without grade 4 lymphopenia n = 220.
|
Characteristic |
Grade 0-3 Lymphopenia (n = 134) |
Grade 4 Lymphopenia (n = 86) |
P
valuea |
| Age, mean (SD) | 68.1 (9.1) | 70.6 (7.7) | .03 |
| PTV Volume (cm3), mean (SD) | 485 (225.61) | 625 (256.48) | <.0001 |
| Sex, No. (%) | .53 | ||
| Female | 28 (20.9) | 15 (17.4) | |
| Male | 106 (79.1) | 71 (82.6) | |
| Stage, No. (%) | .57 | ||
| I | 6 (4.5) | 4 (4.7) | |
| IIA | 46 (34.3) | 23 (26.7) | |
| IIB | 6 (4.5) | 2 (2.3) | |
| III | 71 (53) | 55 (64) | |
| IVA | 5 (3.7) | 2 (2.3) | |
| KPS, No. (%) | .40 | ||
| 70 | 11 (8.2) | 10 (11.6) | |
| 80-100 | 123 (91.8) | 76 (88.4) | |
| Tumor location in esophagus, No. (%) | .02 | ||
| Upper-middle | 39 (29.1) | 13 (15.1) | |
| Lower | 95 (70.9) | 73 (84.9) | |
| Induction chemotherapy, No. (%) | .72 | ||
| No | 98 (73.1) | 61 (70.9) | |
| Yes | 36 (26.9) | 25 (29.1) | |
| Histology, No. (%) | .47 | ||
| Adenocarcinoma | 97 (72.4) | 66 (76.7) | |
| Squamous cell carcinoma | 37 (27.6) | 20 (23.3) | |
| Radiation modality, No. (%) | .01 | ||
| IMRT | 58 (43.3) | 52 (60.5) | |
| PBT | 76 (56.7) | 34 (39.5) |
Abbreviations: PTV, planning treatment volume; KPS, Karnofsky performance status; IMRT, intensity-modulated radiation therapy; PBT, proton beam therapy.
Bolded values are significant.
Table 4.
Odds ratio (OR) and 95% confidence intervals (95% CI) for grade 4 lymphopenia in propensity-matched cohort (n = 220).
|
Characteristic |
Grade 4 lymphopenia, No. (%) |
OR (95% CI) |
P
valuea |
| Age (y) | 86 (39) | 1.036 (1.002-1.070) | .04b |
| Log (PTV) (per 1-unit increase) | 86 (39) | 3.775 (1.958-7.278) | .0001c |
| Sex | .53 | ||
| Female, n = 43 | 15 (35) | 1.00 | |
| Male, n = 177 | 71 (40) | 1.25 (0.62-2.51) | |
| Stage | .16 | ||
| I-II, n = 88 | 29 (33) | 1.00 | |
| III-IVA, n = 132 | 57 (43) | 1.50 (0.85-2.63) | |
| KPS | .40 | ||
| 70, n = 21 | 10 (48) | 1.47 (0.60-3.63) | |
| 80-100, n = 199 | 76 (38) | 1.00 | |
| Tumor location in esophagus | .02 | ||
| Upper-middle, n = 52 | 13 (25) | 1.00 | |
| Lower, n = 168 | 73 (43) | 2.31 (1.15-4.63) | |
| Induction chemotherapy | .72 | ||
| Yes, n = 61 | 25 (41) | 1.12 (0.61-2.04) | |
| No, n = 159 | 61 (38) | 1.00 | |
| Histology | .47 | ||
| Adenocarcinoma, n = 163 | 66 (40) | 1.00 | |
| Squamous cell carcinoma, n = 57 | 20 (35) | 0.79 (0.42-1.49) | |
| Radiation modality | |||
| IMRT, n = 110 | 52 (47) | 1.00 | |
| PBT, n = 110 | 34 (31) | 0.50 (0.29-0.87) | .01d |
Abbreviations: KPS, Karnofsky performance status, PTV, planning target volume.
Boldface type indicates statistical significance on univariate analysis.
In multivariable analysis, OR = 1.039/y increase; 95% CI, 1.003-1.076‘ P = .0339.
In multivariable analysis, OR = 3.47 per 1-unit increase in log (PTV); 95% CI, 1.667-7.212; P = .0009.
In multivariable analysis, OR = 0.469; 95% CI, 0.262-0.840; P = .0109.
Survival Analyses
In the propensity-matched cohort (n = 220), stage III to IVA disease (HR, 2.10; 95% CI, 1.39-3.17; P < .001), greater PTV (HR, 1.47 per 1-unit increase in log [PTV]; 95% CI, 0.99-2.18; P = .05), and greater reduction in lymphocyte count during treatment (HR, 1.55 per 1 unit reduction from baseline to nadir; 95% CI, 1.09-2.20; P = .01) were associated with worse OS (Table 5). On multivariable analysis, no factors remained significantly associated with OS. Age, sex, performance status, tumor location within the esophagus, treatment with induction chemotherapy, and radiation modality were not associated with worse OS. In patients with stage III to IVA disease, IMRT (HR, 1.52; 95% CI, 0.96-2.41; P = .08) showed a marginally significant association with OS on univariable analysis (Figure) but was not significant (HR for IMRT, 1.48; 95% CI, 0.93-2.35; P = .10) on multivariable analysis when adjusting for log (PTV). Stage III to IVA disease (HR, 1.64; 95% CI, 1.15-2.34; P = .007) and greater reduction in lymphocyte count during treatment (HR, 1.62; 95% CI, 1.18-2.22; P = .003) were factors significantly associated with worse DFS on univariable analysis. In patients with stage III or IVA disease, IMRT (HR, 1.50; 95% CI, 0.98-2.31; P = .06) trended toward significant association with DFS on univariable analysis (Supplementary Figure 2 (114.4KB, tif) ) but was not significantly associated with DFS (HR for IMRT, 1.42; 95% CI, 0.92-2.19; P = .11) in a multivariable model adjusting for log (PTV) and lymphocyte count reduction. None of the above clinical or treatment factors were significantly associated with LRRFS.
Table 5.
Hazard ratio (HR) and 95% confidence interval (95% CI) for overall survival in propensity-matched cohort (n = 220).
|
Characteristic |
HR (95% CI) |
P
valuea |
| Age (y) | 1.001 (0.979-1.022) | .95 |
| Log (PTV) (per 1-unit increase) | 1.472 (0.994-2.180) | .05 |
| Lymphocyte reduction (baseline-nadir) (per 1-unit increase) | 1.551 (1.092-2.203) | .01 |
| Sex | .21 | |
| Female | 1.00 | |
| Male | 1.37 (0.84-2.25) | |
| Stage | .0004 | |
| I-II | 1.00 | |
| III-IVA | 2.10 (1.39-3.17) | |
| KPS | .46 | |
| 70 | 1.26 (0.68-2.36) | |
| 80-100 | 1.00 | |
| Tumor location in esophagus | .85 | |
| Upper-middle | 1.00 | |
| Lower | 1.04 (0.68-1.59) | |
| Induction chemotherapy | .61 | |
| Yes | 0.90 (0.58-1.37) | |
| No | 1.00 | |
| Histology | .62 | |
| Adenocarcinoma | 1.00 | |
| Squamous cell carcinoma | 1.11 (0.74-1.67) | |
| Grade 4 lymphopenia | .81 | |
| No | 1.000 | |
| Yes | 1.05 (0.72-1.54) | |
| Radiation modality | .30b | |
| IMRT | 1.00 | |
| PBT | 0.82 (0.56-1.20) | |
Abbreviations: KPS, Karnofsky performance status; IMRT, intensity-modulated radiation therapy; PBT, proton beam therapy.
Boldface type indicates statistical significance on univariate analysis.
In multivariable analysis stratifying by stage adjusting for log (PTV), HR = 0.68; 95% CI, 0.43-1.08; P = 10 in patients with stage III/IVA carcinoma.
Figure.
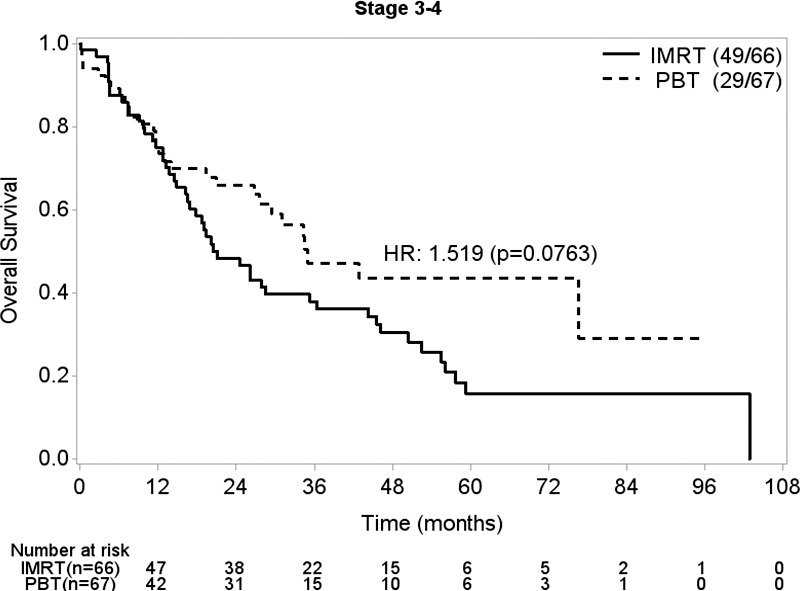
Kaplan-Meier analysis of overall survival in patients with stage III to IVA disease by radiation treatment modality.
Discussion
In this study with 200 patients matched by propensity score and treated definitively with PBT or IMRT and chemotherapy for esophageal cancer, we found that treatment with IMRT compared with PBT, greater PTV, and increased age were predictive of grade 4 lymphopenia. In other prior studies, lymphopenia has been associated with worse clinical outcomes in cancer treatment [1–9], likely through a combination of increased infection risk [17] and blunted antitumor immune-cell response [18–20].
A question of particular interest in this study was whether the modality-specific advantages of PBT over IMRT [21] could also be lymphocyte-sparing, and our study supports that hypothesis. Treatment with IMRT versus PBT, among patients robustly matched for age, volume treated/PTV, clinical stage, KPS, tumor location, and treatment with induction chemotherapy, was significantly associated with worse rates of clinically meaningful grade 4 lymphopenia. In addition, in patients with tumors located lower in the esophagus, radiation modality was significantly associated with lymphocyte reduction on multivariate analysis but that relationship was not significant in patients with tumors located in the upper and middle thoracic. This location dependence suggests that potential lymphocyte-sparing effects of PBT may be most pertinent for limiting integral doses to organs adjacent to the lower esophagus and gastroesophageal junction, such as the spleen. The spleen, in particular, which is a large lymphocyte reservoir, could be more likely to receive integral doses [22, 23]. In addition, circulating lymphocytes in other organs, such as the lung, liver, and stomach, could also be at risk [24, 25]. The relative contribution of dose to each of the lymphocyte-rich organs is a subject of future predictive-modeling analysis. We also found greater PTV to be associated with a greater risk of grade 4 lymphopenia, which supports the hypothesis that a larger volume of low-integral dose to surrounding lymphocyte-harboring organs enhances the risk of lymphopenia. These findings have important implication for treatment planning because beam arrangements could be made to minimize specific organ doses so as to mitigate lymphopenia risk in patients. Additionally, other potential lymphocyte-sparing radiation treatment strategies could be considered, such as hypofractionation [12, 26].
Our study was limited by the retrospective nature of this analysis, and despite the attempt to balance the 2 radiation cohorts with robust propensity-matched methods, there still could be hidden confounding factors that would be less problematic had this been a randomized trial comparison. However, given the fact that the selection for PBT or IMRT was done at a single institutional level, there were minimal biases in the contouring and planning approaches performed per the standard operating procedures of the thoracic and the physics groups. Selection to use PBT was primarily motivated by insurance coverage; therefore, older patients with Medicare tended to be the patients treated with PBT. Occasionally, patients with large PTV because of larger tumors were selected to receive PBT because there would be a smaller integral dose to the organs at risk. Although the results trended toward significance, we did not find that radiation modality was associated with clinical outcomes in this study, which may have been due to insufficient number of events because of the small sample size.
Conclusion
In patients with poorer-risk esophageal cancer treated nonsurgically with definitive CRT, IMRT, as compared with PBT, and greater PTV were strongly associated with grade 4 lymphopenia. A further study to characterize the dosimetric parameters necessary to spare patients grade 4 lymphopenia is ongoing.
Supplementary Material
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts of interest relevant to the current work. B.P.H. reports a consultantship with Ignyta, Inc (San Diego, California), outside the submitted work. S.H.L. reports grants from Elekta (Stockholm, Sweden), STCube Pharmaceuticals (Gaithersburg, Maryland), Hitachi Chemicals (Tokyo, Japan), Peregrine Pharmaceuticals (Tustin, California), and honorarium from AstraZeneca (Cambridge, United Kingdom), outside the submitted work.
Acknowledgments: The statistical analysis was supported in part by a Cancer Center Support Grant (National Cancer Institute Grant P30 CA016672).
Prior Presentations: This work was presented in part at the 99th Annual Meeting of the American Radium Society; May 6-9, 2017; Colorado Springs, Colorado.
References
- 1.Feng JF, Liu JS, Huang Y. Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine (Baltimore) 2014;93:e257. doi: 10.1097/MD.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirahara N, Matsubara T, Mizota Y, Ishibashi S, Tajima Y. Prognostic value of preoperative inflammatory response biomarkers in patients with esophageal cancer who undergo a curative thoracoscopic esophagectomy. BMC Surg. 2016;16:66. doi: 10.1186/s12893-016-0179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messager M, Neofytou K, Chaudry MA, Allum WH. Prognostic impact of preoperative platelets to lymphocytes ratio (PLR) on survival for oesophageal and junctional carcinoma treated with neoadjuvant chemotherapy: a retrospective monocentric study on 153 patients. Eur J Surg Oncol. 2015;41:1316–23. doi: 10.1016/j.ejso.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Yoo EJ, Park JC, Kim EH, Park CH, Shim CN, Lee HJ, Chung HS, Lee H, Shin SK, Lee SK, Lee CG, Lee YC. Prognostic value of neutrophil-to-lymphocyte ratio in patients treated with concurrent chemoradiotherapy for locally advanced oesophageal cancer. Dig Liver Dis. 2014;46:846–53. doi: 10.1016/j.dld.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Cho O, Chun M, Chang SJ, Oh YT, Noh OK. Prognostic value of severe lymphopenia during pelvic concurrent chemoradiotherapy in cervical cancer. Anticancer Res. 2016;36:3541–7. [PubMed] [Google Scholar]
- 6.Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, Campian J, Laheru D, Zheng L, Wolfgang CL, Tran PT, Grossman SA, Herman JM. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2015;38:259–65. doi: 10.1097/COC.0b013e3182940ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendez JS, Govindan A, Leong J, Gao F, Huang J, Campian JL. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J Neurooncol. 2016;127:329–35. doi: 10.1007/s11060-015-2037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, Davoli C, Navaglia F, Zambon CF, Pasquali C, Venza E, Pedrazzoli S, Plebani M. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32:22–8. doi: 10.1097/01.mpa.0000188305.90290.50. [DOI] [PubMed] [Google Scholar]
- 9.Balmanoukian A, Ye X, Herman J, Laheru D, Grossman SA. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30:571–6. doi: 10.3109/07357907.2012.700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A, CROSS Study Group Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 11.Stratton JA, Byfield PE, Byfield JE, Small RC, Benfield J, Pilch Y. A comparison of the acute effects of radiation therapy, including or excluding the thymus, on the lymphocyte subpopulations of cancer patients. J Clin Invest. 1975;56:88–97. doi: 10.1172/JCI108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crocenzi T, Cottam B, Newell P, Wolf RF, Hansen PD, Hammill C, Solhjem MC, To Y-Y, Greathouse A, Tormoen G, Jutric Z, Young K, Bahjat KS, Gough MJ, Crittenden MR. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J Immunother Cancer. 2016;4:45. doi: 10.1186/s40425-016-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen EM, Fan S, Rockwell S, Goldberg ID. The molecular and cellular basis of radiosensitivity: implications for understanding how normal tissues and tumors respond to therapeutic radiation. Cancer Invest. 1999;17:56–72. [PubMed] [Google Scholar]
- 14.Stewart CC, Perez CA. Effect of irradiation on immune responses. Radiology. 1976;118:201–10. doi: 10.1148/118.1.201. [DOI] [PubMed] [Google Scholar]
- 15.Davuluri R, Jiang W, Fang P, Xu C, Komaki R, Gomez DR, Welsh J, Cox JD, Crane CH, Hsu CC, Lin SH. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:128–35. doi: 10.1016/j.ijrobp.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Swisher SG, Moughan J, Komaki RU, Ajani JA, Wu TT, Hofstetter WL, Konski AA, Willet CG. Final results of NRG oncology RTOG 0246: an organ-preserving selective resection strategy in esophageal cancer patients treated with definitive chemoradiation. J Thorac Oncol. 2016;12:368–374. doi: 10.1016/j.jtho.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohira M, Kubo N, Yamashita Y, Sakurai K, Toyokawa T, Tanaka H, Muguruma K, Hirakawa K. Impact of chemoradiation-induced myelosuppression on prognosis of patients with locally advanced esophageal cancer after chemoradiotherapy followed by esophagectomy. Anticancer Res. 2015;35:4889–95. [PubMed] [Google Scholar]
- 18.Liu J, Li F, Ping Y, Wang L, Chen X, Wang D, Cao L, Zhao S, Li B, Kalinski P, Thorne SH, Zhang B, Zhang Y. Local production of the chemokines CCL5 and CXCL10 attracts CD8+ T lymphocytes into esophageal squamous cell carcinoma. Oncotarget. 2015;6:24978–89. doi: 10.18632/oncotarget.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fumagalli LA, Vinke J, Hoff W, Ypma E, Brivio F, Nespoli A. Lymphocyte counts independently predict overall survival in advanced cancer patients: a biomarker for IL-2 immunotherapy. J Immunother. 2003;26:394–402. doi: 10.1097/00002371-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–46. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren S, Partridge M, Bolsi A, Lomax AJ, Hurt C, Crosby T, Hawkins MA. An analysis of plan robustness for esophageal tumors: comparing volumetric modulated arc therapy plans and spot scanning proton planning. Int J Radiat Oncol Biol Phys. 2016;95:199–207. doi: 10.1016/j.ijrobp.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koturbash I, Merrifield M, Kovalchuk O. Fractionated exposure to low doses of ionizing radiation results in accumulation of DNA damage in mouse spleen tissue and activation of apoptosis in a p53/Atm-independent manner. Int J Radiat Biol. 2016;93:148–155. doi: 10.1080/09553002.2017.1231943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Zhao Q, Deng W, Lu J, Xu X, Wang R, Li X, Yue J. Radiation-related lymphopenia is associated with spleen irradiation dose during radiotherapy in patients with hepatocellular carcinoma. Radiat Oncol. 2017;12:90. doi: 10.1186/s13014-017-0824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin SH, Hallemeier CL, Chuong M. Proton beam therapy for the treatment of esophageal cancer. Chin Clin Oncol. 2016;5:53. doi: 10.21037/cco.2016.07.04. [DOI] [PubMed] [Google Scholar]
- 25.Heier HE. The influence of therapeutic irradiation of blood and peripheral lymph lymphocytes. Lymphology. 1978;11:238–42. [PubMed] [Google Scholar]
- 26.Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, Rosati LM, Hacker-Prietz A, Assadi RK, Saeed AM, Pawlik TM, Jaffee EM, Laheru DA, Tran PT, Weiss MJ, Wolfgang CL, Ford E, Grossman SA, Ye X, Ellsworth SG. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;94:571–9. doi: 10.1016/j.ijrobp.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


