Abstract
The emphasis in contemporary medical oncology has been “precision” or “personalized” medicine, terms that imply a strategy to improve efficacy through targeted therapies. Similar attempts at precision are occurring in surgical oncology. Sentinel lymph node (SLN) mapping has recently been introduced into the surgical staging of endometrial cancer with the goal to reduce morbidity associated with comprehensive lymphadenectomy, yet obtain prognostic information from lymph node status. The Society of Gynecologic Oncology’s (SGO) Clinical Practice Committee and SLN Working Group reviewed the current literature for preparation of this document. Literature-based recommendations for the inclusion of SLN assessment in the treatment of patients with endometrial cancer are presented. This article examines:
History and various techniques of SLN mapping in endometrial cancer
Pathology and clinical outcomes from SLN assessment
Controversies and future directions for research in SLN assessment in endometrial cancer
Keywords: Endometrial Cancer, Sentinel Lymph Node Mapping, Surgical Staging, Pathology & Clinical Outcomes, Literature Review, Consensus Recommendation
Introduction
Endometrial cancer is the most common gynecologic cancer in North America, and worldwide there are approximately 320,000 cases diagnosed annually. Following the Federation of International Gynecology and Obstetrics (FIGO) adoption of surgical staging in 1988, pathology that includes information about the primary tumor as well as lymph node status has guided prognosis and use of adjuvant therapies. Surgical staging is associated with risks of lymphedema, lymphocysts, cellulitis, and damage to nearby nerves. Sentinel lymph node (SLN) assessment has been proposed as a more “targeted” alternative to complete pelvic lymphadenectomy in an effort to secure information about lymph node status for treatment planning, yet minimize collateral damage. The purpose of this article is to review the current literature regarding SLN assessment in endometrial cancer and to improve outcomes for women with this disease.
SECTION I: HISTORY OF ENDOMETRIAL CANCER SURGICAL STAGING
The value of staging patients with cancer lies in the ability to assess prognosis, plan therapy, and facilitate communication between health care providers. Surgical staging also serves as a research tool to assess treatments among patient groups and for stratification in clinical trials. Prior to 1950, staging endometrial cancer was quite variable between institutions and expert gynecologists. Following the success of standardized staging for cervical cancer in the 1950s, FIGO assumed responsibility of the Annual Report from the Health Organization of the League of Nations. The first FIGO staging system for endometrial cancer was predicated on two criteria alone. Stage I patients had tumor clinically confined to the uterus, and stage 2 patients had disease that had spread beyond the uterus [1]. FIGO staging of uterine carcinoma has since undergone multiple strategic revisions, most notably in 1962 with expansion to a four-stage system, and in 1988 with a change from clinical to surgical staging [1]. Over the past 60 years, FIGO staging progressively evolved to reflect the significant scientific breakthroughs in understanding the histopathology and associated risks of recurrence associated with various risk factors in endometrial cancer. The staging system now includes tumor grade, depth of myometrial invasion, local and regional spread, lymph node metastasis, and distant metastasis.
The addition of lymph node status to FIGO staging followed the publication of the results of Gynecologic Oncology Group (GOG) study 33 [2] and ultimately contributed to the controversy regarding the clinical significance of lymph node metastasis today. The addition of routine lymphadenectomy led to a significantly increased number of clinical stage I uterine cancers that were upstaged to stage III. However, the risk of lymph node metastasis in early-stage, low-grade tumors is relatively low, and the potential morbidity from routine lymphadenectomy may outweigh population-based clinical benefits. While GOG 33 demonstrated an overall risk of metastasis in pelvic and aortic lymph nodes of 9% and 6%, respectively, well-differentiated tumors had a risk of 3% and 2%, and tumor confined to the endometrium conferred an even lower risk of metastasis at 1% [2]..
Multiple studies have attempted to evaluate the impact of routine lymphadenectomy on survival. Some studies support lymphadenectomy for all patients [3], others in higher grade disease only [4], and others report that the determining factor may be the number of nodes removed [5]. All of these trials were retrospective in nature and led to two large randomized European trials. Benedetti Panici et al [6] identified approximately 10% more cases of nodal metastasis with the inclusion of lymphadenectomy. However, despite the increased detection of metastasis, there was no survival advantage and a significantly higher rate of lymphedema was documented in staged patients [6]. These observations were consistent with the results of the ASTEC trial, which also showed no survival benefits and an increase in lymphedema [7]. These trials were criticized for lacking a standardized lymphadenectomy protocol, as well as for inconsistencies in adjuvant therapy. Nonetheless, these phase 3 trials legitimately called into question the role of routine lymphadenectomy in endometrial cancer.
Mariani et al [8] defined a “low-risk” population in whom staging lymphadenectomy may be safely omitted. Based on the histologic criteria from GOG 33 as well as their own historical cohort of patients treated for endometrial cancer, low risk was defined as grade 1 or 2 disease, less than 50% myometrial invasion, and tumor diameter less than 2 cm. These criteria were then used in a prospective observational study that demonstrated patients with low-risk disease (approximately 30% of all the endometrial cancers treated at the Mayo Clinic) had a less than 1% risk of having a positive lymph node or nodal recurrence, compared to a 16% risk of lymph node involvement for endometrioid adenocarcinoma that did not meet these criteria [8]. The Mayo Clinic low-risk group represents a clinically significant number of women who may be able to avoid staging lymphadenectomy. However, the diagnosis depends on intraoperative frozen section, a practice that has variable levels of reported accuracy [9,10] and may potentially lead to understaging some high-risk cases. In contrast, patients with high-grade histologies (endometrioid grade 3, clear cell, serous, and carcinosarcoma) have a 20–40% risk of lymph node involvement [8,11].
SECTION II: HISTORY OF SLN MAPPING
Although the orderly progression of lymphatic metastases has been hypothesized for several hundred years, the first report of SLN mapping success was in 1977, using lymphangiography of the penis [12]. The reproducibility of SLN mapping with radiocolloid for patients with cutaneous melanomas quickly followed, but was not more widely accepted until blue dyes emerged as a way to augment radiotracers in the late 1980s [13]. Since then, SLN mapping techniques have been developed for several other solid malignancies, including breast, vulva, and cervical cancers. Although the concepts are similar, the path to standardization has been variable due to differences in cancer incidence, rates of lymphatic metastasis, and the prognostic or treatment impact of lymph node status for each disease site. An understanding of historical issues can help guide the development of SLN mapping procedures in endometrial cancer.
Cutaneous melanomas were the first neoplasms for which SLN mapping reached widespread acceptance. Radiocolloid and blue dye SLN mapping techniques were refined at single institutions and subsequently published in the early 1990s [13,14]. These studies included large numbers of patients undergoing SLN mapping followed by completion lymphadenectomy so that false-negative rates could be immediately established. Much like endometrial cancer [7], completion lymphadenectomy has not been shown to improve survival in patients with clinically node-negative cutaneous melanomas [15]. Because of this, subsequent randomized trials have focused on whether completion lymphadenectomy is beneficial for patients with SLN metastases. Although plagued by low accrual, two randomized trials failed to demonstrate a benefit with completion lymphadenectomy in patients with cutaneous melanomas [16,17]. As such, SLN mapping has emerged as the sole mechanism of lymphatic assessment in this setting.
Similar to melanoma, initial reports of SLN mapping for breast cancer using lymphoscintigraphy were published from single institutions in the early 1980s. Refinements of the technique have been aided by the large number of patients with breast cancer and high accrual in clinical trials. Although randomized trials demonstrated the absence of a survival advantage with axillary lymphadenectomy for at least some patients with clinical stage I breast cancer as early as 2002 [18], skepticism in the breast surgery community necessitated further trials to confirm the safety of replacing full dissections with SLN mapping. As such, the NSABP B-32 trial randomized patients undergoing SLN mapping to completion axillary lymphadenectomy versus no further assessment and determined both have an acceptable false-negative rate (9.8%) and no difference in survival between groups [19]. Further randomized trials have confirmed that completion lymphadenectomy does not improve survival in patients with SLN metastasis treated with partial mastectomy and tumor-directed radiation [20] and that the identification of micrometastases with immunohistochemistry does not improve survival [21]. As will be discussed later, these issues remain largely unsettled in endometrial cancer.
In gynecologic oncology, SLN mapping first reached acceptance for the management of vulvar cancer. Unlike breast cancer and melanoma, lymphadenectomy appears to improve survival in patients with clinically node-negative vulvar cancer [22]. Randomized trials such as GOG 173 focused on defining the ability of SLN mapping to identify nodal metastases [23]. Given the rarity of vulvar cancer and low rate of lymphatic metastasis, the development of a randomized trial powered to survival has been infeasible. The prospective GROINSS-V1 trial was designed with a historical control for comparison purpose [24]. This trial demonstrated an acceptable recurrence risk and has effectively established SLN mapping as a standard of care for the management of clinically node-negative T1-T2 (≤4 cm) vulvar cancer.
These results demonstrate the varied approaches that have been taken to establish SLN mapping as an acceptable lymphatic assessment strategy in other malignancies. The various SLN approaches tend to mirror the unique challenges of each disease site. Similarly, the relatively low rate of lymphatic metastasis and questionable survival advantage of lymphadenectomy continue to appropriately influence the evolving role of SLN mapping in endometrial cancer.
SECTION III: SLN TECHNIQUES FOR GYNECOLOGIC CANCERS
Colorimetric Methods
Colorimetric lymphatic mapping refers to the visual detection of lymph channels and nodes using colored dyes in white light. This technique requires the least complex equipment and is applicable to open, laparoscopic, and robotic approaches.
Isosulfan blue is FDA approved for lymphatic mapping. Typically, 3–5cc of a 1% solution are injected into the cervix, after which there is immediate uptake of the dye into lymphatic channels and accumulation in the SLNs within 10–20 minutes. Delay from injection to mapping can cause low detection rates due to transit of dye through the node [25]. Injection should be superficial to minimize uptake of dye into deeper tissues. Disadvantages of isosulfan blue include its expense, limited availability, and the risk (1.1%) of allergic reaction (anaphylaxis) [26]. Premedication can be considered to minimize risks of anaphylaxis, and patients should be monitored for 60 minutes after administration. Anaphylaxis is more likely to occur in patients with a history of bronchial asthma, multiple allergies, or allergies to triphenylmethane dyes. Isosulfan blue, like methylene blue, can interfere with the measurement of oxygen saturation in peripheral blood, leading to falsely low oxygen saturation readings.
Methylene blue is a less expensive alternative to isosulfan blue. This is an off-label use of the dye, however, though there is evidence that suggests equivalency for SLN mapping in other cancers [27]. Two to 4 cc of a 1% solution should be injected. It carries risks of paradoxical methemoglobinemia and serotonin syndrome in patients taking serotonergic psychiatric medications.
Radionuclear Method
The injection of radiolabeled colloid technetium 99 (Tc99) and detection with nuclear imaging and/or intraoperative gamma counters is one of the original techniques of SLN mapping utilized in breast, melanoma, and vulvar cancer management [23,28,29]. It is often used in synergy with a blue dye (or indocyanine green [ICG]) to optimize detection rates [30]. The virtue of radiolabeled isotopes is signal penetration through deep tissue, which can be advantageous in patients with endometrial cancer where nodal basins can be fatty and lymphatic drainage can be unpredictable.
A total of 1 mL of 1 mCi of Tc99 is injected. Both open and laparoscopic gamma probes are available. Preoperative lymphoscintigraphy or three-dimensional single photon emission computed tomography with integrated CT (SPECT/CT) can be used to identify the number and location of SLNs [31] but requires a separate injection procedure in nuclear medicine, adding cost and inconvenience. A gamma-detecting probe identifies areas of “hot” tracer signal intraoperatively. After discriminating the general area of increased uptake, the surgeon employs dissection to visually identify blue (or green) dyes in the area of increased gamma signal. The gamma-detecting probe is then used to quantify the signal strength of the resected SLNs.
Near-infrared Method
ICG is a water soluble tricarbocyanine dye that emits a fluorescent signal in the near-infrared (NIR) light range, and it is FDA cleared for vascular and hepatobiliary imaging. Lymphatic mapping is an off-label use of the drug. Optimal detection of SLNs occurs when the drug is diluted by the surgeon to a 0.5 mg/mL to 1.25 mg/mL concentration using sterile water and 2–4 mL are used [32,33]. NIR imagers are filtered to receive the 830 nM wavelength emitted by ICG and visualize the ICG dye. NIR imagers are available for laparotomy, laparoscopy, and robotic surgery. The ICG signal penetrates tissues, but also allows for real-time visualization during dissection, combining the assets of colorimetric and radionuclear techniques. ICG has been shown superior to blue dyes for detection, particularly in obese patients [34–37]. The only disadvantage of this tracer is the requirement for specialized NIR imaging equipment. The risk of adverse events is extremely low for ICG (1/42,000 anaphylaxis); however, it should be avoided in patients with severe iodine allergy or liver failure, as it is excreted completely by the liver.
Injection Sites
The optimal tracer injection site for endometrial cancer has been investigated and reported from several observational studies (Tables 1 and 2) [30,32,35–58]. Sub-serosal uterine fundus, deeper myometrium [59,60] and hysteroscopically guided sub-endometrial tumor injections have been evaluated [49,61]. While these techniques offer higher rates of para-aortic SLN detection [49], cervical injection has become the most favored technique, as it is straightforward and garners the highest SLN detection rates [62,63]. The tracer should be injected slowly into the submucosa or superficial cervical stroma to maximize lymphatic uptake and minimize staining of deep pelvic tissues. Increasing evidence suggests that cervical injection preserves the accuracy of detection of pelvic metastatic disease [37,64] in comprehensively staged patients, which may result from the confluence of lymphatic pathways from different regions of the uterus exiting the cervix through the lateral parametria. It is likely that some para-aortic lymph nodes are reached only via the lymphatics in the infundibulo-pelvic ligaments through deeper cervical injections; however, the accuracy of para-aortic mapping has not been fully investigated or reported.
Table 1.
Comparison between colorimetric, radioactive and fluorescent tracers
| Site of injection | Tracer | Study | N | Surgical approach | SLN detection rate overall (bilateral) | PA SLN detection Rate | Mean SLN per patient |
|---|---|---|---|---|---|---|---|
| Cervix | Blue | Mais et al. 2010 [48] | 34 | L,1 | 62% (NR) | NR | NR |
| Vidal et al. 2013 [56] | 66 | L,1 | 62% (35%) | 0% | 1.8 | ||
| Tanner et al. 2015 [36] | 57 | R | NR (46%) | NR | NR | ||
| Holloway et al, 2017 [37] | 200 | R | 76% (40%) | 2.5% | 2.8 | ||
| Total Blue | 357 | 71% (40%) | 1.9% | 2.6 (686/266) |
|||
| Technetium99 | Niikura et al. 2013 [49] | 45 | L | 96% (80%) | 0% | 3.1 | |
| Sawicki et al. 2015 [52] | 82 | L | 92% (67%) | 5% | 3.1 | ||
| Total Technetium99 | 127 | 93% (71%) | 3.2% | 3.1 | |||
| Blue & Technetium99 | Ballester et al. 2011 [30] | 125 | L,1 | 89% (62%) | 4% | 3 | |
| Barlin et al. 2012 [38] | 498 | L,1,R | 81% (51%) | 3% | 3 | ||
| How et. al 2012 [44] | 100 | R | 92% (66%) | 15% | 2 | ||
| Bats et al. 2013 [39] | 43 | L,1 | 70% (37%) | 0% | 2.9 | ||
| Lopez-de la Manzanara Cano et al 2014 [47] | 50 | L,1 | 92% (34%) | 7% | 1.5 | ||
| Desai et al. 2014 [41] | 120 | R | 86% (52%) | 0% | 2.6 | ||
| Touhami et al. 2015 [55] | 268 | L,1, R | 94% (74%) | NR | 2 | ||
| Total Blue & Technetium99 | 1,204 | 86% (57%) | 4.1% | 2.6 | |||
| ICG | Jewell et al. 2014 [32] | 227J | R | 95% (79%) | 10% | 3 | |
| Plante et al. 2015 [50] | 50p | l,R | 96% (88%) | 3% | 3 | ||
| Tanner et al. 2015 [36] | 54 | R | NR (77%) | NR | NR | ||
| Rossi et al. 2017 [64] | 340 | R | NR (52%) | 23% | 2 | ||
| TOTAL ICG | 671 | 95% (66%) | 12.7% | 2.4 | |||
| ICG & Technetium99 | Mucke et al. 2014 [58] | 31 | L,1 | 90% (52%) | 23% | 1.3 | |
| Blue & ICG & Technetium99 | How et al. 2015 [43] | 100 | R | 92% (76%) | 8% | 2.9 | |
| Blue & ICG | Holloway et al. 2017 [37] | 180 | R | 96% (84%) | 2.8% | 2.9 | |
| Total combinations with ICG | 311 | 94% (78%) | 6.4% | 2.7 (852/311) | |||
| TOTAL CERVIX | 2,670 | 87% (60%) | 5.8% | 2.8 | |||
| Hysterosco pic | Technetium99 | Solima et al. 2012 [53] | 59 | L,1 | 95% (NR) | 56% | 2.6 |
| Niikura et al. 2013 [49] | 55 | L | 78% (49%) | 56% | 2.8 | ||
| Favero et al. 2015 [42] | 42 | l | 73% (NR) | 40% | 1.7 | ||
| Blue & Technetium99 | Delaloye et al. 2007 [40] | 60 | L,1 | 82% (37%) | 27% | 3.7 | |
| TOTAL HYSTERO SCOPIC | 216 | 82% (42%) | 45% | 2.8 | |||
| Myometrial | Blue | Lopes et al. 2007 [46] | 40 | L | 78% (NR) | 35% | 1.6 |
| Li et al. 2007 [57] | 20 | L | 74% (45%) | 3% | 3.9 | ||
| Niikura et al. 2013 [49] | 51 | L | 63% (49%) | NR | 1.7 | ||
| Sawicki et al. 2015 [52] | 82 | L | 74% (44%) | 10% | 3 | ||
| Technetium99 | Tomé et al. 2013 [54] | 74 | l | 74% (19%) | 34% | 2.8 | |
| Blue & Technetium99 | Robova et al. 2009 [51] | 67 | L | 73% (NR) | NR | NR | |
| TOTAL MYOMET RIAL | 334 | 73% (37%) | 21% | 2.6 |
Abbreviations: n = number of patients, ICG= indocyanine green, SLN = sentinel lymph node, NR = not reported, L= laparotomy, l = laparoscopy, R = robotic, PA SLN= Percentage of detected Para-aortic SLNs.
Table 2.
Diagnostic accuracy of sentinel lymph node detection in function of tracer
| Site of injection |
Tracer | Study | n | % mets |
SLN only |
Sensitivity | NPV | MM only |
ITC only |
|---|---|---|---|---|---|---|---|---|---|
| Cervix | Blue | Mais et al. 2010 [48] | 34 | 18% | 60% | 50% | 85% | NR | NR |
| Vidal et al. 2013 [56] | 66 | 9% | NR | 86% | 98% | NR | NR | ||
| Kim et al. 2013 [45] | 504 | 13% | NR | 98% | 100% | 6% | 30% | ||
| Total Blue | 604 | 13% | 60% | 94% | 99% | 6% | 30% | ||
| Blue & Technetium99 | Bats et al. 2007 [39] | 43 | 23% | 60% | 100% | 100% | 20% | 0 | |
| Ballester et al. 2011 [30] | 125 | 15% | 74% | 84% | 97% | 37% | 5% | ||
| How et. al 2012 [60] | 100 | 11% | 36% | 89% | 99% | 36% | 0 | ||
| Lopez-de la Manzanara Cano et al 2014 [47] | 50 | 6% | 100% | 100% | 100% | 0 | 0 | ||
| Desai et al. 2014 [41] | 120 | 8% | NR | 100% | 100% | 50% | 0 | ||
| Touhami et al. 2015 [55] | 268 | 16% | 35% | 97% | 99% | 16% | 28% | ||
| Total Blue & Technetium99 | 706 | 13% | 51% | 95% | 99% | 27% | 11% | ||
| ICG | Sinno et al. 2014 [35] | 71 | 7% | 60% | 100% | 100% | 0 | 20% | |
| Plante et al. 2015 [50] | 42 | 26% | NR | 93% | 99% | 18% | 73% | ||
| Rossi et al. 2017 [64] | 340 | 12% | 60% | 97% | 100% | 26% | 29% | ||
| ICG & Technetium99 | Mucke et al. 2014 [58] | 31 | 19% | 0 | 100% | 100% | NR | NR | |
| Blue & ICG & Technetium99 | How et al. 2015 [43] | 100 | 10% | 70% | 90% | 99% | 30% | 0 | |
| Blue & ICG Total ICG combinations |
Holloway et al. 2017 [37] | 180 764 |
21% 14.5% |
61% 59% |
98% 97% |
99% 99% |
20% 22% |
38% 29% |
|
| Hysterosc opic | Technetium99 | Solima et al. 2012 [53] | 59 | 15% | NR | 90% | 98% | 30% | 30% |
| Favero et al. 2015 [42] | 42 | 22% | NR | 58% | 89% | NR | NR | ||
| Blue & Technetium99 | Delaloye et al. 2007 [40] | 60 | 15% | 78% | 100% | 100% | NR | NR | |
| Myometrial al | Blue | Lopes et al. 2007 [46] | 40 | 28% | 18% | 75% | 96% | 46% | 0 |
| Li et al. 2007 [57] | 20 | 10% | NR | 100% | 100% | NR | NR | ||
| Technetium99 | Torné et al. 2013 [54] | 74 | 18% | NR | 82% | 98% | NR | NR | |
| Blue & Technetium99 | Robova et al. 2009 [51] | 101 | 5% | NR | 100% | 100% | NR | NR | |
| Mixed | Mixed | Niikura et al. 2013 [49] | 100 | 18% | NR | 100% | 100% | 8% | 3% |
| Mixed | Sawicki et al. 2015 [52] | 188 | 10% | NR | 90% | 98% | NR | NR | |
| Mixed non cervix | 684 | 14% | 54% | 91% | 98% | 22% | 10% |
Abbreviations: n = number of patients, % mets = percentage of patients having metastases in the lymph nodes, SLN only = the sentinel node is the only node that contains metastases, NPV = negative predictive value, MM = micrometastasis, ITC = isolated tumor cells, NR = not reported
SECTION IV: SLN PATHOLOGY
“Ultrastaging” refers to the utilization of enhanced pathology techniques, including deeper serial sections and immunohistochemical (IHC) stains, to increase the detection of malignant cells in SLNs [65]. Strategies for the pathologic processing of SLNs, including the number of level sections examined by routine hematoxylin and eosin (H&E) staining, the depth of sectioning into the tissue block, the interval of microns (μms) between sections, and the use of IHC to identify tumor cells not noted on H&E alone, all vary among institutions and within the published literature. The College of American Pathologists (CAP) guidelines for breast SLN processing are to slice along the long axis of the node at 2-mm intervals and examine all slices microscopically with at least 1 representative H&E level [66]. Additional H&E levels or IHC studies may be employed [66]. In contrast, typical histologic examination of a (non-sentinel) lymph node involves a single H&E section along the long axis of the lymph node (either intact or bisected), with deeper levels or IHC performed at the pathologist’s discretion.
There are no formal evidence-based guidelines for the pathologic assessment of SLNs in endometrial cancer. The algorithm proposed by the group at Memorial Sloan Kettering (MSK) consists of an initial evaluation by routine H&E, and if negative, two adjacent 5-μm sections (one H&E and one cytokeratin AE1/AE3) cut from each paraffin block at each of two levels 50 pm apart [38,45]. Holloway et al [67] used a similar approach with H&E-negative nodes sectioned at 50-μm intervals, resulting in three H&E slides and one stained with AE1/AE3. In another study, six serial sections cut at 40-μm intervals were examined on H&E, as well as an AE1/AE3 taken between the third and fourth levels [55]. Alternatively, endometrial SLNs have been examined by leveling the block at 50-μm intervals with levels 1, 3, and 5 stained with H&E and levels 2 and 4 stained with AE1/AE3 [41]; or by bisecting the node, creating a cytologic smear, and then grossly slicing in 3-mm intervals, which in turn, were sectioned at 200-μm intervals, with H&E-negative nodes stained with AE1/AE3 [68,69]. Likely as a result of differences in patient populations studied, and possibly the surgical and pathologic processing techniques used, identification of low-volume disease (micrometastasis and isolated tumor cells [ITCS]) varies widely between institutions and case series (Table 3) [30,41,45,55,67,69]. Ultrastaging can likely be eliminated in endometrioid adenocarcinoma with no myoinvasion (0.8% metastasis of 242 cases) [45].
Table 3.
Analysis of SLN metastases from recent studies
| Author, year | N (with SLN assessment) | Macro-metastasis | Micro-metastasis | ITC (only) | Total LN positive |
|---|---|---|---|---|---|
| Holloway et al 2016 [67] | 119 | 14 (12%) | 10 (8.4%) | 12 (10%) | 36 (30%) |
| Touhami et al 2015 [55] | 268 | 24 (9%) | 7 (2.6%) | 12 (4.5%) | 43 (16%) |
| Desai et al 2014 [41] * | 103 | 5 (4.9%) | 5 (4.9%) | – | 10 (9.7%) |
| Raimond et al 2014 [69] | 136 | 7 (5%) | 15 (11%) | – | 22 (16%) |
| Kim 2013 [45] ** | 508 | 35 (7%) | 4 (0.8%) | 19 (3.7%) | 64 (13%) |
| Ballester et al 2011 [30] | 111 | 8 (7%) | 7 (6.3%) | 1 (0.9%) | 16 (14%) |
| OVERALL | 1,245 | 93 (7.5%) | 48 (3.9%) | 44 (3.5%) | 185 (14.9%) |
IHC positive only (no description of size of metastasis in the SLN)
includes 6 patients with positive non-SLN
Abbreviations: SLN = sentinel lymph node; N = number of patients; ITC = isolated tumor cells; LN = lymph node
According to the American Joint Committee on Cancer (AJCC) Staging guidelines for the staging of breast cancers, macrometastases are defined as groups of malignant cells >2.0 mm. Micrometastases are defined as >0.2 mm and/or >200 cells, but none greater than 2.0 mm. ITC clusters are small clusters of cells not greater than 0.2 mm, present as either single tumor cells or clusters of <200 cells; ITCs can be detected by H&E or by IHC alone [70]..
Per the AJCC breast cancer guidelines, nodes containing ITCs only are not included as positive lymph nodes but are recommended to be included in the total number of nodes evaluated [70]. The “p” is used for patholologic evaluation of nodes (pN), and the following subcategories have been created for use in breast cancers: pN0 (i-) no regional LN metastases and negative IHC, pN0(i+) malignant cells in lymph nodes <0.2 mm (detected by H&E or IHC including ITC), pN1mi micrometastases >0.2 mm and/or more than 200 cells but <2 mm, and pN1a metastases in 1–3 axillary lymph nodes with at least 1 metastasis greater than 2 mm [70]. The 7th edition of the AJCC Staging Manual does not assign similar sub-categories for lymph node metastases in endometrial cancer [70]. In order to better study the significance of ITCs and micrometastases compared to macro-metastatases in endometrial cancer, we may consider using a similar TNM staging nomenclature in the research setting. Whether pN0(i+) and/or pN1mi should be incorporated into routine pathologic staging or considered as FIGO stage III remains to be determined.
SECTION V: SLN MAPPING IN ENDOMETRIAL CANCER
Several observational studies of SLN mapping in endometrial cancer using either single dyes, combinations of dyes, or Tc-99 radiocolloid injected into the cervix have been reported (Tables 1 and 2) [30,32,35–58]. The reproducibility of the cervical injection technique, high success rate, and low-risk for isolated aortic metastasis has led most investigators to use cervical injections of tracers [71]. ICG, with or without the other tracers injected into the cervix, used with fluorescent imaging emerged as the most consistently effective pelvic SLN detection technique in endometrial cancer [32,36]. With the initial studies of SLN mapping by Abu-Rustum et al, a low false-negative rate was demonstrated [62]. The same investigators described a learning curve with an increase in SLN detection from 77% to 94% (p=0.03) following a 30-case experience [72]. Enhanced pathologic analysis with serial sectioning and IHC increased the detection of metastasis by approximately two-fold compared to routine H&E findings in patients undergoing SLN mapping, largely through the detection of micrometastases and ITCs that were not identified on the initial H&E examinations [30,45,67,69].
In a 3-year retrospective analysis of 507 low- and high-risk histology cases undergoing SLN mapping, a gradual decrease in the number of completion lymphadenetomy procedures was identified along with a decrease in the average number of lymph nodes removed [73]. There was no difference in the annual number of cases identified with lymph node metastasis (Y1, 7.0%; Y2, 7.9%; Y3, 7.5%; p=1.0), despite the decreasing proportion of lymphadenectomy cases (Y1, 65.0%; Y2, 35.0%; Y3, 23.0%; p<0.001). The authors suggested that the SLN algorithm may reduce the need for standard lymphadenectomy and did not appear to adversely affect the detection of stage IIIC disease. It has also been recognized that approximately 5% of SLNs are located in areas not routinely dissected with pelvic lymphadenectomy, such as presacral and deep internal iliac lymph nodes [43]. More recently, staging results from patients undergoing lymphadenectomy (N=661) versus SLN mapping plus lymphadenectomy (N=119) were retrospectively compared [67]. Despite equivalency in demographics and uterine tumor pathology risk factors for metastasis, the SLN group had more lymph node metastasis (30.3% vs. 14.7%, p<0.001), more stage IIIC disease (30.2% vs. 14.5%, p<0.001), more GOG high-risk cases (32.8% vs. 21.8%, p=0.013), and received more adjuvant therapy (28.6% vs. 16.3%, p<0.01). The SLN was the only metastasis in 18 (50%) of mapped cases with positive nodes, and the false-negative rate was 2.8%. Performance of SLN mapping with staging lymphadenectomy increased the detection of lymph node metastasis (OR 3.29, 1.87–5.82; p<0.001) [67].
The National Comprehensive Cancer Network (NCCN) SLN Algorithm
The primary objective of SLN mapping in endometrial cancer is to identify the lymph nodes most at risk for metastasis in order to limit complete lymphadenectomy procedures and their associated morbidities. To assure accuracy of staging, SLN mapping requires a high rate of SLN detection, high sensitivity for detection of metastasis, and a low false-negative rate. Suggested reasons for mapping failure include lymphatic obstruction by tumor in cases with clinically positive nodes [38], obesity, and use of blue dye only [36]. Failure to map, therefore, requires side-specific lymphadenectomy in order to assess lymph node status. Any suspiciously enlarged or firm lymph nodes should also be removed irrespective of mapping results. Barlin et al described a reduction in the false-negative rate in patients mapped with blue dye from 15% to 2% when an SLN algorithm that included side-specific lymphadenectomy for mapping failure was followed [38]. The algorithm was published in the 2014 NCCN guidelines for endometrial cancer. Other investigators have confirmed similar reductions in false-negative SLN mapping associated with the use of the NCCN SLN algorithm [56,74,75].
Key Points of the SLN Algorithm and the NCCN Guidelines Version 1.2017
Expertise of the surgeon and attention to technical detail are important factors for mapping success.
Superficial and deep cervical injection of dye emerged as a useful and validated mapping technique.
Complete evaluation of the peritoneal cavity is required (SLN mapping is for clinical stage I, apparent uterine-confined disease).
SLN dissection begins with evaluation of the retroperitoneal spaces and identification of the sentinel drainage pathways that emanate from the parametria, followed by excision of the most proximal lymph nodes in the sentinel pathway.
Any suspicious lymph nodes should be removed regardless of SLN mapping and frozen section analysis may influence the decision to perform para-aortic lymphadenectomy in some cases. However, routine frozen section of SLNs is not advised because of relatively low sensitivity for detection of metastasis in normal appearing lymph nodes, cost, and potential alteration of ultrastaging pathology.
Performance of hemi-pelvic side-specific lymphadenectomy for mapping failure has been shown to reduce false-negative staging.
Enhanced pathology evaluation of SLNs with serial sectioning and IHC stains increases the detection of low-volume metastasis. See section VII for a discussion on the controversies of detecting ITC metastasis.
Oncologic Outcomes with the SLN Algorithm Compared to Lymphadenectomy
There are currently few clinical studies that compare recurrence patterns and survival in women with endometrial cancer staged by the SLN algorithm versus pelvic and aortic lymphadenectomy. Raimond et al reported that SLN biopsies used with lymphadenectomy detected 10% (3-fold) more metastasis than lymphadenectomy and allowed stratification of patients to pelvic radiation versus brachytherapy [69]. There was no difference in recurrence-free survival for SLN-mapped cases (node + or -) and unmapped cases; however, variations in treatments administered under non-protocol physician discretion and an underpowered study prevented making conclusions about SLN effect on survival. The multi-institutional SENTI-ENDO study also evaluated long-term outcomes and the impact of SLN biopsy on survival in 125 patients with clinical stage I or II disease [76]. Similar to the Raimond study, no conclusions could be made about oncologic outcomes other than an effect on choice of adjuvant therapy. Even in “best case” scenarios, not all recurrences can be prevented with staging lymphadenectomy (SLN or complete), but side wall recurrences in the nodal basins should be minimized if there is any value in the identification of appropriate lymph nodes and their treatment. In this context, a recent publication showed an improved 4-year recurrence free survival in a mixed population of patients with low and high risk histologies. How et al reported a 68% reduction of pelvic sidewall recurrences in patients staged with SLN biopsies followed by completion lymphadenectomy compared to routine lymphadenectomy procedures (HR 0.32, p=0.007) [77]. Future studies comparing patterns of disease recurrence in patients with high risk histologies undergoing the SLN algorithm alone without completion lymphadenectomy, to traditional lymphadenectomy will be necessary to confirm the efficacy of the SLN algorithm.
In a comparison of complete lymphadenectomy at the Mayo Clinic (Rochester, MN) to the SLN algorithm at Memorial Sloan Kettering Cancer Center (New York, NY), pelvic node metastasis was identified in 2.6% and 5.1% of patients, respectively (p=0.03), and aortic node metastases in 1.0% and 0.8% respectively (p=0.75). Myometrial invasion was absent in 29% and 57% of tumors, respectively. Despite some differences in patient characteristics and adjuvant therapy, the 3-year disease-free survival rates were not different (96.8% [(95% CI, 95.2–98.5] and 94.9% [95% CI, 92.4–97.5], respectively]. These data support the use of the SLN algorithm for staging patients with endometrioid adenocarcinoma with less than 50% myometrial invasion [78]. The oncologic outcomes of patients with deeply invasive high-grade lesions from the same institutions are currently being analyzed. In a presentation at the 2016 IGCS biennial meeting, Soliman et al [79] reported a SLN detection rate of 89% from a prospective study of high-grade or deeply invasive endometrial cancer for which SLN mapping was followed by completion pelvic and aortic lymphadenectomy. They also confirmed that SLN mapping accurately identified positive nodes when combined with a side-specific lymphadenectomy per the NCCN algorithm [80], with a false-negative rate of 4.5% [79].
Similar survival comparisons have been reported for patients with carcinosarcoma managed with the SLN algorithm versus lymphadenectomy [81]. In one study, of 136 patients with uterine carcinosarcoma, 48 had surgical staging with SLN mapping and 88 had routine lymphadenectomy consisting of pelvic and/or para-aortic lymph node dissection. The median number of lymph nodes removed was 8 and 20, respectively (p ≤ 0.001); however, the median number of positive nodes was similar between the groups (p=0.2). There was no difference in median progression-free survival between the SLN and lymphadenectomy groups (23 vs. 23.2 months, respectively; p=0.7). High-risk uterine papillary serous carcinoma has also been evaluated in a cohort of 248 patients (153 using the SLN algorithm, 95 with routine lymphadenectomy) [82]. For the SLN versus lymphadenectomy groups, the median nodes removed were 12 (range, 1–50) and 21 (range, 1–75), respectively (p<0.001). There were no differences in adjuvant therapy or 2-year progression-free survival (77% vs.71%, respectively, p=0.3) [82]. These data suggest the possible safety of the NCCN SLN algorithm in the surgical staging of high-risk histologies, however larger multi-institutional studies with long-term follow up should be performed before lymphadenectomy is abandoned in high-grade disease.
SECTION VI: CONTROVERSIES IN SLN MAPPING FOR ENDOMETRIAL CANCER
While the current information from SLN mapping studies in endometrial cancer appear quite promising, there are many controversies. The accuracy of the technique across practitioners, appropriate patient selection, optimal treatment algorithm to differentially manage high- and low-grade patients, the role of para-aortic dissection, and the clinical significance of ITC node metastasis require further research.
Is SLN Biopsy Accurate in Detecting Metastatic Disease?
In order to be an acceptable staging procedure, SLN biopsy must have high sensitivity and negative predictive values. The FIRES study included 344 patients with endometrial cancer (100 of whom had high-grade disease) who were SLN mapped and staged by 19 surgeons from 10 institutions [64]. The findings of this trial were consistent with prior smaller series and retrospective analyses with respect to accuracy, with a sensitivity of 97.2% and a negative predictive value of 99.7%. False-negative SLNs appeared more prevalent in patients who failed to map bilaterally. To minimize false-negatives, we recommend close adherence with the NCCN SLN algorithm, which includes completion lymphadenectomy for unmapped sides [38, 80] and the removal of any suspiciously enlarged non-mapped lymph nodes. As with all cancers, patients being offered SLN biopsy for endometrial cancer should be counseled regarding the potential risk for missed occult disease. However, even with a low false-negative rate, SLN mapping increases the detection of metastasis overall compared to routine lymphadenectomy [67,69].
Patient Eligibility and the Integration of Staging Algorithms
Evidence suggests that patients with either low- or high-grade histologies may be candidates for SLN mapping. Oncologic outcomes from institutions that apply SLN-only techniques are comparable those of institutions who practice radical lymphadenectomy approaches inclusive of infrarenal para-aortic dissection for patients with endometrioid carcinomas that are less than 50% myo-invasive [78]. “Low-risk” endometrial cancer is often a retrospective diagnosis, not always available intra-operatively. Nevertheless, comprehensive lymphadenectomy offers little opportunity for clinical benefit in low-risk histology populations. Accurate identification of the minority of seemingly low-risk patients who harbor node metastasis will significantly alter adjuvant therapy prescription and may affect their prognosis. At a minimum, morbidity is likely reduced for patients with low-risk histology using the NCCN SLN algorithm compared to patients undergoing routine lymphadenectomy [80].
Retrospective data support that when high-grade cancers such as carcinosarcoma and uterine papillary serous carcinoma are staged with SLN biopsy, oncologic outcomes appear similar to historical cohorts [81,82]. Pre- and intraoperative assessment for suspicious nodes or extra-uterine disease should be conducted in high-risk (Type II and G3 endometriod) patients, and close adherence to the SLN mapping algorithm is critically important. Until more prospective registry trial information documents further safety and efficacy of SLN mapping in high-risk disease, consideration of “add on” completion lymph node dissections is an acceptable approach [83].
Determining Adjuvant Therapy and the Role of Para-aortic Node Dissection with SLN Biopsy
Staging lymphadenectomy serves an important role in guiding adjuvant therapy. Improved survival has been observed when chemotherapy is prescribed for metastatic endometrial cancer [84], and the location and volume of lymph node metastases can be used to aid in the prescription of tumor-directed radiation therapy for the purpose of local control [85] and possibly survival [86, 87]. When adopting the sentinel lymph node technique, clinicians are provided with qualitatively different information regarding the extent and distribution of lymph node metastases compared to traditional lymphadenectomy, and this may pose challenges for determining optimal adjuvant therapies.
One challenge clinicians face is uncertainty about the disease status of normal-appearing nonsentinel lymph nodes, and the potential for residual metastatic disease. Studies of comprehensively staged patients have shown that residual metastatic disease is present in non-SLN’s in approximately 40% of patients {37, 87]. Careful attention to the appearance of the non-SLN per the surgical algorithm is critically important, and suspiciously enlarged nodes should be removed. It is assumed that any lower volume residual disease in non-SLN will be controlled with adjuvant therapy, and the early studies in high risk histology patients discussed in Section V. seem to support this assumption [79, 81, 82]. Nevertheless, more studies with longer followup are desirable to confirm.
A second challenge with SLN mapping is uncertainly about whether nodal metastases are exclusively pelvic or co-exist with para-aortic disease. Approximately half of patients with positive lymph nodes have disease in both pelvic and para-aortic regions [89, 90]. If clinicians prefer to treat node-positive patients with adjuvant radiation therapy, the uncertainty of paraaortic node status in patients who only receive pelvic SLN biopsies can render decisions about the extent of radiation fields difficult. While approximately 10% of patients have SLNs identified in para-aortic regions [Table 2 & ref. 77], the sensitivity and false negative rates for detection of para-aortic metastases have not been described. Para-aortic lymphadenectomy should be considered for patients with grossly positive pelvic nodes, high-risk histologies, and deep myo-invasion, if the information gained will influence the extent of radiation fields.
Finally, use of the SLN surgical algorithm may be associated with failure to diagnose isolated positive para-aortic disease. The risk for isolated para-aortic nodal metastases is approximately 3% [88, 89]. Failure to identify para-aortic metastases potentially results in failure to prescribe appropriate adjuvant therapy. This issue is particularly relevant with SLN detection using cervical injection of dyes, because of the lower rates of para-aortic SLN detection compared to fundal or intra-tumoral injections [49, 51]. In the FIRES trial, completion para-aortic dissection was performed in 58% of all patients and 74% of patients with high grade cancers. No cases of missed isolated para-aortic nodal metastases were observed among patients who mapped at least one SLN and underwent para-aortic lymphadnectomy, however not all patients underwent an infra-renal dissection. Isolated para-aortic metastases were correctly identified in the para-aortic sentinel nodes following cervical injection in 3(<1%) cases [64].
In order to overcome these challenges with SLN mapping, the following strategies can be considered by clinicians. Preoperative imaging can be performed on patients at high risk for lymph node metastases (high grade tumors) to identify suspicious lymph nodes in the para-aortic region that should be surgically evaluated regardless of mapping results. In addition, frozen section analysis to identify invasion greater than 50% identifies patients at high risk for paraaortic metastasis, as well as positive pelvic nodes. Intra-operatively, surgeons should closely inspect the para-aortic region for the identification of true SLN’s (as opposed to secondary echelon nodes) particularly among those patients who appear to have failed to map a pelvic SLN. Among patients at higher risk for occult para-aortic disease (high grade histologies, deeply invasive uterine tumors, positive pelvic nodes) surgeons can elect to perform para-aortic lymphadenectomy, and rely on the SLN algorithm exclusively for pelvic nodal evaluation.
Post-operatively, pelvic node positive patients who did not undergo para-aortic lymphadenectomy should receive imaging (with PET or CT scans) to evaluate for gross residual non-SLN disease. Radiographic findings can help guide tumor directed radiation, however PET and CT scans suffer from lower sensitivity than lymphadenectomy and cannot detect disease less than 0.5mm. The most controversial option would be re-operation to complete the lymphadenectomy in SLN-positive patients for diagnostic and therapeutic purposes. This option carries with it the greatest potential risk to patients, and should only be contemplated if the results of re-operation would substantially alter the prescription of adjuvant therapy or for purposes of debulking significantly enlarged lymph nodes that were missed at the initial staging procedure. It is important to note that lymphadenectomy with removal of normal-appearing lymph nodes has not been associated with a survival advantage [7]. A more conservative approach is to utilize the results of pelvic SLN sampling and pre or post-operative imaging to guide chemotherapy prescription and radiation fields, reserving extended radiation fields to the para-aortic region for patients with proximal iliac SLN metastases, positive para-aortic findings on imaging, or high grade cancers.
Ultimately the dilemmas that clinicians face in the determination of adjuvant therapy for metastatic endometrial cancer are not novel to the era of the SLN algorithm. Historically, not all patients undergo comprehensive staging [91], and oncologists commonly rely on limited information for determining adjuvant therapy. Additionally, the role of radiation and chemotherapy in the adjuvant setting is not conclusive and is currently being evaluated in the phase III GOG #258 trial that compares chemotherapy to chemo-radiation plus chemotherapy. We await the results of ongoing cooperative group trials that address the optimal therapies for patients with node-positive disease.
Should Adjuvant Treatment for ITCs and/or Micrometastasis be offered?
At this time, the significance of low-volume metastases in endometrial cancer is unclear. Current NCCN breast cancer guidelines state that routine IHC is not recommended, and that treatment decisions should be based on H&E results. However, both the biology and natural history of breast and endometrial cancers are quite different, and the application of breast cancer guidelines to endometrial cancer is untested. For endometrial cancer, low-volume metastases found with ultrastaging make up approximately half of the lymph node metastases identified through SLN assessments (Table 3) [30,41,45,55,67,69]. Occult (IHC positive) lymph node metastases in SLNs or non-SLNs are associated with high-risk uterine features such as lymph-vascular space invasion and deep myometrial invasion, and are associated with a higher rate of recurrence [92,93]. Recurrence rates for patients with SLN micrometastases who are treated with adjuvant therapy approximate those of patients without metastases, but it is uncertain what impact adjuvant therapy has on these patients’ outcomes [94]. The presence of ITCs or micrometastases may represent a prognostic biomarker in terms of survival outcomes, but it is still unknown whether the presence of ITCs should be used as a predictive biomarker, independent of other histopathology risk factors for metastasis or recurrence.
What Criteria Constitute Proficiency for the SLN Technique?
In the absence of learning curve data in endometrial cancer, surgeons should consider following the American Society of Clinical Oncology (ASCO) guidelines for application of the technique in breast cancer [95]. Surgeons should complete at least 20 SLN procedures with concomitant completion lymphadenectomy prior to adopting an SLN algorithm. Quality outcomes that include SLN detection rates (bilateral, unilateral or failed), rates of “empty” SLN specimens, and false-negative SLNs should be continuously monitored and approximate or surpass those reported in the literature. Following the NCCN SLN algorithm increases the detection of metastases and reduces the false-negative rate [80]. It should be cautioned that in low-risk populations (<5% risk for metastasis), documentation of an individual surgeon’s acceptable false-negative rate will require more than 20 cases because of the low frequency of events.
SECTION VII: FUTURE DIRECTIONS
The NCCN SLN mapping algorithm holds great promise as a modern staging strategy for apparent uterine-confined endometrial carcinoma [80]. While awaiting the launch of randomized surgical trials that include an SLN component, several NCI-designated United States cancer centers have embarked on collaborative SLN studies of their endometrial cancer databases, and the results continue to be analyzed. For example, in a comparison of patient cohorts from two separate institutions, the validity of the SLN algorithm was demonstrated with no apparent detriment in surgical staging or oncologic outcomes when patients who underwent staging with the SLN algorithm from one center were compared to patients managed with comprehensive pelvic and aortic lymphadenectomy at another center [78]. Similar collaborative efforts are ongoing to analyze data for higher risk endometrial cancer histology, including deeply myoinvasive carcinomas, as well as serous and clear cell carcinoma. Moreover, large singleinstitution prospective studies utilizing SLN cervical injection followed by pelvic and para-aortic lymphadenectomy to prospectively describe the detection rate and false-negative rate are ongoing, and the encouraging results of these investigations have been recently presented at the SGO and International Gynecologic Cancer Society (IGCS) Annual Meetings [37,79]. With increasing utilization of the SLN algorithm for staging throughout the United States, Canada and Europe, several investigators have proposed and launched registry studies to describe outcomes, quality, cost assessment, and survival data. In addition, several investigators are working on survey questionnaires for patients who underwent lymphadenectomy versus SLN staging using the algorithm to compare long-term morbidity and obtain more data on the incidence of lower extremity lymphedema with both approaches. Hopefully, these ongoing efforts and investigations will add to our knowledge of the potential benefits and side effects of SLN mapping.
Several proposals for a surgical randomized trial with an SLN algorithm component have been presented at the NRG and other national and international cooperative group meetings. One of the concepts that received enthusiasm was a proposal to study SLN mapping in a higher risk group for lymph node metastasis. This resulted in the creation of the STATEC (Selective Targeting of Adjuvant Therapy in Endometrial Cancer) Trial, a randomized phase 3 study comparing hysterectomy with pelvic and para-aortic node dissection with optional SLN mapping versus hysterectomy without para-aortic and pelvic node dissection in patients with intermediate-to high-risk early-stage endometrial cancer.
The significance of ITCs in SLNs as the only finding of extra-uterine disease and the options of treatment for these patients remains the subject of much discussion and interest. Designing a prospective randomized trial for adjuvant therapy for these select cases is probably not feasible from statistical and cost perspectives. The preferred approach to study these issues may be to reach consensus on diagnostic definitions among gynecologic pathologists and standardize adjuvant therapy among SLN investigators, and then follow the outcomes from prospective registries in order to understand the clinical behavior of low-volume metastasis.
The recent advent of NIR fluorescent technology in various laparoscopic and robotic platforms initiated a surge in the use of SLN mapping with ICG as a preferred mapping agent over blue dye or technetium. Several industry device manufacturers are actively developing novel imaging technologies with enhanced computer software to produce more precise and reliable fluorescent imaging. The colored segmented fluorescence (CSF) mode (Pinpoint®, Novadaq, Ontario) allows surgeons to see the operative field in four different modes (picture in picture), including a high-definition colored image, SPY (black and white with highest precision), green overlay pinpoint, and CSF with a color gradient of fluorescent heat map intensity uptake (Figure 1). The FILM trial, a prospective, randomized, open-label multicenter study assessing the safety and utility of PINPOINT® NIR fluorescence imaging in the identification of SLNs in patients with uterine and cervical malignancies, should complete enrollment in 2017.
Figure 1.
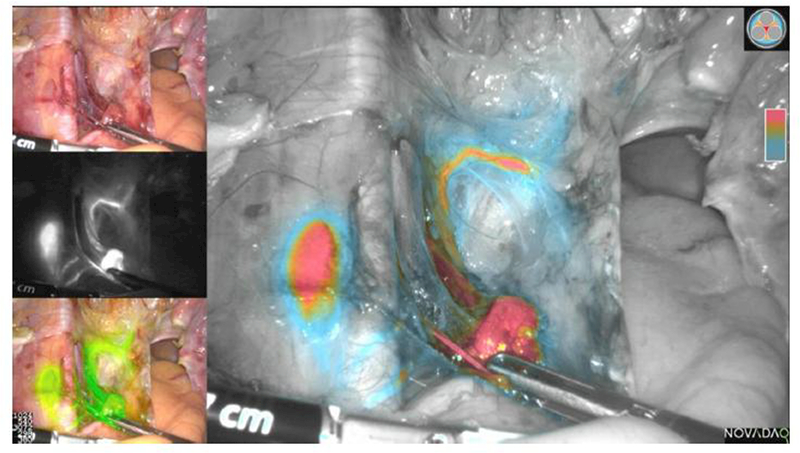
Example of left pelvic sentinel lymph node (SLN) following indocyanine green (ICG) cervical injection in endometrial cancer. The lymphatic trunk, SLN, and secondary node are seen. Colored segmented fluorescence (CSF) mode (Novadaq, Ontario) allows the surgeon to see the operative field in four different modes (picture in picture), including a high-definition colored image (left upper quadrant), SPY (black and white with highest precision), green overlay PINPOINT®, and CSF with a color gradient of fluorescent intensity uptake (red highest, blue lowest).
Investigations going beyond colored dye cervical injections are underway in several institutions. Following the preliminary work by Thorek and Grimm [96], the utilization of positron (FDG) lymphography has been piloted in a recent prospective study at Memorial Sloan Kettering with the objective to investigate whether an FDG cervical injection can map SLN metastasis, and not just the SLN. Other novel ongoing phase 1 SLN trials include one studying the injection of the nanoparticle C-dots (Cornell dots, Cornell University) into the cervix for the development of nanoparticles for molecular imaging in humans [97]. These and similar studies with potentially targeted molecular probes that can be injected systemically or interstitially are currently among the most novel strategies for developing modem surgical staging tools for uterine malignancy. Hopefully, as more specific and targeted molecular approaches are validated for endometrial cancer, including a better understanding of modem molecular profiling and its impact on adjuvant therapy recommendations, these technologies might replace surgical staging for women with endometrial carcinoma. Until that time, SLN mapping with targeted lymph node assessment represents a rational alternative to complete lymphadenectomy for staging endometrial cancer.
SECTION VIII: CONSENSUS RECOMMENDATIONS
Based on the current literature, we recommend that:
For patients with endometrial cancer, SLN mapping by cervical injection of tracers accurately predicts the presence of pelvic lymph node metastasis and has a low (<5%) false-negative rate when the NCCN surgical algorithm is closely followed. It is recommended that completion lymphadenectomy be performed as an “add on” until an individual surgeon’s experience documents literature-comparable success of SLN detection and a <5% falsenegative rate.
Use of ICG dye with NIR fluorescent imaging has similar rates of mapping success to those of radiocolloid Tc-99 combined with blue dye. Radiocolloid Tc-99 combined with dye remains an acceptable approach. When available, cervical injection of ICG dye with infrared imaging is preferable because of technical ease, high success, and reliability.
Patients with low-grade endometrioid adenocarcinoma (grade 1 or 2) are appropriately staged following the NCCN SLN algorithm guidelines (Version 1.2017): SLN mapping can be performed in lieu of routine pelvic lymphadenectomy for patients with apparent uterine-confined gradel and 2 endometrioid cancers.
SLN mapping increases the overall detection of metastasis compared to routine lymphadenectomy. As with all cancers, however, patients should be counseled regarding the potential risk for missed occult disease using SLN biopsy for staging endometrial cancer.
SLN mapping is accurate for detecting pelvic nodal metastasis and some aortic SLNs. Decisions about completion para-aortic dissection should be at the attending surgeon’s discretion based on individualized patient characteristics and tumor-based risk criteria (depth of invasion, histology, and pelvic node status).
Pathologic processing of each SLN should include serial sectioning along the longitudinal plane of the node at 2-mm intervals and microscopic examination of all slices with at least 1 representative H&E level. Pathologic ultrastaging (deeper level sections and/or immunohistochemical studies) increases the detection of ITCs and micrometastasis. The clinical significance of increased detection of ITCs in this setting is currently uncertain and deserves study in well-designed clinical trials.
Incorporating the NCCN SLN mapping algorithm into the staging of high-grade endometrial cancer (grade 3 endometrioid, serous, clear cell, or carcinosarcoma) is feasible and currently utilized by several institutions, with encouraging early results. Completion lymphadenectomy with para-aortic assessment is reasonable in patients with high-grade disease until more data regarding the safety and efficacy of SLN biopsies alone become available.
Highlights.
SLN mapping compared to staging lymphadenectomy in EC may reduce morbidity
Literature-based recommendations for the inclusion of SLN assessments are presented
History and various techniques of SLN mapping in endometrial cancer are described
Pathology and clinical outcomes from SLN assessment are reviewed
Controversies and future directions for research in SLN assessment are discussed
Footnotes
Conflict of Interest Statement
- Dr. Holloway reports personal fees from Intuitive Surgical, Inc., outside the submitted work;
- Dr. Boggess reports personal fees from Intuitive Surgical, Inc., outside the submitted work;
- Dr. Lowery reports personal fees from AstraZeneca, Inc., outside the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mikuta JJ. International Federation of Gynecology and Obstetrics staging of endometrial cancer 1988. Cancer. 1993;71:1460–3. [DOI] [PubMed] [Google Scholar]
- 2.Rungruang B, Olawaiye AB. Comprehensive surgical staging for endometrial cancer. Rev Obstet Gynecol. 2012;5:28–34. [PMC free article] [PubMed] [Google Scholar]
- 3.Kilgore LC, Partridge EE, Alvarez RD, Austin JM, Shingleton HM, Noojin F 3rd, et al. Adenocarcinoma of the endometrium: Survival comparisons of patients with and without pelvic node sampling. Gynecol Oncol. 1995;56:29–33. [DOI] [PubMed] [Google Scholar]
- 4.Trimble EL, Kosary C, Park RC. Lymph node sampling and survival in endometrial cancer. Gynecol Oncol. 1998;71:340–3. [DOI] [PubMed] [Google Scholar]
- 5.Chan JK, Cheung MK, Huh WK, Osann K, Husain A, Teng NN, et al. Therapeutic role of lymph node resection in endometrioid corpus cancer: A study of 12,333 patients. Cancer. 2006;107:1823–30. [DOI] [PubMed] [Google Scholar]
- 6.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–16. [DOI] [PubMed] [Google Scholar]
- 7.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet. 2009;373:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: A paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Case AS, Rocconi RP, Straughn JM Jr., Conner M, Novak L, Wang W, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006;108:1375–9. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Bandyopadhyay S, Semaan A, Shah JP, Mahdi H, Morris R, et al. The role of frozen section in surgical staging of low risk endometrial cancer. PLoS ONE. 2011;6:e21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altman AD, Ferguson SE, Atenafu EG, Kobel M, McAlpine JN, Panzarella T, et al. Canadian high risk endometrial cancer (CHREC) consortium: Analyzing the clinical behavior of high risk endometrial cancers. Gynecol Oncol. 2015;139:268–74. [DOI] [PubMed] [Google Scholar]
- 12.Cabanas RM. An approach for the treatment of penile carcinoma. Cancer. 1977;39:456–66. [DOI] [PubMed] [Google Scholar]
- 13.Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9. [DOI] [PubMed] [Google Scholar]
- 14.Albertini JJ, Cruse CW, Rapaport D, Wells K, Ross M, DeConti R, et al. Intraoperative radio-lympho-scintigraphy improves sentinel lymph node identification for patients with melanoma. Ann Surg. 1996;223:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sim FH, Taylor WF, Pritchard DJ, Soule EH. Lymphadenectomy in the management of stage I malignant melanoma: A prospective randomized study. Mayo Clin Proc. 1986;61:697–705. [DOI] [PubMed] [Google Scholar]
- 16.Leiter U, Stadler R, Mauch C, Hohenberger W, Brockmeyer N, Berking C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17:757–67. [DOI] [PubMed] [Google Scholar]
- 17.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17. [DOI] [PubMed] [Google Scholar]
- 18.Martelli G, Boracchi P, De Palo M, Pilotti S, Oriana S, Zucali R, et al. A randomized trial comparing axillary dissection to no axillary dissection in older patients with T1N0 breast cancer: Results after 5 years of follow-up. Ann Surg. 2005;242:1–6; discussion 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA. 2011;305:569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langer I, Guller U, Viehl CT, Moch H, Wight E, Harder F, et al. Axillary lymph node dissection for sentinel lymph node micrometastases may be safely omitted in early-stage breast cancer patients: Long-term outcomes of a prospective study. Ann Surg Oncol. 2009;16:3366–74. [DOI] [PubMed] [Google Scholar]
- 22.Stehman FB, Bundy BN, Thomas G, Varia M, Okagaki T, Roberts J, et al. Groin dissection versus groin radiation in carcinoma of the vulva: A Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 1992;24:389–96. [DOI] [PubMed] [Google Scholar]
- 23.Levenback CF, Ali S, Coleman RL, Gold MA, Fowler JM, Judson PL, et al. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: A gynecologic oncology group study. J Clin Oncol. 2012;30:3786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oonk MH, van Hemel BM, Hollema H, de Hullu JA, Ansink AC, Vergote I, et al. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: results from GROINSS-V, a multicentre observational study. Lancet Oncol. 2010;11:646–52. [DOI] [PubMed] [Google Scholar]
- 25.Kushner DM, Connor JP, Wilson MA, Hafez GR, Chappell RJ, Stewart SL, et al. Laparoscopic sentinel lymph node mapping for cervix cancer - a detailed evaluation and time analysis. Gynecol Oncol. 2007;106:507–12. [DOI] [PubMed] [Google Scholar]
- 26.Albo D, Wayne JD, Hunt KK, Rahlfs TF, Singletary SE, Ames FC, et al. Anaphylactic reactions to isosulfan blue dye during sentinel lymph node biopsy for breast cancer. Am J Surg. 2001;182:393–8. [DOI] [PubMed] [Google Scholar]
- 27.Blessing WD, Stolier AJ, Teng SC, Bolton JS, Fuhrman GM. A comparison of methylene blue and lymphazurin in breast cancer sentinel node mapping. Am J Surg. 2002;184:341–5. [DOI] [PubMed] [Google Scholar]
- 28.Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–53. [DOI] [PubMed] [Google Scholar]
- 29.Gipponi M, Di Somma C, Peressini A, Solari N, Gliori S, Nicolo G, et al. Sentinel lymph node biopsy in patients with Stage I/II melanoma: Clinical experience and literature review. J Surg Oncol. 2004;85:133–40. [DOI] [PubMed] [Google Scholar]
- 30.Ballester M, Dubernard G, Lecuru F, Heitz D, Mathevet P, Marret H, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: A prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011;12:469–76. [DOI] [PubMed] [Google Scholar]
- 31.Perissinotti A, Paredes P, Vidal-Sicart S, Torne A, Albela S, Navales I, et al. Use of SPECT/CT for improved sentinel lymph node localization in endometrial cancer. Gynecol Oncol. 2013;129:42–8. [DOI] [PubMed] [Google Scholar]
- 32.Jewell EL, Huang JJ, Abu-Rustum NR, Gardner GJ, Brown CL, Sonoda Y, et al. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies. Gynecol Oncol. 2014;133:274–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi EC, Ivanova A, Boggess JF. Robotically assisted fluorescence-guided lymph node mapping with ICG for gynecologic malignancies: A feasibility study. Gynecol Oncol. 2012;124:78–82. [DOI] [PubMed] [Google Scholar]
- 34.Holloway RW, Bravo RA, Rakowski JA, James JA, Jeppson CN, Ingersoll SB, Ahmad S. Detection of sentinel lymph nodes in patients with endometrial cancer undergoing robotic-assisted staging: A comparison of colorimetric and fluorescence imaging. Gynecol Oncol. 2012;126:25–9. [DOI] [PubMed] [Google Scholar]
- 35.Sinno AK, Fader AN, Roche KL, Giuntoli RL 2nd, Tanner EJ. A comparison of colorimetric versus fluorometric sentinel lymph node mapping during robotic surgery for endometrial cancer. Gynecol Oncol. 2014;134:281–6. [DOI] [PubMed] [Google Scholar]
- 36.Tanner EJ, Sinno AK, Stone RL, Levinson KL, Long KC, Fader AN. Factors associated with successful bilateral sentinel lymph node mapping in endometrial cancer. Gynecol Oncol. 2015;138:542–7. [DOI] [PubMed] [Google Scholar]
- 37.Holloway RW, Ahmad S, Kendrick JE, Bigsby GE, Brudie LA, Ghurani GB, et al. A prospective cohort study comparing colorimetric and fluorescent imaging for sentinel lymph node mapping in endometrial cancer. Ann Surg Oncol. 2017; DOI: 10.1245/s10434-017-5825-3. [DOI] [PubMed] [Google Scholar]
- 38.Barlin JN, Khoury-Collado F, Kim CH, Leitao MM Jr., Chi DS, Sonoda Y, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;125:531–5. [DOI] [PubMed] [Google Scholar]
- 39.Bats AS, Mathevet P, Buenerd A, Orliaguet I, Mery E, Zerdoud S, et al. The sentinel node technique detects unexpected drainage pathways and allows nodal ultrastaging in early cervical cancer: Insights from the multicenter prospective SENTICOL study. Ann Surg Oncol. 2013;20:413–22. [DOI] [PubMed] [Google Scholar]
- 40.Delaloye JF, Pampallona S, Chardonnens E, Fiche M, Lehr HA, De Grandi P, et al. Intraoperative lymphatic mapping and sentinel node biopsy using hysteroscopy in patients with endometrial cancer. Gynecol Oncol. 2007;106:89–93. [DOI] [PubMed] [Google Scholar]
- 41.Desai PH, Hughes P, Tobias DH, Tchabo N, Heller PB, Dise C, et al. Accuracy of robotic sentinel lymph node detection (RSLND) for patients with endometrial cancer (EC). Gynecol Oncol. 2014;135:196–200. [DOI] [PubMed] [Google Scholar]
- 42.Favero G, Pfiffer T, Ribeiro A, Carvalho JP, Baracat EC, Mechsner S, et al. Laparoscopic sentinel lymph node detection after hysteroscopic injection of technetium-99 in patients with endometrial cancer. Int J Gynecol Cancer. 2015;25:423–30. [DOI] [PubMed] [Google Scholar]
- 43.How J, Gotlieb WH, Press JZ, Abitbol J, Pelmus M, Ferenczy A, et al. Comparing indocyanine green, technetium, and blue dye for sentinel lymph node mapping in endometrial cancer. Gynecol Oncol. 2015;137:436–42. [DOI] [PubMed] [Google Scholar]
- 44.How J, Lau S, Press J, Ferenczy A, Pelmus M, Stern J, et al. Accuracy of sentinel lymph node detection following intra-operative cervical injection for endometrial cancer: A prospective study. Gynecol Oncol. 2012;127:332–7. [DOI] [PubMed] [Google Scholar]
- 45.Kim CH, Soslow RA, Park KJ, Barber EL, Khoury-Collado F, Barlin JN, et al. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int J Gynecol Cancer. 2013;23:964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopes LA, Nicolau SM, Baracat FF, Baracat EC, Goncalves WJ, Santos HV, et al. Sentinel lymph node in endometrial cancer. Int J Gynecol Cancer. 2007;17:1113–7. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-De la Manzanara Cano C, Cordero Garcia JM, Martin-Francisco C, Pascual-Ramirez J, Parra CP, Cespedes Casas C. Sentinel lymph node detection using 99mTc combined with methylene blue cervical injection for endometrial cancer surgical management: A prospective study. Int J Gynecol Cancer. 2014;24:1048–53. [DOI] [PubMed] [Google Scholar]
- 48.Mais V, Peiretti M, Gargiulo T, Parodo G, Cirronis MG, Melis GB. Intraoperative sentinel lymph node detection by vital dye through laparoscopy or laparotomy in early endometrial cancer. J Surg Oncol. 2010;101:408–12. [DOI] [PubMed] [Google Scholar]
- 49.Niikura H, Kaiho-Sakuma M, Tokunaga H, Toyoshima M, Utsunomiya H, Nagase S, et al. Tracer injection sites and combinations for sentinel lymph node detection in patients with endometrial cancer. Gynecol Oncol. 2013;131:299–303. [DOI] [PubMed] [Google Scholar]
- 50.Plante M, Touhami O, Trinh XB, Renaud MC, Sebastianelli A, Grondin K, et al. Sentinel node mapping with indocyanine green and endoscopic near-infrared fluorescence imaging in endometrial cancer. A pilot study and review of the literature. Gynecol Oncol. 2015;137:443–7. [DOI] [PubMed] [Google Scholar]
- 51.Robova H, Charvat M, Strnad P, Hrehorcak M, Taborska K, Skapa P, et al. Lymphatic mapping in endometrial cancer: Comparison of hysteroscopic and subserosal injection and the distribution of sentinel lymph nodes. Int J Gynecol Cancer. 2009;19:391–4. [DOI] [PubMed] [Google Scholar]
- 52.Sawicki S, Lass P, Wydra D. Sentinel lymph node biopsy in endometrial cancer: Comparison of 2 detection methods. Int J Gynecol Cancer. 2015;25:1044–50. [DOI] [PubMed] [Google Scholar]
- 53.Solima E, Martinelli F, Ditto A, Maccauro M, Carcangiu M, Mariani L, et al. Diagnostic accuracy of sentinel node in endometrial cancer by using hysteroscopic injection of radiolabeled tracer. Gynecol Oncol. 2012;126:419–23. [DOI] [PubMed] [Google Scholar]
- 54.Torne A, Pahisa J, Vidal-Sicart S, Martinez-Roman S, Paredes P, Puerto B, et al. Transvaginal ultrasound-guided myometrial injection of radiotracer (TUMIR): A new method for sentinel lymph node detection in endometrial cancer. Gynecol Oncol. 2013;128:88–94. [DOI] [PubMed] [Google Scholar]
- 55.Touhami O, Trinh XB, Gregoire J, Sebastianelli A, Renaud MC, Grondin K, et al. Predictors of non-sentinel lymph node (non-SLN) metastasis in patients with sentinel lymph node (SLN) metastasis in endometrial cancer. Gynecol Oncol. 2015;138:41–5. [DOI] [PubMed] [Google Scholar]
- 56.Vidal F, Leguevaque P, Motton S, Delotte J, Ferron G, Querleu D, et al. Evaluation of the sentinel lymph node algorithm with blue dye labeling for early-stage endometrial cancer in a multicentric setting. Int J Gynecol Cancer. 2013;23:1237–43. [DOI] [PubMed] [Google Scholar]
- 57.Li B, Li XG, Wu LY, Zhang WH, Li SM, Min C. A pilot study of sentinel lymph node identification in patients with endometrial cancer. Bull Cancer. 2007;94:E1–4. [PubMed] [Google Scholar]
- 58.Mucke J, Klapdor R, Schneider M, Langer F, Gratz KF, Hillemanns P. Isthmocervical labelling and SPECT/CT for optimized sentinel detection in endometrial cancer: Technique, experience and results. Gynecol Oncol. 2014;134:287–92. [DOI] [PubMed] [Google Scholar]
- 59.Burke TW, Levenback C, Tornos C, Morris M, Wharton JT, Gershenson DM. Intraabdominal lymphatic mapping to direct selective pelvic and paraaortic lymphadenectomy in women with high-risk endometrial cancer: Results of a pilot study. Gynecol Oncol. 1996;62:169–73. [DOI] [PubMed] [Google Scholar]
- 60.Holub Z, Jabor A, Kliment L. Comparison of two procedures for sentinel lymph node detection in patients with endometrial cancer: A pilot study. Eur J Gynaecol Oncol. 2002;23:53–7. [PubMed] [Google Scholar]
- 61.Perrone AM, Casadio P, Formelli G, Levorato M, Ghi T, Costa S, et al. Cervical and hysteroscopic injection for identification of sentinel lymph node in endometrial cancer. Gynecol Oncol. 2008;111:62–7. [DOI] [PubMed] [Google Scholar]
- 62.Abu-Rustum NR, Khoury-Collado F, Pandit-Taskar N, Soslow RA, Dao F, Sonoda Y, et al. Sentinel lymph node mapping for grade 1 endometrial cancer: Is it the answer to the surgical staging dilemma? Gynecol. Oncol 2009;113:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rossi EC, Jackson A, Ivanova A, Boggess JF. Detection of sentinel nodes for endometrial cancer with robotic assisted fluorescence imaging: Cervical versus hysteroscopic injection. Int J Gynecol Cancer. 2013;23:1704–11. [DOI] [PubMed] [Google Scholar]
- 64.Rossi EC, Kowalski LD, Scalici J, Cantrell L, Schuler K, Hanna RK, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384–392. [DOI] [PubMed] [Google Scholar]
- 65.Cochran AJ. Prediction of outcome for patients with cutaneous melanoma. Pigment Cell Res. 1997;10:162–7. [DOI] [PubMed] [Google Scholar]
- 66.College of American Pathologists. Protocol for the Examination of Specimens From Patients With Invasive Carcinoma of the Breast. Accessed November 21, 2016 http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebCont ent/pdf/cp-breast-invasive-16protocol-3300.pdf
- 67.Holloway RW, Gupta S, Stavitzski NM, Zhu X, Takimoto EL, Gubbi A, et al. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecol Oncol. 2016;141:206–10. [DOI] [PubMed] [Google Scholar]
- 68.Ballester M, Naoura I, Chereau E, Seror J, Bats AS, Bricou A, et al. Sentinel node biopsy upstages patients with presumed low- and intermediate-risk endometrial cancer: Results of a multicenter study. Ann Surg Oncol. 2013;20:407–12. [DOI] [PubMed] [Google Scholar]
- 69.Raimond E, Ballester M, Hudry D, Bendifallah S, Darai E, Graesslin O, et al. Impact of sentinel lymph node biopsy on the therapeutic management of early-stage endometrial cancer: Results of a retrospective multicenter study. Gynecol Oncol. 2014;133:506–11. [DOI] [PubMed] [Google Scholar]
- 70.Compton CC, Byrd DR, Garcia-Aguilar J, Kurtzman SH, Olawaiye A, Washington MK. AJCC Cancer Staging Atlas. New York, NY: Springer; 2012. [Google Scholar]
- 71.Kang S, Yoo HJ, Hwang JH, Lim MC, Seo SS, Park SY. Sentinel lymph node biopsy in endometrial cancer: Meta-analysis of 26 studies. Gynecol Oncol. 2011;123:522–7. [DOI] [PubMed] [Google Scholar]
- 72.Khoury-Collado F, Glaser GE, Zivanovic O, Sonoda Y, Levine DA, Chi DS, et al. Improving sentinel lymph node detection rates in endometrial cancer: How many cases are needed? Gynecol Oncol. 2009;115:453–5. [DOI] [PubMed] [Google Scholar]
- 73.Leitao MM Jr, Khoury-Collado F, Gardner G, Sonoda Y, Brown CL, Alektiar KM, et al. Impact of incorporating an algorithm that utilizes sentinel lymph node mapping during minimally invasive procedures on the detection of stage IIIC endometrial cancer. Gynecol Oncol. 2013;129:38–41. [DOI] [PubMed] [Google Scholar]
- 74.Ehrisman J, Secord AA, Berchuck A, Lee PS, Di Santo N, Lopez-Acevedo M, et al. Performance of sentinel lymph node biopsy in high-risk endometrial cancer. Gynecol Oncol Rep. 2016;17:69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hagen B, Valla M, Aune G, Ravlo M, Abusland AB, Araya E, et al. Indocyanine green fluorescence imaging of lymph nodes during robotic-assisted laparoscopic operation for endometrial cancer. A prospective validation study using a sentinel lymph node surgical algorithm. Gynecol Oncol. 2016;143:479–83. [DOI] [PubMed] [Google Scholar]
- 76.Darai E, Dubernard G, Bats AS, Heitz D, Mathevet P, Marret H, et al. Sentinel node biopsy for the management of early stage endometrial cancer: Long-term results of the SENTI-ENDO study. Gynecol Oncol. 2015;136:54–9. [DOI] [PubMed] [Google Scholar]
- 77.How J, Gauthier C, Abitbol J, Lau S, Salvador S, Gotlieb R et al. Impact of sentinel lymph node mapping on recurrence patterns in endometrial cancer. Gynecol Oncol. 2017. 144 (3): 503–509. [DOI] [PubMed] [Google Scholar]
- 78.Zahl Eriksson AG, Ducie J, Ali N, McGree ME, Weaver AL, Bogani G, et al. Comparison of a sentinel lymph node and a selective lymphadenectomy algorithm in patients with endometrioid endometrial carcinoma and limited myometrial invasion. Gynecol Oncol. 2016;140:394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soliman P, Westin S, Dioun S, Sun C, Euscher E, Munsell M, et al. Sentinel lymph node mapping accurately identifies positive nodes in women with high risk endometrial cancer: A prospective validation trial. Int J Gynecol Cancer. 2016;26(Suppl. 3):68. [Google Scholar]
- 80.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Uterine Neoplasms, Version 1.2017. 2016.
- 81.Schiavone MB, Zivanovic O, Zhou Q, Leitao MM Jr., Levine DA, Soslow RA, et al. Survival of patients with uterine carcinosarcoma undergoing sentinel lymph node mapping. Ann Surg Oncol. 2016;23:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schiavone MB, Scelzo C, Straight CE, Zhou Q, Alektiar K, Makker V, et al. Survival of patients with serous uterine carcinoma undergoing sentinel lymph node mapping. Ann Surg Oncol. 2017; DOI: 10.1245/s10434-017-5816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller DS. Patients with endometrial cancer at risk for lymphatic metastasis should undergo pelvic and periaortic lymphadenectomy as part of their initial surgery. Cancer. 2017;123:192–6. [DOI] [PubMed] [Google Scholar]
- 84.Randall M, Filiaci V, Muss H, Spirtos N, Mannel R, Fowler J, Thigpen JT, Benda J. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 24(1): 36–44, 2006. [DOI] [PubMed] [Google Scholar]
- 85.Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 2006; 103:155–159 [DOI] [PubMed] [Google Scholar]
- 86.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of paraaortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375:1165–72. [DOI] [PubMed] [Google Scholar]
- 87.Secord AA, Geller MA, Broadwater G, Holloway RW, Shuler K, Dao NY, et al. A multicenter evaluation of adjuvant therapy in women with optimally resected stage IIIC endometrial cancer. Gynecol Oncol. 2013;128:65–70. [DOI] [PubMed] [Google Scholar]
- 88.Khoury-Collado F, Murray MP, Hensley ML, Sonoda Y, Alektiar KM, Levine DA, et al. Sentinel lymph node mapping for endometrial cancer improves the detection of metastatic disease to regional lymph nodes. Gynecol Oncol. 2011;122:251–4. [DOI] [PubMed] [Google Scholar]
- 89.Kumar S, Podratz KC, Bakkum-Gamez JN, Dowdy SC, Weaver AL, McGree ME, et al. Prospective assessment of the prevalence of pelvic, paraaortic and high paraaortic lymph node metastasis in endometrial cancer. Gynecol Oncol. 2014;132:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.James JA, Rakowski JA, Jeppson CN, Stavitzski NM, Ahmad S, Holloway RW. Robotic transperitoneal infra-renal aortic lymphadenectomy in early-stage endometrial cancer. Gynecol Oncol. 2015;136:285–92. [DOI] [PubMed] [Google Scholar]
- 91.Foote JR, Gaillard S, Broadwater G, et al. Disparities in the surgical staging of high-grade endometrial cancer in the United States. Gynecol Oncol Res Pract 2017;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Todo Y, Suzuki Y, Azuma M, Hatanaka Y, Konno Y, Watari H, et al. Ultrastaging of paraortic lymph nodes in stage IIIC1 endometrial cancer: A preliminary report. Gynecol Oncol. 2012;127:532–7. [DOI] [PubMed] [Google Scholar]
- 93.Yabushita H, Shimazu M, Yamada H, Sawaguchi K, Noguchi M, Nakanishi M, et al. Occult lymph node metastases detected by cytokeratin immunohistochemistry predict recurrence in node-negative endometrial cancer. Gynecol Oncol. 2001;80:139–44. [DOI] [PubMed] [Google Scholar]
- 94.St Clair CM, Eriksson AG, Ducie JA, Jewell EL, Alektiar KM, Hensley ML, et al. Low-volume lymph node metastasis discovered during sentinel lymph node mapping for endometrial carcinoma. Ann Surg Oncol. 2016;23:1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20. [DOI] [PubMed] [Google Scholar]
- 96.Thorek DL, Abou DS, Beattie BJ, Bartlett RM, Huang R, Zanzonico PB, et al. Positron lymphography: Multimodal, high-resolution, dynamic mapping and resection of lymph nodes after intradermal injection of 18F-FDG. J Nucl Med. 2012;53:1438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bradbury MS, Phillips E, Montero PH, Cheal SM, Stambuk H, Durack JC, et al. Clinically-translated silica nanoparticles as dual-modality cancer-targeted probes for image-guided surgery and interventions. Integr Biol. 2013;5:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


