Abstract
Objectives
UNBS5162 is a novel naphthalimide that binds to DNA by intercalation and suppresses CXCL chemokine elaboration. A Phase I study of UNBS5162 was conducted to establish pharmacokinetics (PK), maximum tolerated dose (MTD), dose-limiting toxicity, safety and anti-tumor activity in patients with advanced solid tumors or lymphoma.
Methods
UNBS5162 was administered in a 3 + 3 dose escalation scheme by intravenous infusion over 1 h weekly for 3 weeks of a 4-week cycle. Safety, serial serum PK and tolerability were captured throughout the study. Response Evaluation Criteria in Solid Tumors was utilized every 2 cycles to assess for anti-tumor response.
Results
Twenty-four patients with metastatic carcinoma and 1 patient with lymphoma were treated at eight dose levels (18–234 mg/m2). All patients were evaluable for tolerability and toxicity. Grade 3 toxicities include nausea (n = 1), fatigue (n = 1) and anorexia (n = 1). Prolongation of QTc [Hodges] was observed in 6 cases (Gr 1 = 2; Gr 2 = 2; Gr 3 = 2). Cmax and area under the curve increased linearly with dose with a t1/2 of 30–60 min. 16 patients completed 2 cycles of therapy, all with pharmacodynamics at 8 weeks.
Conclusions
The MTD or dose-limiting toxicity for UNBS5162 was not reached due to the magnitude of QTc prolongation at the highest dose of 234 mg/m2/week that led to study termination.
Keywords: Naphthalimide, Maximum tolerated dose, Dose-limiting toxicity, QTc, Chemokines
Introduction
Naphthalimides are DNA intercalating agents that have been evaluated clinically as potential anti-cancer agents [1] due to high pre-clinical anti-tumor activity against a variety of human solid and hematologic tumor cells. Amonafide, the first naphthalimide evaluated in the clinic failed to enter phase III trials due to dose-limiting myelosuppression [1]. Bone marrow toxicity was due to metabolism through N-acetyl transferase 2 to the toxic metabolite, N-acetyl-amonafide [2, 3]. UNBS3157 (2,2,2-trichloro-N-({2-[2(dimethylamino)ethyl]-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-5-yl}carbamoyl)acetamide) is a modified naphthalimide designed to avoid the specific activating metabolism that induces hematologic toxicity. UNBS3157 is rapidly and almost totally hydrolyzed in physiological saline into UNBS5162, which accounts for its anti-cancer properties without generating amonafide [4]. UNBS5162, when administered in a metronomic approach in vitro, almost completely diminished expression of pro-angiogenic chemokines CXCL1, CXCL2, CXCL3, CXCL6 and CXCL8 [4]. In vivo, UNBS5162 significantly increased survival in an orthotopic human PC-3 prostate cancer xenograft mouse model at approximately the same magnitude as paclitaxel and had a synergistic therapeutic benefit when administered in combination with paclitaxel [4]. Additionally, UNBS5162 in combination with paclitaxel significantly increased survival in an orthotopic A549 NSCLC xenograft mouse model [5]. In vivo, it had been shown to increase effectiveness of radiotherapy in an SC MXT-HI mouse mammary tumor model metastasizing to the liver [5].
Given the poor objective response rates and dismal survival in patients with relapsed and refractory solid tumors and lymphoma, a phase I clinical trial was conducted with UNBS5162 to establish pharmacokinetics (PK), maximum tolerated dose (MTD), dose-limiting toxicity (DLT) safety and anti-tumor activity.
Materials and methods
Patient selection
Eligibility requirements were pathologically confirmed advanced solid tumors or lymphoma, refractory to or intolerant of established therapy known to provide clinical benefit, age ≥18 years, life expectancy of ≥12 weeks, Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, one or more metastatic tumors measurable by RECIST, acceptable organ function (blood counts, renal and hepatic) and able to comply with study procedures. In females with child-bearing potential a negative pregnancy test within 72 h of the first dose was required. Subjects were excluded if they had New York Heart Association class III or IV heart failure, cardiac disease [myocardial infarction within 6 months prior to day 1 of infusion, unstable arrhythmia or evidence of ischemia on electrocardiogram (ECG)], known brain metastases (unless previously treated and well controlled for 3 months), major surgery within 4 weeks prior to day 1 or minor surgery within 2 weeks prior to day 1 (other than diagnostic surgery), active, uncontrolled infection (bacterial, viral or fungal) requiring systemic therapy, pregnant or lactating, received radiation therapy, surgery or an investigational therapy within 1 month prior to study entry (6 weeks for mitomycin-C or nitrosourea), received chemotherapy prior to study entry within 3–5 half-lives of that chemotherapy agent or 4 weeks prior to study entry (whichever is shorter) with resolution of any side-effects from that previous therapy, other severe concurrent nonmalignant disease (like kidney or liver disease) that could compromise protocol objectives in the judgment of the investigator and/or sponsor, were receiving any other investigational agent, exhibited allergic reactions to compounds with a similar structure (e.g., naphthalimides), had QTc > 450 ms using Hodges formula, using any medication known to prolong QTc interval, using medication which were potent inducers and inhibitors of the cytochrome P450 enzymes or on anticoagulant therapy, were HIV-positive or have AIDS or infected with hepatitis C virus. All patients gave informed consent to participate and this study was conducted in accordance to the declaration of Helsinki and applicable guidelines on good clinical practice.
Study design
A comprehensive Investigational New Drug-enabling toxicology program was conducted with UNBS5162 to support a first-in-human phase 1 clinical trial in cancer patients. This program included single-dose, repeat-dose non-pivotal, and repeat-dose pivotal studies in mice, rats and dogs up to 4 weeks in duration. Toxicology studies conducted in rats and dogs used the intended clinical route and schedule of administration. Using the algorithm described in the Food and Drug Administration’s General Guide for Starting Dose Selection for a Cytotoxic Agent in Cancer Patients, values for the dose severely toxic to 10 % of rodents (STD10) and the non-rodent highest non-severely toxic dose (HNSTD) were derived from the definitive UNBS5162 rat and dog toxicology studies and used to calculate a clinical starting dose of 18 mg/m2/week UNBS5162 (Investigator Brochure).
This study was approved by the institutional review boards of the participating institutions and conducted using the modified Fibonacci 3 + 3 dose escalation design. This was a phase I, single-agent, open-label, multi-center, dose-escalation study to determine MTD and DLTs of UNBS5162. Toxicities were graded according to National Cancer Institute Common Toxicity Criteria for Adverse Events, Version 3.0 (NCI CTCAE) [6]. In addition, secondary objectives were to determine the safety and tolerability of UNBS5162 and to establish its PK and pharmacodynamic (PD) profile and to observe any anti-tumor activity. DLTs were assessed only during the 1st cycle and were febrile neutropenia, grade 4 neutropenia for >4 days, grade 3 or 4 thrombocytopenia, grade 3 or 4 non-hematologic toxicities.
UNBS5162 was administered by intravenous (IV) infusion over 1 h weekly for 3 weeks followed by 1 week of no treatment. An individual cycle of therapy was defined as 4 weeks. Patients who successfully completed 1 cycle of treatment without any evidence of significant treatment-related toxicity or clinical evidence of progressive disease were permitted to continue treatment based on tolerability and response. The starting dose of UNBS5162 was 18 mg/m2/week for 3 weeks followed by 1 week rest period. After the first patient received the dose for 3 weeks and did not develop any NCI CTCAE grade 2 or higher toxicity after completing 2 weeks of treatment, 2 additional patients were enrolled and administered the same dose. If none of the 3 patients experienced a NCI CTCAE grade 2 or higher toxicity after 1 cycle of treatment, subsequent dose escalations proceeded. At least three patients were enrolled at each dose level. When one patient experienced a treatment-related toxicity qualifying as a DLT, 3 additional patients were enrolled at that dose level. If no additional DLTs were observed, dose escalation was resumed. However, if 2 of 3 patients experience treatment related toxicity, MTD was considered to have been exceeded. MTD was defined as the dose at which ≤1 of 6 patients experienced a DLT during cycle 1, with the next higher dose having at least 2/6 patients experiencing DLT during cycle 1. The MTD cohort was permitted to enroll up to 12 patients to further evaluate safety and tolerability.
Only 1 dose reduction for any hematologic or non-hematologic toxicity per patient was permitted. Patients who required a second dose reduction due to toxicity were discontinued from treatment and followed per study protocol. Patients were also discontinued if they required a delay of >3 weeks due to any drug-related grade 3 or 4 non-hematologic toxicity or DLT.
Pre-treatment and follow-up studies
A complete history and physical examination, ECOG performance status (PS) assessment and laboratory studies (including complete blood count, liver function tests, chemistry analyses) were performed prior to enrollment and during each cycle of treatment. Laboratory tests were performed every week with treatment. 12-lead ECGs were obtained on cycle 1, day 1 (immediately prior to infusion, at the end of infusion and 1, 4 and 8 h after the end of infusion), and on days 8 and 15 (immediately prior to and at the end of infusion).
Plasma pharmacokinetic assays
Blood samples for PK analysis were collected on cycle 1 day 1 at the following time points: before dosing, 30 min after the start of the infusion, at the end of infusion, at 1, 2, 4, 6, 8, 24 h after the completion of the infusion and 30 min prior to dosing on days 8 and 15 (weeks 2 and 3). All patients with measurable plasma concentrations of UNBS5162 were included in the PK analysis. PK parameters were derived using a 2-compartment model using WinNonlin Professional Version 5.2.1 (Pharsight Corp, Mountain View, CA, USA). Nonlinear regression was used to fit the parameters of the 2-compartment model to the concentration–time data. Plasma samples were assayed for UNBS5162 using a validated LC–MS/MS method.
Results
Patient characteristics
From September 2007 to May 2009, 25 patients with advanced malignancy were enrolled in this study. Baseline characteristics of the patients are shown in Table 1. Enrolled patients had a median age of 67 year (range 23–81 year) with an ECOG PS of zero in 56 % and of one in 44 %. Two-thirds of the patients enrolled were males (64 %). Patients with a variety of tumor types were enrolled with the most common being gastrointestinal malignancies (40 %) (colorectal = 8, gastric = 1, pancreatic = 1) (Table 1). The median number of cycles administered per patients was 2 (range = 1–5). There were no dose reductions in any patient throughout the study.
Table 1.
Patient demographics
| Characteristic | Total (n = 25) |
|---|---|
| Age (years) | |
| Median | 67 |
| Range | 23–81 |
| Sex | |
| Male, n (%) | 16 (64) |
| Race, n (%) | |
| African–American | 1 (4) |
| Caucasian | 20 (72) |
| Hispanic | 6(24) |
| ECOG | |
| 0 | 15 (56) |
| 1 | 12 (44) |
| Primary tumor site | |
| Pancreas | 1 |
| Colorectal | 8 |
| Gastric | 1 |
| GIST | 1 |
| Anal | 1 |
| Lung (NSCLC) | 2 |
| Prostate | 2 |
| Breast | 1 |
| Ovary | 1 |
| Vaginal | 1 |
| Lymphoma (DLBCL) | 1 |
| Head/neck (Adeno-cystic) | 1 |
| Melanoma | 1 |
| Sarcoma | 2 |
| Neuroendocrine | 1 |
Safety and tolerability
Of the 25 patients treated, 23 (92 %) experienced a treatment-related adverse event. Table 2 lists the most frequently reported adverse event (occurring in ≥10 % of patients). There were three grade 3 adverse events afflicting one patient per side-effect (nausea, fatigue, anorexia). There were 5 serious adverse events reported in patients during the study but none of them were determined to be related to UNBS5162. Two patients died while on study and the deaths were not related to the study medication (1 patient from disease progression after completing 1 cycle of treatment and 1 patient from pneumonia after receiving 2 cycles of treatment).
Table 2.
All adverse events (≥10 % incidence) per dose: all grades for cycle 1 only
| Dose level (mg/m2/week) | No. of patients | Nausea (n) | Dizziness | Fatigue | Constipation |
|
| |||||
| 18 | 3 | 3 | 0 | 3 | 1 |
| 36 | 4 | 2 | 1 | 1 | 1 |
| 59.4 | 3 | 0 | 1 | 1 | 1 |
| 90 | 3 | 1 | 0 | 1 | 1 |
| 126 | 3 | 1 | 1 | 0 | 1 |
| 162 | 3 | 2 | 0 | 2 | 1 |
| 198 | 3 | 0 | 0 | 1 | 1 |
| 234 | 3 | 0 | 0 | 0 | 1 |
|
| |||||
| Dose level (mg/m2/week) | No. of patients | Vomiting | Anorexia | Chills | Back pain |
|
| |||||
| 18 | 3 | 2 | 0 | 1 | 0 |
| 36 | 4 | 0 | 2 | 1 | 0 |
| 59.4 | 3 | 0 | 0 | 1 | 0 |
| 90 | 3 | 0 | 0 | 1 | 1 |
| 126 | 3 | 1 | 0 | 0 | 1 |
| 162 | 3 | 2 | 3 | 0 | 1 |
| 198 | 3 | 1 | 0 | 0 | 0 |
| 234 | 3 | 0 | 0 | 0 | 1 |
|
| |||||
| Dose level (mg/m2/week) | No. of patients | ECG QTc prolongation | Chest paina | Abdominal distention | Abdominal pain |
|
| |||||
| 18 | 3 | 0 | 0 | 0 | 0 |
| 36 | 4 | 0 | 1 | 0 | 0 |
| 59.4 | 3 | 1 | 1 | 0 | 0 |
| 90 | 3 | 1 | 0 | 0 | 0 |
| 126 | 3 | 0 | 0 | 1 | 1 |
| 162 | 3 | 1 | 1 | 0 | 1 |
| 198 | 3 | 1 | 0 | 1 | 1 |
| 234 | 3 | 2 | 0 | 1 | 0 |
|
| |||||
| Dose level (mg/m2/week) | No. of patients | Diarrhea | GERD | Dyspnea | Insomnia |
|
| |||||
| 18 | 3 | 0 | 1 | 0 | 1 |
| 36 | 4 | 1 | 0 | 0 | 0 |
| 59.4 | 3 | 1 | 1 | 0 | 1 |
| 90 | 3 | 0 | 0 | 1 | 0 |
| 126 | 3 | 1 | 0 | 1 | 0 |
| 162 | 3 | 0 | 1 | 0 | 0 |
| 198 | 3 | 0 | 0 | 1 | 1 |
| 234 | 3 | 0 | 0 | 0 | 0 |
No patient experienced treatment related grade 3–5 AEs
GERD gastroesophageal reflux disease
Chest pain-musculoskeletal in nature
Pharmacokinetics
Data from 24 of the 25 patients treated with UNBS5162 were included in the PK analysis. The plasma concentration of UNBS5162 increased with dose as measured by Cmax and area under the curve (AUC) with a t1/2 of 30–60 min. However, at the 2 highest dose levels (cohort 7 and 8), there was no apparent increase in plasma concentration. A summary of compartmental PK parameters for UNBS5162 is presented in Table 3.
Table 3.
Summary of compartmental PK parameters for UNBS5162 by treatment group [Mean (SD)]
| Group | Dose (mg) | K10 (h−1) | Cmax (ng/mL) | t1/2 α (h) | t1/2 β (h) | AUC (h ng/mL) | CL (L/h) | Vss (L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 30.2 | 0.632 (0.080) | 419.6 (146.2) | 0.51 (0.08) | 7.48 (2.29) | 958 (245) | 32.4 (5.3) | 206 (36) |
| 2 | 64.6 | 0.499 (0.251) | 612.6 (214.7) | 0.64 (0.21) | 8.19 (0.51) | 1644 (279) | 39.9 (6.2) | 313 (33) |
| 3 | 125.0 | 0.737 (0.240) | 1428.9 (643.7) | 0.42 (0.12) | 6.94 (1.67) | 3194 (1143) | 43.1 (16.9) | 280 (142) |
| 4 | 169.9 | 0.564 (0.154) | 1821.4 (893.6) | 0.63 (0.16) | 7.34 (0.91) | 4252 (1709) | 43.7 (14.1) | 278 (99) |
| 5 | 252.9 | 1.138 (0.370) | 3847.7 (738.5) | 0.33 (0.10) | 6.56 (1.04) | 6640 (2006) | 39.7 (6.7) | 202 (53) |
| 6 | 311.0 | 0.963 (0.322) | 3981.3 (1986.6) | 0.28 (0.03) | 7.57 (1.62) | 10127 (4411) | 35.1 (15.8) | 245 (109) |
| 7 | 433.7 | 1.352 (0.370) | 6376.6 (321.3) | 0.32 (0.06) | 6.78 (1.34) | 11631 (2161) | 37.6 (4.9) | 162 (45) |
| 8 | 491.2 | 1.075 (0.231) | 5913.7 (267.5) | 0.33 (0.02) | 7.94 (0.21) | 12786 (1852) | 39.2 (10.6) | 233 (31) |
K10 elimination rate, Cmax peak concentration, t1/2 α half-life associated with the rapid macro-rate constant alpha, t1/2 β half-life associated with the slow macro-rate constant beta, AUC area under the curve, CL total body clearance, Vss volume of distribution at steady state
QTc prolongation
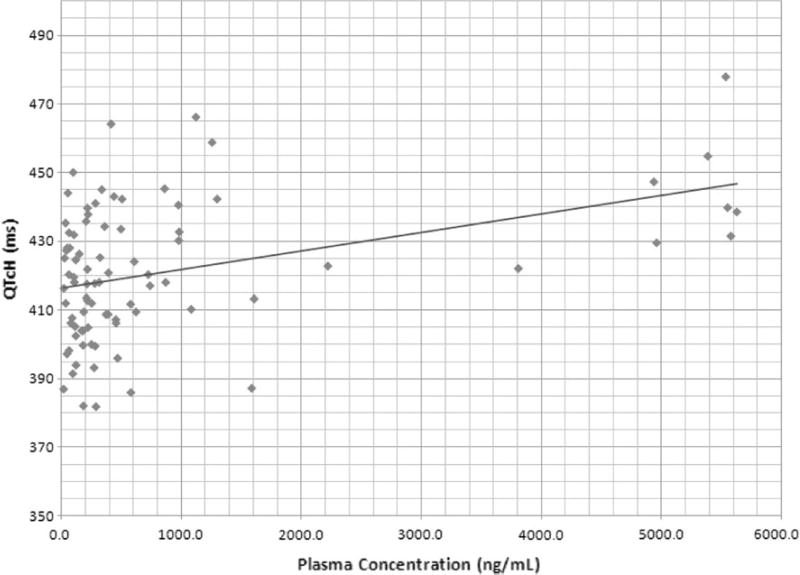
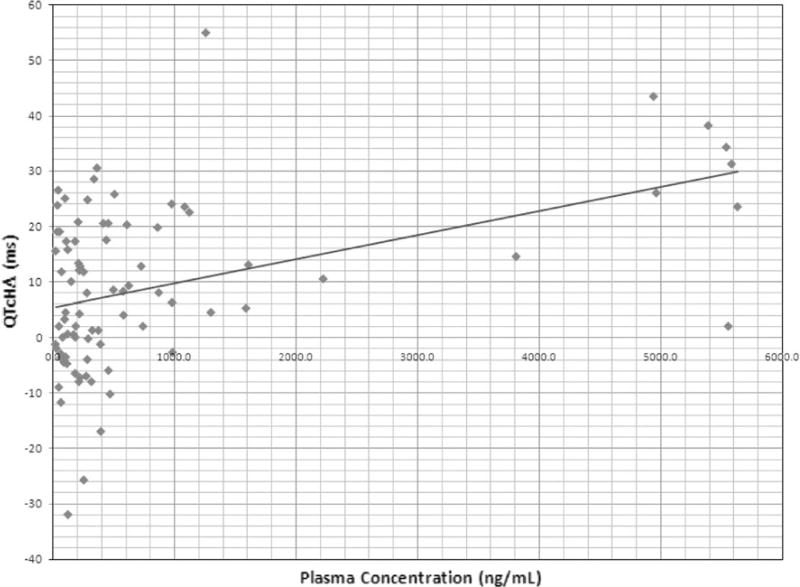
A total of 323 ECGs were obtained from 25 patients. QT intervals were calculated using the Hodges formula [7]. Prolongation of QTcH was observed in 6 cases [(Gr 1 = 2, Gr 2 = 2 and Gr 3 = 2) prolongations as defined by CTCAE criteria]; however, none of them were associated with any clinically significant events. Grade 3 QTcH noted in 2 patients were 501 ms (with 162 mg/m2/week dose) and 511 ms (with 234 mg/m2/week dose). None of patients were taking any concomitant medication known to be associated with QT prolongation. Figures 1 and 2 show the relationship of plasma concentration of UNBS5162 and QTcH (Fig. 1) and ΔQTcH (Fig. 2). Results indicate a highly significant linear relationship between QTcH (p = 0.0001) and ΔQTcH (0.00001) with plasma levels of UNBS5162. In some patients the prolongation of QT interval persisted for several hours after the decline in plasma concentration of UNBS5162. All patients were asymptomatic. These results demonstrate that despite pre-clinical data suggesting a low risk of QTc prolongation associated with UNBS5162 administration, the statistically significant relationship between plasma concentrations of UNBS5162 and QTc prolongation, and the magnitude of QTc prolongation at the highest doses administered (234 mg/m2) warranted the study being terminated by the sponsor. The MTD was not reached.
Fig. 1.
Relationship between simultaneous plasma concentration of UNBS5162 and QTcH. QTc interval >450 ms using Hodges formula was considered QTc prolongation
Fig. 2.
Relationship between simultaneous plasma concentration of UNBS5162 and ΔQTcH. ΔQTcH >30 is considered significant and QTc prolongation appears to be more frequent at the highest dose evaluated
Anti-tumor response
Sixteen patients were available for response evaluation. All patients progressed after 2 cycles of therapy.
Discussion
UNBS5162 is an antagonist of CXCF chemokine expression which has shown promising activity in vitro and in vivo against various human tumor cell lines [1, 4]. This phase I, multi-center study was conducted to evaluate PK, MTD and DLT of UNBS5162. Twenty-five patients were enrolled and no significant DLT was observed; however, QTc prolongations were noted in 6 of 25 patients which resulted in stopping enrollment. A significant relationship was noted between the plasma concentration of UNBS5162 and QTc prolongation (Figs. 1, 2). In Fig. 1, QTc interval >450 ms using Hodges formula was considered QTc prolongation. In Fig. 2, ΔQTcH >30 was considered significant. QTc prolongation appears to be more frequent at the highest dose evaluated.
In vitro evaluation of UNBS5162 and its effect on QTc prolongation was assessed in a human embryonic kidney cell line (HEK293) transfected with human ether-a-go–go cDNA with drug concentrations of 10, 30 and 100 µM. UNBS5162 inhibited the human ether-a-go-go-related gene (hERG) potassium current by (mean ± SEM; n = 3) 0.6 ± 0.1 % at 10 µM, 10.4 ± 0.5 % at 30 µM and 56.5 ± 1.3 % at 100 µM versus 0.8 ± 0.4 % in control. The IC50 was 87.9 µM (Hill coefficient = 2.0) for UNBS5162 on hERG potassium current. The cardiovascular safety was further evaluated in vivo in anesthetized dogs following IV administration of UNBS5162 at 5 and 15 mg/kg. There was no effect on blood pressure, heart rate, femoral blood flow or ECG intervals.
However, in the Phase I study 6 patients had prolonged QTc intervals which were attributed to UNBS5162 (none of the patients were taking any other medications associated with QTc prolongation). In 3 patients the prolongation of the QTc interval persisted for many hours after the plasma levels of UNBS5162 decreased. This is likely due to tight binding of the drug (or metabolite) to the potassium channel. We realize that the number of patients and observations are insufficient to evaluate a potential hypothesis for the resulting PK relationship to the plasma concentrations of UNBS5162 to QTc effects (Table 3). However, a regression analysis of all available drug concentration data and ECG tracings showed a highly statistically significant relationship between UNBS5162 plasma concentrations and QTcH and ΔQTcH (Figs. 1, 2).
There was no evidence of myelotoxicity (neutropenia, thrombocytopenia or lymphopenia), hepatotoxicity (elevated aminotransferase or bilirubin) or nephrotoxicity (elevated blood urea nitrogen or creatinine) at the doses administered in this study (Table 2). All evaluable patients had progressive disease at the end of cycle 2. MTD was not reached as the study was halted to further evaluate QTc prolongations.
Further development novel formulations of naphthalimides with unique mechanisms of action have been developed. Quinamed™, a substituted naphthalimide (ChemGenex Therapeutics, Inc., Menlo Park, CA, USA) interferes with important anti-cancer targets including topoisomerase II and signaling proteins in the EGFR pathway. The IV formulation is in phase II clinical trials for the treatment of advanced solid tumors. Formulation development studies are evaluating novel routes and dosage for naphthalimides in pre-clinical models. Mice (C3H) bearing subcutaneous RIF-1 tumor (100 mm3) treated with Quinamed in different formulations (oral, intravenous, subcutaneous or intra-peritoneal) showed efficacy by tumor growth delay. Administration of Quinamed formulations CGX-571-13, CGX-571-17 and CGX-571-11 at 60 mg/kg all gave T/C ratio of 1.2 after oral, intramuscular or intra-peritoneal administration, respectively. Repeated oral administration of CGX-571-13 at 60 mg/kg (×4) increased the T/C ratio to 1.3 without weight loss or toxicities. These data suggest Quinamed formulations for oral or subcutaneous routes of administration are feasible and warrant future clinical development [8]. A novel class of multi-targeted naphthalimides designed and synthesized showed inhibition of topoisomerase II (topo II) with induction of lysosomal membrane permeabilization (LMP) and associated apoptosis in cancer cell lines. Compounds 7a–d and 8a–d potently inhibited the growth of 5 tested cancer cell lines with IC50 ranging from 2 to 10 µM with an activity profile superior to amonafide [9].
A novel amonafide analog, M(2)-A 2-(2-(dimethyl-arnino)ethyl)-6-(thiophene-2-ylmethylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione potently inhibits topoisomerase II(alpha). Investigation showed that M(2)-A induces G2/M cell cycle arrest through inhibition of the PI3K/Akt pathway. M(2)-A inhibited proliferation of HL60 with an IC50 of 18.86 µM. The expression of cyclin B1 and CDK1 changed in response to M(2)-A treatment of HL60 cells. Furthermore, M(2)-A treatment of HL60 cells inhibited NF-κB nuclear translocation, up-regulated Bax, down-regulated Bc1–2, and activated caspase-3, -9 activity leading to apoptosis. Moreover, phosphorylation of PI3K(p85) and Akt decreased following M(2)-A treatment. These data suggest that M(2)-A may have therapeutic potential in leukaemia [10].
Since amonafide-1-malate (amonafide) is active in the presence of MDR-1/P-gp efflux pumps, a frequent cause of treatment failure in secondary acute myeloid leukemia (AML). Two phase I clinical trials enrolled 43 patients with relapsed/refractory or secondary AML or chronic myeloid leukemia in blast crisis to investigate amonafide alone or in combination with cytarabine. 3 of 17 patients on monotherapy and 10 of 26 patients on combination therapy achieved a complete remission. Single-agent and combination therapy together showed responses in 9 of 20 patients with poor-risk secondary AML with a tolerable safety profile [11]. This phase 1 dose-escalation trial demonstrated that despite pre-clinical data suggesting low risk of QTc prolongation with UNBS5162 administration, there was a statistically significant relationship noted in study patients. At the dose cohorts evaluated there were no response in this population of advanced solid tumor patients, although the agent was well tolerated. The magnitude of QTc prolongation at the highest doses warranted the study drug to be terminated by the sponsor.
Acknowledgments
We wish to thank the patients and their families, clinical research support services (CRSS) at the Arizona Cancer Center, Mayo Clinic, AZ, USA and Virginia G. Piper Cancer Center at Scottsdale Healthcare, Nikki Barkett RN and Drias Pharmaceuticals for support.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
Daruka Mahadevan, Arizona Cancer Center, Tucson, AZ, USA.
Donald W. Northfelt, Mayo Clinic, Scottsdale, AZ, USA
Pavani Chalasani, Arizona Cancer Center, Tucson, AZ, USA.
Diane Rensvold, Arizona Cancer Center, Tucson, AZ, USA.
Sandra Kurtin, Arizona Cancer Center, Tucson, AZ, USA.
Daniel D. Von Hoff, Virginia G. Piper Cancer Center at Scottsdale Healthcare, Scottsdale, AZ, USA
Mitesh J. Borad, Mayo Clinic, Scottsdale, AZ, USA
Raoul Tibes, Mayo Clinic, Scottsdale, AZ, USA.
References
- 1.Brana MF, Ramos A. Naphthalimides as anti-cancer agents: synthesis and biological activity. Curr Med Chem Anticancer Agents. 2001;1:237–255. doi: 10.2174/1568011013354624. [DOI] [PubMed] [Google Scholar]
- 2.Ratain MJ, Mick R, Berezin F, et al. Phase I study of amonafide dosing based on acetylator phenotype. Cancer Res. 1993;53:2304–2308. [PubMed] [Google Scholar]
- 3.Ratain MJ, Rosner G, Allen SL, et al. Population pharmacodynamic study of amonafide: a cancer and leukemia group B study. J Clin Oncol. 1995;13:741–747. doi: 10.1200/JCO.1995.13.3.741. [DOI] [PubMed] [Google Scholar]
- 4.Mijatovic T, Mahieu T, Bruyère C, et al. UNBS5162, a novel naphthalimide that decreases CXCL chemokine expression in experimental prostate cancers. Neoplasia. 2008;10(6):573–586. doi: 10.1593/neo.08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Quaquebeke E, Mahieu T, Dumont P, et al. 2,2,2-Trichloro-N-({2-[2-(dimethylamino)ethyl]-1,3-dihydro-1H-benzo[de]isoquinolin-5yl}carbamoyl) acetamide (UNBS3157), a novel non-hematotoxic naphthalimide derivative with potent anti-tumor activity. J Med Chem. 2007;50:4122–4134. doi: 10.1021/jm070315q. [DOI] [PubMed] [Google Scholar]
- 6.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 7.Hodges M, Salerno Q, Erlien D. Bazett’s QT correction reviewed. Evidence that a linear QT correction for heart rate is better. J Am Coll Cardiol. 1983;1:694. [Google Scholar]
- 8.Michaels S, Brown DM. Preclinical development of novel naphthalimide formulations. Experimental and Molecular Therapeutics 41: Antiangiogenesis. Proc Am Assoc Cancer Res. 2004;45 Abstract #4576. [Google Scholar]
- 9.Chen Z, Liang X, Zhang H, et al. A new class of naphthalimide-based antitumor agents that inhibit topoisomerase II and induce lysosomal membrane permeabilization and apoptosis. J Med Chem. 2010;53(6):2589–2600. doi: 10.1021/jm100025u. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Wu A, Xu Y, et al. M(2)-A induces apoptosis and G(2)-M arrest via inhibiting PI3K/Akt pathway in HL60 cells. Cancer Lett. 2009;283(2):193–202. doi: 10.1016/j.canlet.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 11.Allen SL, Kolitz JE, Lundberg AS, et al. Phase I trials of amonafide as monotherapy and in combination with cytarabine in patients with poor-risk acute myeloid leukemia. Leuk Res. 2010;34(4):487–491. doi: 10.1016/j.leukres.2009.07.038. [DOI] [PubMed] [Google Scholar]