Abstract
Skeletal muscle has an extraordinary regenerative capacity due to the activity of tissue-specific muscle stem cells (MuSCs). Consequently, these cells have received the most attention in studies investigating the cellular processes of skeletal muscle regeneration. However, efficient capacity to rebuild this tissue also depends on additional cells in the local milieu, as disrupting their normal contributions often leads to incomplete regeneration. Here, we review these additional cells that contribute to the regenerative process. Understanding the complex interactions between and among these cell populations has the potential to lead to therapies that will help promote normal skeletal muscle regeneration under conditions where this process is suboptimal.
Introduction
Skeletal muscle makes up approximately 35% of the total mass of a human, making it collectively one of the largest organ systems (Janssen et al., 2000). This organ provides an individual with a means to generate power for motion, a depot for energy stores, and a system for thermoregulation (Frontera and Ochala, 2014; Rowland et al., 2014). A defining characteristic of skeletal muscle is its remarkable capacity for regeneration, a highly coordinated cellular process that initiates with an inflammatory response, proceeds through a necrotic cycle, and continues on a regenerative course that leads to a fully restored tissue similar to uninjured skeletal muscle (Chargé and Rudnicki, 2004; Tidball, 2011). Interference with these synchronized processes generally results in fatty, fibrotic, and/or boney deposition, which prevents full anatomical and functional restoration. The normal process of aging and pathological processes, such as those that occur in the muscular dystrophies and heterotopic ossification, are notable examples of when this process is perturbed and leads to atypical tissue infiltration in skeletal muscle (Davies et al., 2017; Mann et al., 2011; Pretheeban et al., 2012). Regardless of the cause, failure of muscle to regenerate properly can have a severe impact on the organism. Therefore, the process of muscle regeneration following injury has been an intensely investigated area with the hope that an increased understanding will lead to therapies to restore optimal skeletal muscle structure and function when the regenerative process is impaired.
The areas of focus for this review are the cellular processes following injury and, specifically, the necessary influence of non-myogenic cells on the muscle stem cells (MuSCs; also known as satellite cells) during regeneration. MuSCs have garnered the most attention in this process and, thus, the molecular regulation and fate determinants of MuSCs have been extensively reviewed (Fukada, 2018; Relaix and Zammit, 2012; Segalés et al., 2016). Additionally, while the extracellular matrix (ECM) has proven to be rich in signaling factors and an integral participant in regeneration (Bentzinger et al., 2013), this lies outside of the cellular focus of this review. Hence, we will briefly review the historical findings of MuSC necessity in regeneration and then focus our attention on supporting cells.
The Stem Cells – MuSCs
Over half a century ago, Alexander Mauro first identified the MuSC in electron micrographs of skeletal muscle noting a cell with its own plasmalemma sharing the basal lamina of a muscle fiber (Mauro, 1961). He termed this cell a “satellite cell” and predicted that it contributed to muscle regeneration as a cellular source for myofiber repair. In the decades since, MuSCs have been studied in detail both in vitro and in vivo, and at all stages of life. Collectively, these studies have shown that MuSCs originate from multipotent mesodermal progenitors of the embryonic dermamyotome and become committed to myogenesis at late stages of embryogenesis (Lepper and Fan, 2010). Adult MuSCs maintain this commitment in most normal and pathological environments (Joe et al., 2010; Kanisicak et al., 2009; Lounev et al., 2009). A notable exception is the propensity of aged MuSCs to adopt fibrogenic phenotypes in the setting of aged or dystrophic muscle (Biressi et al., 2014; Brack et al., 2007). Adult MuSCs largely remain mitotically quiescent in homeostatic conditions with very low turnover rates over the course of a lifespan (Chakkalakal et al., 2012). In this state, MuSCs remain closely apposed to myofibers, receiving signals directly from the myofibers and from factors in the local milieu (Yin et al., 2013). Communication with adjacent endothelial cells (ECs), perhaps by direct cell-cell contact, has also been suggested (Christov et al., 2007), and additional data supports a role for this interaction in the maintenance of MuSC quiescence (Abou-Khalil et al., 2009). However, following injury to skeletal muscle, MuSCs activate and enter the cell cycle. Their rapid proliferation (Fig. 1) produces daughter cells that either self-renew to maintain the MuSC pool or differentiate to become myoblasts for myofiber production and repair (Motohashi and Asakura, 2014). These properties satisfy the prerequisites of a tissue-specific adult stem cell and confirm Mauro’s hypothesis of MuSC contribution to the regenerative process.
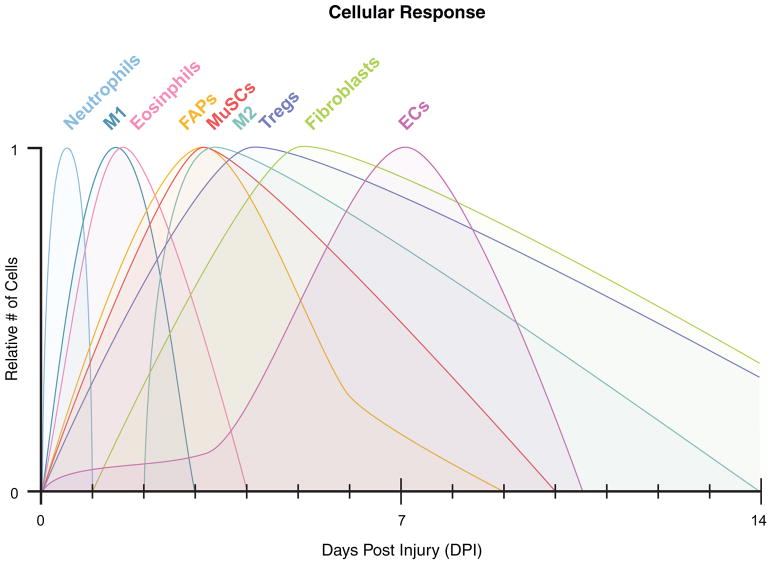
Figure 1. Cellular dynamics during the regenerative response of skeletal muscle.
Following injury, there is a cascade of cellular expansion followed by reduction that occurs during the first 14 days of the regenerative response. For many of these populations in the regenerative milieu, the maximum expansion varies with the type of injury and estimates vary depending on the assay used to assess cell numbers or percentages. Thus, the relative abundance of each population of cells is normalized to 1 on the y-axis. M1 and M2 – macrophages; FAPs – fibroadipogenic progenitors; MuSCs – muscle stem cells; Tregs – regulatory T cells; ECs – endothelial cells.
Many of these findings would not have been possible without three scientific advances: 1) transgenic tools allowing the genetic targeting of MuSCs (Kanisicak et al., 2009; Lepper et al., 2009); 2) fluorescence activated cell sorting providing a means to isolate highly pure MuSCs (Fukada et al., 2004; Liu et al., 2015; Yi and Rossi, 2011); and 3) single fiber isolation that maintains the immediate MuSC niche (Bischoff, 1986). The field has benefited particularly from the specificity of the expression in skeletal muscle of Pax7 in adult MuSCs (Seale et al., 2000) (Table 1), exploiting this gene in many recombineering strategies for lineage tracing, genetic manipulation, and cell ablation experiments. In fact, in vivo MuSC ablation studies have confirmed the necessary role of MuSCs in skeletal muscle regeneration (Fry et al., 2015; Lepper et al., 2011; Murphy et al., 2011; Sambasivan et al., 2011). In mice depleted of MuSCs, there is a complete lack of formation of nascent fibers following injury, which supports the role of MuSCs as the chief myogenic progenitors in the adult. The isolation of MuSCs by FACS has allowed for the precise molecular characterization of MuSC quiescence, activation, proliferation, and differentiation, processes that continue to be studied in great detail (Almada and Wagers, 2016). Of course, isolating cells removes them from the influences of their niche, some of which can be maintained by studying MuSCs on isolated single fibers, an ex vivo preparation which preserves the sublaminar environment (Bischoff, 1986). In recent years, cells found in the interstitial spaces (i.e., between myofibers) have gained attention as additional, integral constituents of the MuSC niche, providing essential support for MuSC function (discussed in detail below). However, the roles of all of these cellular players remains incompletely characterized, largely because of the complexity of cell-cell interactions both at rest and during regeneration, and also because of the incomplete mapping and characterization of all of the cellular components.
Table 1.
Genes that identify cells of the regenerative milieu.
| Cell Type | Markers | References |
|---|---|---|
| Muscle Stem Cells (MuSCs) | Pax7, Vcam1, SM/C2.6, α7-Integrin/CD34 | Fukada et al., 2004; Joe et al., 2010; Liu et al., 2015; Seale et al., 2000 |
| Fibroadipogenic Progenitors (FAPs) | Sca-1, PDGFRα, Tie-2 | Joe et al., 2010; Uezumi et al., 2010; Wosczyna et al., 2012 |
| M1 Macrophages | CD45, CD11b, CD68, Ly6c-high | Arnold et al., 2007; Villalta et al., 2009 |
| M2 Macrophages | CD45, CD11b, CD163, CD206 | Arnold et al., 2007; Villalta et al., 2009 |
| Eosinophils | CD45, CD11b, Siglec F | Heredia et al., 2013 |
| Regulatory T Cells (Tregs) | CD25, Fox3p, CD4 | Burzyn et al., 2013; Kuswanto et al., 2016 |
| Neutrophils | Ly6C, F4/80 negative | Tidball et al., 2010 |
| Vascular Endothelial Cells (ECs) | CD31, VwF, VE-Cadherin | Abou-Khalil et al., 2009; Christov et al., 2007; Wosczyna et al., 2012 |
| Pericytes | CD146, NG2, ALP, PDGFRβ | Dellavalle et al., 2007; Kostallari et al., 2015; Wosczyna et al., 2012; Zhu et al., 2008 |
| Fibroblasts | αSMA, ER-TR7, FSP-1, Tcf4 | Joe et al., 2010; Mathew et al., 2011; Uezumi et al., 2010 |
The Support Group – Cells of the Regenerative Milieu
Following injury to skeletal muscle, there is a temporally defined cellular response involving both tissue-resident cells and cells infiltrating from the general circulation. Although many different methods have been used to induce muscle injury in order to study the regenerative response, we will not address the differences between them but will focus on the processes that are common to the regenerative process in response to most types of injury.
Interstitial Cells with Myogenic Potential
Before enumerating cellular participants with the potential to influence MuSC dependent myogenesis, it is important to mention that there are cell populations in muscle that have previously been shown to possess myogenic potential, but without the ability to rescue myogenesis following injury in the absence of MuSCs (Lepper et al., 2011; Murphy et al., 2011; Sambasivan et al., 2011). Cells such as myoendothelial cells (Zheng et al., 2007), CD133+ cells (Torrente et al., 2004), PW1 interstitial cells (PICs) (Mitchell et al., 2010), muscle-derived stem cells (MDSCs) (Qu-Petersen, 2002), Twist2+ progenitor cells (N. Liu et al., 2017), and muscle side population (SP) cells (Majka et al., 2003) are members of this category. The ability of these cells to support MuSCs during regeneration has not yet been determined and, thus, they will not be discussed in detail. Many of these cells receive continued interest in the realm of myogenic cell transplantation therapies as a substitute for MuSCs, since using MuSCs has been ineffective due to low engraftment, a limited ability to migrate, and a large amount cell death following transplantation (Negroni et al., 2011; Tedesco et al., 2010). It is possible that future experiments in which these cells either are depleted or genetically targeted to modulate secreted factors may reveal novel roles in the process of regenerative myogenesis.
Fibroadipogenic Progenitors (FAPs)
The interstitial space between myofibers is rich with progenitor cells many of which have only been identified in the past decade. Mesenchymal progenitor cells that have received considerable attention are termed fibroadipogenic progenitors (FAPs) (Joe et al., 2010; Uezumi et al., 2010) (Table 1). As the name suggests, these cells are believed to be the chief mediators of fatty and fibrotic tissue accumulation in skeletal muscle as seen in muscle of aged individuals or muscular dystrophy patients (Uezumi et al., 2011). These cells are multipotent, capable not only of adipogenesis and fibrogenesis, but also of chondrogenesis and osteogenesis (Wosczyna et al., 2012). Consequently, these cells are thought to be more broadly mesenchymal in nature (Table 1).
FAPs share many molecular and functional properties with mesenchymal stem cells (MSCs) and, thus, many of their proposed functions in regeneration have been aligned with those of MSCs. The MSC classification is somewhat ambiguous since members are molecularly and functionally heterogeneous. However, many of the cells that are classified as such share a minimum set of characteristics (e.g., express cell surface markers like PDGFRα and CD73, and have the ability to differentiate into multiple mesodermal lineages) (Dominici et al., 2006). There is also ample evidence that the majority of these cells are necessary constituents of the regenerative milieu in numerous tissues (Park et al., 2012; Stzepourginski et al., 2017). FAPs meet these necessary characteristics and, for these reasons, together with data from in vitro studies (Joe et al., 2010; Uezumi et al., 2010), have been assumed to have a role in supporting the regenerative process in injured skeletal muscle.
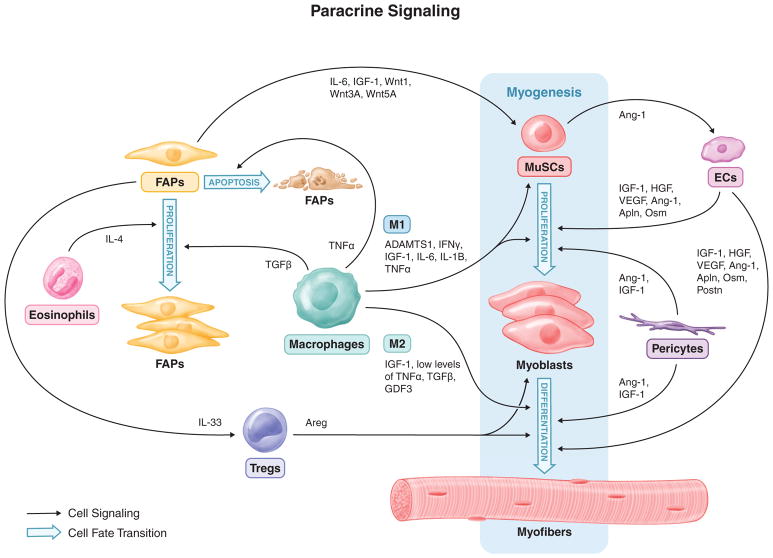
Following skeletal muscle injury in mice, FAPs rapidly expand, mirroring the proliferative dynamics of MuSCs (Fig. 1). Around three days post injury (dpi), FAPs reach a maximum in number and then quickly recede to normal levels around 7 dpi (Joe et al., 2010; Lemos et al., 2015; 2012; Uezumi et al., 2010). Genetically targeting these cells remains difficult due to the lack of a unique marker and therefore, the necessity of these cells in the regenerative process has not been definitely determined. Nevertheless, numerous studies have coupled in vivo assays with ex vivo analyses to suggest FAPs support MuSC differentiation and a regenerative milieu with the optimal extracellular matrix remodeling and the correct cellular constituents. The initial publication describing these cells used a co-culture assay to show that FAPs increase the amount of myogenic differentiation observed with MuSC-derived myoblasts (Joe et al., 2010). The authors attributed this increase in myogenesis to FAP-dependent expression and secretion of insulin-like growth factor-1 (IGF-1), interleukin-6 (IL-6), Wnt1, Wnt3a, and Wnt5a, which are presumed to target myogenic cells (Fig. 2). In fact, IL-6 was the most significantly increased following injury, at about 10-fold when compared to cells in homeostatic conditions. Multiple cell types in the regenerative milieu are known to secrete IL-6 making it difficult to assess the influence of this cytokine being secreted from specific cells (Muñoz-Cánoves et al., 2013). Future IL-6 loss-of-function experiments in co-cultures of FAPs and MuSCs or the in vivo deletion of IL-6 specifically in FAPs during muscle regeneration will be needed to assess the direct impact of FAP-derived IL-6 on myogenesis. Consequently, the increase in myogenesis observed in the co-cultures between MuSCs and FAPs has yet to be specifically attributed to FAPs secreting IL-6 or the other noted factors.
Figure 2. Paracrine signaling between MuSCs and the cells of the regenerative milieu.
The regenerative milieu is a dynamic singling environment with at least one goal being the nurturing of MuSCs to expand, differentiate to myoblasts, and finally fuse to repair damaged myofibers and create nascent myofibers. The cells of the regenerative milieu have a coordinated response to secrete a multitude of cytokines that target MuSCs and one another to accomplish this regenerative endeavor and rebuild functional skeletal muscle. M1 and M2 – macrophages; FAPs – fibroadipogenic progenitors; MuSCs – muscle stem cells; Tregs – regulatory T cells; ECs – endothelial cells; Apln – Apelin; Postn – Periostin, Osm – Oncostatin M.
In addition to FAPs supporting the regenerative dynamics of MuSCs, there is also evidence of crosstalk with infiltrating immune cells to aid in supporting an environment conducive to regeneration. A recent publication revealed that FAP numbers during regeneration are regulated by cytokines from inflammatory macrophages (Lemos et al., 2015). Following the surge of FAP proliferation around 3 dpi, macrophages target FAPs for apoptosis by secreting tumor necrosis factor α (TNFα) (Fig. 1 and 2). These inflammatory macrophages then switch to a pro-regenerative phenotype, secreting transforming growth factor β (TGFβ) to induce the secretion of extracellular matrix by FAPs. However, if this precise switch between macrophage subtypes is perturbed and a hybrid phenotype secreting both factors is established, TGFβ will dominate the response leading to increased ECM deposition by FAPs and pathological fibrosis. Thus, FAPs play a critical role in maintaining the local environment during regeneration. Additionally, a recent study (discussed in the eosinophils section below) revealed the ability of FAPs to clear necrotic debris through phagocytosis (Heredia et al., 2013). To confirm whether FAPs do indeed exhibit this phagocytic activity in vivo, additional experiments will need to demonstrate this process occurring during and being necessary for the regenerative process.
Whereas there is evidence suggesting that FAPs may be necessary for effective skeletal muscle regeneration, molecular heterogeneity makes FAPs difficult to genetically target for loss-of-function studies. Without these types of analyses, cause-and-effect relationships remain to be determined. Furthermore, their functional heterogeneity lead to a confusing relationship between cells that share some molecular similarities, but have varying fate potentials. For example, a recent report identified Twist2+ mesenchymal-like cells in the interstitial space of muscle (N. Liu et al., 2017). The authors found that these cells may be similar to FAPs in terms of surface markers, but that they are functionally distinct, participating in type II myofiber regeneration. Similarly, FAPs have been proposed to be a subpopulation of Sca1+ PICs, but again, these cells have the capacity for myogenic differentiation (Pannérec et al., 2013). However, other reports of FAPs do not observe myogenic potential from isolated cells (Joe et al., 2010; Uezumi et al., 2010; Wosczyna et al., 2012). These inconsistent results could be due to a technical variance, such as one assay being more permissive to myogenesis than another, or a difference in the cell populations studied due to differing isolation protocols. Regardless, these types of discrepancies denote the difficulty encountered when trying to determine the definitive biological roles of these cells. Future single cell sequencing of FACS-isolated FAPs may lead to a better understanding of population heterogeneity and possibly provide markers to subdivide these populations for genetic targeting in vivo and functional analyses. If this additional fractionation into unique cell populations can be accomplished, investigators will be better able to assess the relationships between FAPs and other cells that share some of these molecular and functional features.
Macrophages
The most immediate cellular response to muscle injury is the infiltration of immune cells (Tidball, 2011). These include cells of the myeloid and lymphoid lineages. Recent publications have confirmed macrophages as key components of the early mononuclear cell infiltrate in the skeletal muscle regenerative response (Fig. 2). The canonical macrophage functions of free radical production to stimulate the breakdown of damaged tissue and phagocytosis to clear the area of debris have been well established (Tidball, 2005). However, macrophages have now been revealed to signal to MuSCs and FAPs, and thus to be important mediators of the cellular response. For simplicity, macrophages will be discussed using the M1 and M2 terminology, signifying the transition from an inflammatory to a regenerative phenotype. However, it is now apparent that this transition is not represented by distinct phenotypes, but more of a continuum of phenotypes (Novak and Koh, 2013).
Inflammatory M1 macrophages are the first to appear in the degenerative milieu (Table 1, Fig. 1). There, they secrete cytokines, such as a disintegrin-like and metalloproteinase with thrombospondin type 1 motif (ADAMTS1) (Du et al., 2017), interferon γ (IFNγ) (Cheng et al., 2008; Kelić et al., 1993), IGF-1 (Tonkin et al., 2015), IL-6 (Wang et al., 2008), IL-1β (Broussard et al., 2004), and TNFα (Langen et al., 2004) to stimulate MuSC proliferation while simultaneously limiting MuSC differentiation and fusion (Saclier et al., 2013a; Tidball and Villalta, 2010) (Fig. 2). During this phase of regeneration, there is thus a prominent scavenger activity with the phagocytoses of debris by immune cells and an active proliferation of MuSCs. Furthermore, inflammatory (presumably M1) macrophages were recently shown to secrete TNFα during regeneration to target FAPs for apoptosis, thereby minimizing the transition of FAPs to detrimental fibrocytes (discussed in the FAP section above) (Lemos et al., 2015) (Fig. 2). Following this phase, M2 macrophages become the dominant macrophage phenotype appearing in the tissue (Table 1, Fig. 1). A recent publication supports an IL-10 dependent M1-to-M2 phenotype transition versus independent lineages for the origin of these two macrophage populations (Deng et al., 2012). If this transition is delayed and the M1 phenotype prolonged, inflammatory cytokines persist and myogenesis is impaired, demonstrating the importance of this precisely timed phenotypic switch. Once M2 macrophages are present, they mark the beginning of the regenerative phase and support myogenesis by initially secreting high levels of IGF-1, which supports ongoing MuSC proliferation (Tonkin et al., 2015), and then low levels of TNFα, TGFβ (Saclier et al., 2013b), and growth differentiation factor 3 (GDF3) (Varga et al., 2016), which foster myogenic differentiation (Juban and Chazaud, 2017; Novak and Koh, 2013; Saclier et al., 2013a) (Fig. 2). There is evidence that having too many M2 macrophages in skeletal muscle may result in fibrosis (X. Wang et al., 2015). According to this study, the M2a subtype of pro-regenerative macrophages accumulates in aged muscle leading to increased fibrosis. While this result may be related to the aging milieu, a prolonged M2 phase following injury in young animals may likewise lead to aberrant regeneration.
To accurately assess the role of macrophages in the regenerative process, cell ablation experiments have been conducted by multiple groups and all observe decreased myogenesis and increased fibrosis following injury in skeletal muscle in the absence of macrophage populations (Lemos et al., 2015; X. Liu et al., 2017; Shen et al., 2008; Xiao et al., 2016). Consistent with the functions of macrophages discussed above, macrophage ablation led to an increase in inflammatory cytokines and a decrease in pro-myogenic factors. These macrophage ablation studies did not discriminate between M1 and M2 phenotypes, and they resulted in the depletion of macrophages throughout the entire organism. Therefore, the effects of systemic depletion of macrophages on tissue regeneration also needs to be considered in addition to the effects of local depletion. Mouse models that conditionally and locally target macrophages (e.g., CD11b-diptheria toxin receptor (DTR) mouse combined with local administration of diphtheria toxin (DT)) will provide methods to assess regeneration in the absence of macrophages only in muscle and this will help reduce the systemic influence of depletion on the regenerative process. Moreover, more precise targeting of macrophage phenotypes (i.e., M1 and M2) for depletion and genetic manipulation will greatly increase the understanding of the function of each cell type in the regenerative progression.
Additional Immune Cells – Eosinophils, Regulatory T Cells (Tregs), and Neutrophils
In addition to macrophages, other immune cells that are enriched in regenerating muscle have recently received attention (Fig. 1). Recent studies have identified eosinophils and a special type of T-cell, regulatory T cells (Tregs), as necessary components for effective muscle restoration (Burzyn et al., 2013; Heredia et al., 2013) (Table 1). Eosinophils are part of the early immune response and have been shown to aid in the regenerative response by secreting IL-4 to target FAPs for proliferation while simultaneously preventing the adipogenic lineage conversion of these cells (Heredia et al., 2013) (Fig. 2). Therefore, there is an indirect impact on MuSCs through supporting the pro-regenerative functions of FAPs (described above). Additionally, these studies revealed FAPs have phagocytic ability that aid in the removal of necrotic debris. Therefore, eosinophil dependent expansion of FAPs helps establish a local environment that collectively supports the MuSC regenerative response.
Recent reports have also demonstrated the accumulation of Tregs in regenerating muscle around four days following injury (Burzyn et al., 2013; Kuswanto et al., 2016) (Fig. 1). It appears that FAPs are key regulators of Tregs homing to regenerating muscle, specifically secreting IL-33 to attract these special T-cells to the tissue (Kuswanto et al., 2016) (Fig. 2). This timing is consistent with when FAPs reach a peak at three days following injury (Joe et al., 2010), just prior to Treg accumulation. Further studies where Tregs were ablated using a conditional DTR approach found that the regenerative response was negatively impacted in the absence of these cells (Burzyn et al., 2013). Specifically, prolonged inflammation, decreased number of regenerating fibers, and increased fibrosis were noted. Of course, the regenerative response is multifaceted and implies the presence of crosstalk between numerous local cells and Tregs. Indeed, the authors identified a growth factor, Amphiregulin (Areg), that is expressed by Tregs and targets MuSCs that express the Areg receptor, epidermal growth factor receptor (EGFR), to increase cellular survival and support differentiation (Fig. 2). The accumulation of Tregs four days following injury is suggestive of a role in MuSC differentiation, as MuSCs peak at this time and are ready to support the regenerative process by terminally differentiating and contributing to fiber growth and repair. Additional studies are needed to determine if eosinophil-secreted Areg truly functions in both cell survival and differentiation processes. The molecular mechanism for Treg-dependent skeletal muscle regeneration will begin to become more clear as their roles in different phases of this complex process are teased apart.
The earliest immune cells to infiltrate skeletal muscle following injury are neutrophils (Fig. 1, Table 1) (Butterfield et al., 2006; Tidball and Villalta, 2010). Although these cells are not yet known to directly target MuSCs, a brief discussion of these cells is warranted due to their necessary proinflammatory role in early regeneration. Neutrophils generate reactive oxygen species to aid in establishing the early necrotic environment and the subsequent degradation of injured tissues (Butterfield et al., 2006; Tidball and Villalta, 2010). Additionally, proinflammatory cytokines, such as IFNγ, IL-1β, TNFα, IL-1, and IL-8, are secreted from neutrophils and act as chemokines to stimulate additional immune cell infiltration (Tidball and Villalta, 2010; Yang and Hu, 2018). Whereas evidence is lacking to suggest that these cytokines secreted from neutrophils directly target MuSCs, other cell types appearing later in the regenerative milieu (see macrophage section above) have been shown to secrete these factors to influence MuSC proliferation. It will be interesting to assess MuSC activation and proliferation in a neutrophil depleted background, or in a background in which neutrophils are genetically modified to remove one or many of these cytokines, to determine if these early signals alter later MuSC regenerative dynamics.
Endothelial Cells
As the degenerative process transitions to a regenerative phase, nascent vessels begin to appear through the process of angiogenesis. Capillary sprouting as well as ECs derived from circulating endothelial progenitor cells rebuild vasculature from existing and nascent structures (Asahara et al., 1997; Hershey et al., 2001; Majka et al., 2003; Scholz et al., 2003). Vascular ECs (Table 1) gradually increase following injury, peaking around 7 dpi (Fig. 1, Wosczyna et al., unpublished observations). These cells are believed to support the regenerative process through paracrine influence on MuSCs, secreting factors such as angiopoietin-1 (Ang-1) (Mofarrahi et al., 2015), IGF-1 (Christov et al., 2007), hepatocyte growth factor (HGF) (Tatsumi et al., 1998), and vascular endothelial growth factor (VEGF) (Bryan et al., 2008) (Fig. 2). These growth factors influence both MuSC proliferation and differentiation. However, most of the supporting data for these effector functions comes from in vitro assays where ECs are co-cultured with MuSCs (Latroche et al., 2015); in vivo experiments investigating the role of many of these factors in the regenerative processes are limited. More recent in vitro work using ECs co-cultured with MuSCs has demonstrated that ECs secrete Apelin and Oncostatin M to increase MuSC proliferation and differentiation, and Periostin to increase MuSC differentiation (Latroche et al., 2017) (Fig. 2). In these studies, the authors also attempted to translate these findings to in vivo myogenesis by injecting blocking antibodies or inhibitors for each of these proteins into regenerating skeletal muscle. Decreases in myogenin positive cells, embryonic myosin heavy chain positive fibers, and myofiber cross-sectional area were observed, indicating that these proteins are involved in in vivo myogenic regeneration. However, other cells in the regenerative milieu secrete these factors (Latroche et al., 2017), thus limiting the ability to attribute the effects solely to the EC-specific expression of these proteins. The possibility of cellular crosstalk between ECs and MuSCs is further supported by the finding that in the human pathology, amyopathic dermatomyositis, there is a loss of vasculature structures in skeletal muscle (Emslie-Smith and Engel, 1990) and concomitant loss of MuSCs without other known cellular changes (Christov et al., 2007). Furthermore, MuSCs have been shown to express tyrosine-protein kinase receptor-2 (Tie-2), the receptor for Ang-1, a ligand highly expressed by ECs (Abou-Khalil et al., 2009) (Fig. 2). When this interaction is inhibited during muscle regeneration with a Tie-2 blocking antibody, MuSCs exhibit enhanced proliferation. Conversely, when Ang-1 is overexpressed in muscle during regeneration, MuSCs are less proliferative. These findings suggest that ECs encourage MuSCs to return to quiescence after the initial expansion in the first few days of regeneration.
The ability to clearly assess EC necessity in the regenerative process has been confounded by the requirement for endothelium in the processes of almost all organ systems. Therefore, it is difficult to determine if a phenotype observed in one tissue following a genetic manipulation in ECs is due to local perturbation or if it is secondary to a systemic influence. While the findings summarized above are highly suggestive of a necessary EC influence on MuSC regenerative kinetics, tissue-specific targeting will be needed to determine the actual role of ECs in this process.
Pericytes
Another progenitor cell that sits in close relation to MuSCs are pericytes. Pericytes share a basal lamina with the vasculature ECs and express markers such as chondroitin sulfate proteoglycan 4 (NG2) and PDGFRβ (Crisan et al., 2008; Dellavalle et al., 2007) (Table 1). Previous interest in skeletal muscle pericytes has been mainly due to their myogenic potential and, accordingly, their prospect as a cellular source for myogenic rebuilding in pathological conditions (Tedesco et al., 2010). However, recent findings suggest a new and necessary role of NG2-positive pericytes in skeletal muscle regeneration (Kostallari et al., 2015). In these studies, the authors used an NG2-Cre mouse model to genetically target and ablate pericytes and observed an increase in MuSC activation and a small decrease in myofiber diameter, suggesting a role in both MuSC quiescence and in differentiation of MuSC progeny. Of course, separating MuSC activation and differentiation solely by functional outcome is difficult, as they are not mutually exclusive. However, experiments suggested that pericytes specifically express Ang-1 and IGF-1 to regulate MuSC activation and myofiber hypertrophy, respectively (Kostallari et al., 2015) (Fig. 2). While the authors demonstrated transgene expression specificity, the NG2-Cre mouse was originally designed to target pericytes of the brain (Zhu et al., 2008). Undoubtedly, the dosing of NG2-Cre;R26-iDTR mice with DT, even when administered intramuscularly, causes off target ablation of pericytes in other tissues. Therefore, the systemic effect of pericyte ablation on skeletal muscle dynamics cannot be excluded. Furthermore, identifying pericytes by cell surface markers remains challenging and limits the ability to isolate these cells by FACS for in vitro assays or even characterize their numbers over a regenerative time course.
Fibroblasts
The last group of cells in the regenerative milieu that will be discussed are fibroblasts (Fig. 1). These cells have been proposed to be important in MuSC-dependent myogenesis but continue to lack a clear molecular identity (Table 1). Therefore, the influence of fibroblasts on MuSC-dependent myogenesis remains difficult to assess. Due to molecular heterogeneity, the majority of assays conducted with fibroblasts have been completed in vitro with cells isolated based on their ability to adhere quickly to plastic tissue culture vessels (Richler and Yaffe, 1970). This approach has been used to demonstrate that fibroblasts can increase the fusion of co-cultured myoblasts (Mathew et al., 2011), suggesting that fibroblasts contribute to MuSC differentiation. Furthermore, in vivo depletion of TCF4+ cells (believed to be mostly fibroblasts) results in impaired muscle regeneration (Murphy et al., 2011). However, expression of TCF4 has been shown in multiple other cellular compartments, including MuSCs and ECs (Mathew et al., 2011; The Tabula Muris Consortium et al., 2018), suggesting that the effects of the depletion of the TCF4 lineage may not be simply due to the depletion of fibroblasts. Identifying fibroblasts by a single marker remains a challenge and continues to limit the conclusions of the necessity of these cells in regenerative myogenesis.
Conclusions
In the field of skeletal muscle research, we are fortunate to have a well-defined MuSC and its readily identifiable niche on the myofiber. This has allowed the field to experiment with the resident cells in the interstitium and the immune infiltrating cells during regeneration. The results of these studies have led to the identification of the essential roles of cells like macrophages and FAPs in optimal MuSC performance during regeneration. In this review, we have brought together current information regarding cells that are capable of influencing MuSC regenerative dynamics. Furthermore, there is evidence of crosstalk between many of these cells signifying the importance of a cooperative cellular milieu. As studies that target these cellular populations genetically and pharmacologically reveal their central roles, the details of this complexity will continue to evolve.
Given the complex milieu of regenerating muscle, reducing the number of cell-cell interactions in simple assays, such as in vitro co-culture tests, allows for the teasing apart of cellular interactions and molecular processes. Many of these processes can then be tested in a more complex environment, with the benchmark being in vivo, and the architecture of the complete milieu will begin to emerge. The goal will be to develop a dynamic model of the inner workings of the regenerative environment, allowing for a better understanding of the regenerative process. The ultimate goal, of course, is to use this knowledge to develop better therapies to mitigate functional loss associated with impaired regenerative capacity in the setting of organismal aging or disease pathology.
Wosczyna and Rando present a Review of the complex cellular milieu surrounding regenerating muscle. They describe the cell types themselves as well as how they contribute to the regulation of muscle stem cells in the regeneration process.
Acknowledgments
We would like to thank the members of the Rando lab for insightful discussions. The authors’ efforts were supported by the Glenn Foundation for Aging Research, grants from the NIH (T32 AG000266 and K99 AG053438 to MNW; P01 AG036695, TR01 AG047820, and R37 (MERIT Award) AG023806 to TAR), and grants from the Department of Veterans Affairs to TAR. Figures 1 and 2 were illustrated by Eva Mae Natividad Baucom.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Khalil R, Le Grand F, Pallafacchina G, Valable S, Authier FJ, Rudnicki MA, Gherardi RK, Germain S, Chrétien F, Sotiropoulos A, Lafuste P, Montarras D, Chazaud B. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell. 2009;5:298–309. doi: 10.1016/j.stem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almada AE, Wagers AJ. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nat Rev Mol Cell Biol. 2016;17:267–279. doi: 10.1038/nrm.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12:75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biressi S, Miyabara EH, Gopinath SD, Carlig PMM, Rando TA. A Wnt-TGFβ2 axis induces a fibrogenic program in muscle stem cells from dystrophic mice. Sci Transl Med. 2014;6:267ra176–267ra176. doi: 10.1126/scitranslmed.3008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol. 1986;115:129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Broussard SR, McCusker RH, Novakofski JE, Strle K, Shen WH, Johnson RW, Dantzer R, Kelley KW. IL-1beta impairs insulin-like growth factor i-induced differentiation and downstream activation signals of the insulin-like growth factor i receptor in myoblasts. J Immunol. 2004;172:7713–7720. doi: 10.4049/jimmunol.172.12.7713. [DOI] [PubMed] [Google Scholar]
- Bryan BA, Walshe TE, Mitchell DC, Havumaki JS, Saint-Geniez M, Maharaj AS, Maldonado AE, D’Amore PA. Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol Biol Cell. 2008;19:994–1006. doi: 10.1091/mbc.E07-09-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–95. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train. 2006;41:457–465. [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargé SBP, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Cheng M, Nguyen MH, Fantuzzi G, Koh TJ. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am J Physiol, Cell Physiol. 2008;294:C1183–91. doi: 10.1152/ajpcell.00568.2007. [DOI] [PubMed] [Google Scholar]
- Christov C, Chrétien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Davies OG, Liu Y, Player DJ, Martin NRW, Grover LM, Lewis MP. Defining the balance between regeneration and pathological ossification in skeletal muscle following traumatic injury. Front Physiol. 2017;8:194. doi: 10.3389/fphys.2017.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189:3669–3680. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Du H, Shih CH, Wosczyna MN, Mueller AA, Cho J, Aggarwal A, Rando TA, Feldman BJ. Macrophage-released ADAMTS1 promotes muscle stem cell activation. Nat Commun. 2017;8:669. doi: 10.1038/s41467-017-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie-Smith AM, Engel AG. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol. 1990;27:343–356. doi: 10.1002/ana.410270402. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2014;96:183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2015;21:76–80. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada SI, Higuchi S, Segawa M, Koda KI, Yamamoto Y, Tsujikawa K, Kohama Y, Uezumi A, Imamura M, Miyagoe-Suzuki Y, Takeda S, Yamamoto H. Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp Cell Res. 2004;296:245–255. doi: 10.1016/j.yexcr.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Fukada SI. The roles of muscle stem cells in muscle injury, atrophy and hypertrophy. J Biochem. 2018;163:353–358. doi: 10.1093/jb/mvy019. [DOI] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey JC, Baskin EP, Glass JD, Hartman HA, Gilberto DB, Rogers IT, Cook JJ. Revascularization in the rabbit hindlimb: dissociation between capillary sprouting and arteriogenesis. Cardiovasc Res. 2001;49:618–625. doi: 10.1016/s0008-6363(00)00232-7. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FMV. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juban G, Chazaud B. Metabolic regulation of macrophages during tissue repair: insights from skeletal muscle regeneration. FEBS Lett. 2017;19:71–3021. doi: 10.1002/1873-3468.12703. [DOI] [PubMed] [Google Scholar]
- Kanisicak O, Mendez JJ, Yamamoto S, Yamamoto M, Goldhamer DJ. Progenitors of skeletal muscle satellite cells express the muscle determination gene, MyoD. Dev Biol. 2009;332:131–141. doi: 10.1016/j.ydbio.2009.05.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelić S, Olsson T, Kristensson K. Interferon-gamma promotes proliferation of rat skeletal muscle cells in vitro and alters their AChR distribution. J Neurol Sci. 1993;114:62–67. doi: 10.1016/0022-510x(93)90050-9. [DOI] [PubMed] [Google Scholar]
- Kostallari E, Baba-Amer Y, Alonso-Martin S, Ngoh P, Relaix F, Lafuste P, Gherardi RK. Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence. Development. 2015;142:1242–1253. doi: 10.1242/dev.115386. [DOI] [PubMed] [Google Scholar]
- Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, Mathis D. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity. 2016;44:355–367. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen RCJ, Van Der Velden JLJ, Schols AMWJ, Kelders MCJM, Wouters EFM, Janssen-Heininger YMW. Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J. 2004;18:227–237. doi: 10.1096/fj.03-0251com. [DOI] [PubMed] [Google Scholar]
- Latroche C, Gitiaux C, Chrétien F, Desguerre I, Mounier R, Chazaud B. Skeletal muscle microvasculature: a highly dynamic lifeline. Physiology. 2015;30:417–427. doi: 10.1152/physiol.00026.2015. [DOI] [PubMed] [Google Scholar]
- Latroche C, Weiss-Gayet M, Muller L, Gitiaux C, Leblanc P, Liot S, Ben Larbi S, Abou-Khalil R, Verger N, Bardot P, Magnan M, Chrétien F, Mounier R, Germain S, Chazaud B. Coupling between myogenesis and angiogenesis during skeletal muscle regeneration is stimulated by restorative macrophages. Stem Cell Reports. 2017;9:2018–2033. doi: 10.1016/j.stemcr.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FMV. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21:786–794. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- Lemos DR, Paylor B, Chang C, Sampaio A, Underhill TM, Rossi FMV. Functionally convergent white adipogenic progenitors of different lineages participate in a diffused system supporting tissue regeneration. Stem Cells. 2012;30:1152–1162. doi: 10.1002/stem.1082. [DOI] [PubMed] [Google Scholar]
- Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48:424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cheung TH, Charville GW, Rando TA. Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat Protoc. 2015;10:1612–1624. doi: 10.1038/nprot.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Garry GA, Li S, Bezprozvannaya S, Sanchez-Ortiz E, Chen B, Shelton JM, Jaichander P, Bassel-Duby R, Olson EN. A Twist2-dependent progenitor cell contributes to adult skeletal muscle. Nat Cell Biol. 2017:1–24. doi: 10.1038/ncb3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu Y, Zhao L, Zeng Z, Xiao W, Chen P. Macrophage depletion impairs skeletal muscle regeneration: The roles of regulatory factors for muscle regeneration. Cell Biol Int. 2017;41:228–238. doi: 10.1002/cbin.10705. [DOI] [PubMed] [Google Scholar]
- Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment ADA, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J of bone and joint surgery Volume. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majka SM, Jackson KA, Kienstra KA, Majesky MW, Goodell MA, Hirschi KK. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest. 2003;111:71–79. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Muñoz-Cánoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138:371–384. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophysic Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Pannérec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- Mofarrahi M, McClung JM, Kontos CD, Davis EC, Tappuni B, Moroz N, Pickett AE, Huck L, Harel S, Danialou G, Hussain SNA. Angiopoietin-1 enhances skeletal muscle regeneration in mice. Am J Physiol Regul Integr Comp Physiol. 2015;308:R576–89. doi: 10.1152/ajpregu.00267.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi N, Asakura A. Muscle satellite cell heterogeneity and self-renewal. Front Cell Dev Biol. 2014;2:1. doi: 10.3389/fcell.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Cánoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280:4131–4148. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negroni E, Vallese D, Vilquin JT, Butler-Browne G, Mouly V, Trollet C. Current advances in cell therapy strategies for muscular dystrophies. Expert Opin Biol Ther. 2011;11:157–176. doi: 10.1517/14712598.2011.542748. [DOI] [PubMed] [Google Scholar]
- Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannérec A, Formicola L, Besson V, Marazzi G, Sassoon DA. Defining skeletal muscle resident progenitors and their cell fate potentials. Development. 2013;140:2879–2891. doi: 10.1242/dev.089326. [DOI] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretheeban T, Columbia T, Lemos D, Paylor B, Zhang RH, Rossi F. Role of stem/progenitor cells in reparative disorders. Fibrogenesis Tissue Repair. 2012;5:20. doi: 10.1186/1755-1536-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu-Petersen Z. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139:2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- Richler C, Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970;23:1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Rowland LA, Bal NC, Periasamy M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol Rev. 2014;90:1279–1297. doi: 10.1111/brv.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saclier M, Cuvellier S, Magnan M, Mounier R, Chazaud B. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 2013a;280:4118–4130. doi: 10.1111/febs.12166. [DOI] [PubMed] [Google Scholar]
- Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013b;31:384–396. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- Scholz D, Thomas S, Sass S, Podzuweit T. Angiogenesis and myogenesis as two facets of inflammatory post-ischemic tissue regeneration. Mol Cell Biochem. 2003;246:57–67. [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Segalés J, Perdiguero E, Muñoz-Cánoves P. Regulation of muscle stem cell functions: a focus on the p38 MAPK signaling pathway. Front Cell Dev Biol. 2016;4:e114388–15. doi: 10.3389/fcell.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Li Y, Zhu J, Schwendener R, Huard J. Interaction between macrophages, TGF-beta1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury. J Cell Physiol. 2008;214:405–412. doi: 10.1002/jcp.21212. [DOI] [PubMed] [Google Scholar]
- Stzepourginski I, Nigro G, Jacob JM, Dulauroy S, Sansonetti PJ, Eberl G, Peduto L. CD34 +mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci USA. 2017;114:E506–E513. doi: 10.1073/pnas.1620059114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120:11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quake SR, Wyss-Coray T, Darmanis S. The Tabula Muris Consortium. Single-cell transcriptomic characterization of 20 organs and tissues from individual mice creates a Tabula Muris. 2018. pp. 1–26. [DOI] [Google Scholar]
- Tidball JG. Mechanisms of muscle injury, repair, and regeneration. Compr Physiol. 2011;1:2029–2062. doi: 10.1002/cphy.c100092. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–53. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–87. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, Schneider MD, Musarò A, Rosenthal N. Monocyte/macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol Ther. 2015;23:1189–1200. doi: 10.1038/mt.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D’Antona G, Tonlorenzi R, Porretti L, Gavina M, Mamchaoui K, Pellegrino MA, Furling D, Mouly V, Butler-Browne GS, Bottinelli R, Cossu G, Bresolin N. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest. 2004;114:182–195. doi: 10.1172/JCI20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada SI, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada SI. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- Varga T, Mounier R, Patsalos A, Gogolák P, Peloquin M, Horvath A, Pap A, Daniel B, Nagy G, Pintye E, Póliska S, Cuvellier S, Larbi SB, Sansbury BE, Spite M, Brown CW, Chazaud B, Nagy L. Macrophage PPARγ, a lipid activated transcription factor controls the growth factor GDF3 and skeletal muscle regeneration. Immunity. 2016;45:1038–1051. doi: 10.1016/j.immuni.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wu H, Zhang Z, Liu S, Yang J, Chen X, Fan M, Wang X. Effects of interleukin-6, leukemia inhibitory factor, and ciliary neurotrophic factor on the proliferation and differentiation of adult human myoblasts. Cell Mol Neurobiol. 2008;28:113–124. doi: 10.1007/s10571-007-9247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wehling-Henricks M, Samengo G, Tidball JG. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell. 2015;14:678–688. doi: 10.1111/acel.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012;27:1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Liu Y, Chen P. Macrophage Depletion Impairs Skeletal Muscle Regeneration: the Roles of Pro-fibrotic Factors, Inflammation, and Oxidative Stress. Inflammation. 2016;39:2016–2028. doi: 10.1007/s10753-016-0438-8. [DOI] [PubMed] [Google Scholar]
- Yang W, Hu P. Skeletal muscle regeneration is modulated by inflammation. Journal of Orthopaedic Translation. 2018;13:25–32. doi: 10.1016/j.jot.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L, Rossi F. Purification of progenitors from skeletal muscle. J Vis Exp. 2011 doi: 10.3791/2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Price F, Rudnicki MA. Satellite Cells and the Muscle Stem Cell Niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, Yap S, Pollett JB, Drowley L, Cassino T, Gharaibeh B, Deasy BM, Huard J, Péault B. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]