Abstract
Posttraumatic stress disorder (PTSD) is a chronic, debilitating condition for which effective medications are scant and little is known about neural correlates of risk versus resilience. Oxytocin is a hypothalamic neuropeptide that has demonstrated promise in modulating neurobiological and behavioral correlates of PTSD. Cognitive deficits in areas such as working memory and executive control are highly prevalent among individuals with PTSD and oxytocin might modulate these impairments in individuals with PTSD. Using a double-blind, placebo-controlled design, this study employed functional magnetic resonance imaging (fMRI) and the N-back working memory task to examine the effects of oxytocin (24 IU) versus placebo on working memory and dorsolateral prefrontal cortex connectivity among individuals with PTSD (n=16) as compared to a trauma-exposed control group (n=18). Results indicate that individuals with PTSD on oxytocin performed better in the 2-back condition of the N-back task compared to individuals with PTSD on placebo. Results also indicate that connectivity between dorsolateral prefrontal cortex and anterior cingulate increased in the 2-back condition among individuals with PTSD on oxytocin as compared to placebo. These findings provide preliminary evidence of an effect of oxytocin on working memory among individuals with PTSD and insights into the neurobiological mechanisms underlying this association. Future studies are necessary to understand the mechanisms responsible for working memory deficits in PTSD and to examine the potential of oxytocin for use as a treatment for PTSD. NCT01963078
Keywords: Oxytocin, PTSD, resilience, working memory, translational research
Introduction
Posttraumatic Stress Disorder (PTSD) is a chronic, debilitating psychiatric disorder affecting approximately 8% of the general U.S. population (Kessler, Petukhova, Sampson, Zaslavsky, & Wittchen, 2012). Exposure to trauma during childhood and adolescence is highly prevalent and greatly increases the risk for developing PTSD (Gilbert et al., 2009; Green et al., 2010). With regard to treatment, several behavioral interventions have demonstrated efficacy in the treatment of PTSD. However, there remains a scarcity of effective pharmacological treatments available (Jonas et al., 2013; The Management of Posttraumatic Stress Disorder Work Group, 2017). Thus, developing more effective pharmacologic treatment options for PTSD is a significant priority (Ipser & Stein, 2012; Sofuoglu, Rosenheck, & Petrakis, 2014).
Abundant literature demonstrates that one problematic area of PTSD symptomatology, and a potential target for PTSD intervention development, is executive functioning (Aupperle, Melrose, Stein, & Paulus, 2012; Polak, 2012; Woon, Farrer, Braman, Mabey, & Hedges, 2017).. Childhood trauma in particular is known to result in problematic neurobiological changes that impair executive functioning (Kaufman, Plotsky, Nemeroff, & Charney, 2000; Majer, Nater, Lin, Capuron, & Reeves, 2010; Nemeroff, 2004; Philip et al., 2016). Executive functioning impairments are associated with distress and functional problems including sleep disturbance, intrusive thoughts, and maladaptive rumination (Bomyea & Lang, 2015; Bomyea, Stein, & Lang, 2015; Cox, Ebesutani, & Olatunji, 2016; Martindale, Morissette, Rowland, & Dolan, 2017), all of which are known contributors to severely negative sequelae of PTSD, including suicidality (Bomyea & Lang, 2015). In addition, emerging literature suggests that executive functioning is both a prognostic indicator of PTSD treatment outcomes as well as a marker of successful symptom reduction (Haaland, Sadek, Keller, & Castillo, 2016; Scott et al., 2017; Walter, Palmieri, & Gunstad, 2010). Working memory is among the most saliently impaired aspects of executive functioning in PTSD (Scott et al., 2015). Thus, identifying pathways to improve elements of executive functioning such as working memory may be critical to reducing PTSD symptomatology.
Neural correlates of executive functioning and working memory, in particular, include the dorsolateral prefrontal cortex (DLPFC), anterior cingulate, and inferior parietal cortex (Hartley & Speer, 2000). These and other brain regions have been identified as necessary components for accurate rehearsal, maintenance or manipulation of information in working memory (Barbey, 2013; Frank, Loughry, & O’Reilly, 2001; Schaefer et al., 2006). Notably, one recent preliminary study of healthy adults compared to resilient adults exposed to childhood adversity found greater activation in the superior temporal gyrus/insula, left inferior parietal lobule, and middle temporal and parahippocampal gyrus during the n-back task among individuals with childhood adversity (Philip et al., 2016). While the use of neuroimaging has expanded extensively in recent medication development efforts for PTSD, relatively few studies have examined neural mechanisms of risk and resiliency for PTSD (van der Werff, van den Berg, Pannekoek, Elzinga, & Van Der Wee, 2013). This scarcity of knowledge might contribute to the limited pharmacological treatment options available for PTSD.
Examination of the neuropeptide oxytocin for the prevention and treatment of PTSD has expanded tremendously in recent years with promising findings (Koch et al., 2014; Olff, Langeland, Witteveen, & Denys, 2010; van Zuiden et al., 2016). Preclinical and clinical research shows that oxytocin has anxiolytic effects (K. MacDonald & MacDonald, 2010; Missig, Ayers, Schulkin, & Rosen, 2010) and attenuates anxiety and fear responses even among individuals with anxiety and PTSD (Acheson et al., 2013; Eckstein et al., 2014; Frijling et al., 2014). Recent literature has demonstrated that oxytocin might attenuate neurobiological underpinnings of PTSD (Flanagan, Baker, McRae, Brady, & Moran-Santa Maria, 2015; Koch et al., 2016; Nawijn et al., 2016). Oxytocin may also enhance behavioral PTSD treatment (Flanagan, Sippel, Wahlquist, Moran-Santa Maria, & Back, in press) and prevent PTSD (van Zuiden et al., 2016). Furthermore, research indicates that dysregulation of the corticolimbic brain circuitry is centrally involved in the pathophysiology of PTSD (Bethlehem, van Honk, Auyeung, & Baron-Cohen, 2013; Brown et al., 2013; Stevens et al., 2013) and recent neuroimaging studies show that oxytocin might modulate connectivity networks with compromised function in PTSD (Bethlehem et al., 2013; Cohen et al., 2010; Dodhia et al., 2014; Frijling et al., 2016; Kirsch et al., 2005; Koch et al., 2016). A growing number of previous studies have examined neural correlates of working memory specifically among individuals with PTSD (Honzel, Justus, & Swick, 2014; McDermott et al., 2016; Tian et al., 2014), and many clinical studies have found desired effects of oxytocin on behavioral and neural factors associated with executive function among individuals with PTSD, such as social memory and fear extinction and recall (Acheson et al., 2013; Guzmán et al., 2014). However, the only studies that have examined the effects of oxytocin on working memory specifically have focused on individuals with schizophrenia (Feifel, MacDonald, Cobb, & Minassian, 2012; Michalopoulou et al., 2015).
This preliminary study addresses several gaps in the literature examining oxytocin and PTSD. First, no clinical studies to our knowledge have directly examined the effects of oxytocin on working memory performance among individuals with PTSD, nor have any studies examined neural correlates of these potential effects. In particular, no studies to our knowledge have examined modulation of functional connectivity by oxytocin in a working memory task in PTSD or trauma exposure. Most of the existing relevant studies have used resting state fMRI with the amygdala as a seed region or region-of-interest (Dodhia et al., 2014; Frijling et al., 2016; Koch et al., 2016). Two of these studies showed enhanced coupling between the amygdala and various prefrontal regions on oxytocin (compared to placebo) but this only occurred for males in the Dodhia et al. study. Frijling et al. reported reduced amygdala-prefrontal connectivity on oxytocin, but this was assessed using resting state fMRI following trauma scripts. These mixed findings necessitate additional research on the effects of oxytocin on modulating functional connectivity in PTSD.
In addition, the present study employs a trauma-exposed control group (i.e., a group of individuals who experienced an event or events prior to age 18 that met DSM-IV definition of Criterion A, but did not develop PTSD) rather than a group of healthy controls, which enables us to differentiate working memory function between these two groups and to examine oxytocin’s ability to mitigate this risk factor. It is important to utilize a trauma-exposed control group because while it is estimated that at least half of the population will experience a potentially traumatic event in their life, less than 20% of those individuals will meet the diagnostic criteria for PTSD (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995). Thus, identifying factors that differentiate individuals who develop PTSD from those who are exposed to trauma but do not develop PTSD is critical to identify and target prevention and intervention efforts (Highland et al., 2015; Nievergelt et al., 2015; Pietrzak et al., 2010; Seal et al., 2009).
Using a double-blind, placebo-controlled design, this study employed functional magnetic resonance imaging (fMRI) and the N-back working memory task to examine the effects of a single dose of intranasal oxytocin (24 IU) versus placebo on 1) working memory performance and 2) executive control system function (i.e., DLPFC connectivity) during the working memory task among individuals with PTSD (n=16) as compared to a trauma-exposed control group (n=18). Given the important role of the DLPFC in working memory, this region served as the seed region in a functional connectivity analysis. The analysis focused on connectivity between the DLPFC and two regions-of-interest: the left inferior parietal cortex and the anterior cingulate cortex. Both of these regions are recruited specifically in 2-back working memory tasks (Lenartowicz & McIntosh, 2005). In addition, oxytocin receptors have been reported in the anterior cingulate cortex in humans (Boccia et al, 2013). We expected that participants in the PTSD group might exhibit poorer working memory compared to participants in the trauma-exposed control group. We also hypothesized that oxytocin will improve working memory function and increase DLPFC connectivity with the other two components of the working memory network (inferior parietal and anterior cingulate cortex) in PTSD patients compared to a trauma-exposed control group.
Methods
Participants
Thirty-eight individuals who responded to local media advertisements enrolled in the larger human laboratory study used a within-subjects crossover design to examine the effects of oxytocin on amygdala reactivity to negative emotional cues between individuals with PTSD resulting from childhood trauma versus individuals who had childhood trauma exposure but did not develop PTSD. Four participants (three women in the PTSD group and one woman from the trauma-exposed control group) were omitted from analyses based on poor quality of structural or functional images. Thus, our final sample was comprised of 34 individuals. Inclusion criteria for all study participants included (1) scores of moderate to severe (>3) on a minimum of one of the five trauma domains of the Childhood Trauma Questionnaire (Bernstein et al., 2003) and (2) experiencing, witnessing, or confronting an event(s) that involved actual or threatened death or serious injury, or a threat to the physical integrity of themselves, or others and the person’s response involved intense fear, helplessness, and/or horror (i.e., Criterion A DSM-IV for PTSD), prior to the age of 18. Sixteen participants (7 women; 9 men) comprised the PTSD group while 18 participants (11 women; 7 men) comprised the trauma-exposed control group. Participants in the PTSD group and those in the trauma-exposed control group were matched on age, education, and smoking status.
Exclusion criteria included (1) pregnancy, breastfeeding, or ineffective means of birth control; (2) evidence of or a history of head trauma, neurological disorders, seizures, unconsciousness; (3) current psychotic bipolar disorder; (4) past 30-day illicit drug use as evidenced by subject report and urine drug screen on the day of the fMRI scan; (5) unwillingness or inability to maintain abstinence from alcohol for 24 hours and illicit drugs for 72 hours prior to the study visits; (6) ferrous metal implants/pacemaker; (7) claustrophobia; (8) history of or current significant hematological, endocrine, cardiovascular, pulmonary, renal, gastrointestinal diseases. Consistent with existing literature, use of hormonal birth control was assessed among women (Scheele, Plota, Stoffel-Wagner, Maier, & Hurlemann, 2015).
Procedures
Participants completed IRB-approved written informed consent on day 1 of the larger human laboratory study, before any study procedures occurred. Participation in the larger study lasted approximately two weeks and included a screening visit, questionnaires, a physical examination, a urine pregnancy test for women, and two scanning visits on two consecutive days. Participants were randomly assigned in a double-blind manner to receive either oxytocin or placebo on days 1 and 2 in a counter-balanced fashion. The n-back task, analyzed in the current study, was completed only on day 2. Thus, the manipulation of oxytocin versus placebo was cross-sectional for the present analyses.
Measures
Inclusion and exclusion criteria, including PTSD diagnosis, were determined by the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998) and the substance use module of the Structured Clinical Interview for DSM-IV (SCID-IV; First, Spitzer, Gibbon, Williams, & Benjamin, 1994). Participants completed these diagnostic interviews prior to completing the remaining interviews and self-report assessments and all other study procedures.
Posttraumatic stress disorder (PTSD) symptom severity was evaluated using the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995) for the first eight participants enrolled in the study (3 participants in the PTSD group; 5 participants in the trauma-exposed control group) and by the Posttraumatic Diagnostic Scale (PDS; (Foa, Cashman, Jaycox, & Perry, 1997) for all remaining participants. The CAPS is a standardized structured clinical interview that indexes past-month PTSD diagnostic status and provides a continuous measure of PTSD symptom severity consistent with DSM-IV diagnostic criteria (American Psychiatric Association, 1994). The PDS is a self-report measure comprised of 49 items that yield a total PTSD symptom severity score in the past 30 days. Childhood trauma was established as a criterion A event by interviewers. CAPS interviews were linked to participants’ index trauma consistent with standardized procedures and participants who completed the PDS were instructed to reference their index event when responding to questions. CAPS and PDS scores are reported here in order to characterize the sample, but were not included in any study analyses.
Severity of childhood trauma exposure was assessed using the Childhood Trauma Questionnaire (Bernstein et al., 2003). The CTQ is a 25-item self-report questionnaire comprised of five domains of childhood abuse and neglect: sexual abuse, physical abuse, emotional abuse, emotional neglect, and physical neglect. Responses are rated on a 5-point scale from (1) never true, to (5) very often true and summed to obtain a total score in addition to total scores of each subscale.
Medication administration
The dose of intranasal oxytocin used in this study (24 IU) was chosen based on previous literature examining the effect of acute oxytocin administration on neural circuitry where dosages of 24 and 27 IU were given (Domes et al., 2007; Domes et al., 2010; Kirsch et al., 2005). The research pharmacy at the principal investigator’s home institution compounded the oxytocin and placebo (saline) nasal sprays and was responsible for treatment randomization. Under the supervision of the research staff, participants self-administered their medication approximately 45-minutes prior to the scanning session.
Working memory task
The n-back task required participants to focus on a set of serially presented letters on a screen and to respond using an MRI-compatible button box. The 0-back condition was used as a control condition, where participants indicated the presence of the letter “X” by pressing the button under their index finger; otherwise, they pressed the button under their middle finger. The 1-back condition required a button press with the index finger when the same letter was repeated in sequence; otherwise, participants pressed the button under the middle finger. The 2-back condition required a button press with the index finger when a letter matched the letter presented before the prior letter; otherwise, participants pressed the button under the middle finger. The participants were given instructions for completing the task and were allowed to practice prior to beginning the task in the MRI scanner to ensure they understood the task design.
Participants completed a single functional run of the n-back task, with 12 task blocks and 4 rest blocks. Three task blocks, with a counterbalanced order of 0-, 1- and 2-back conditions, were followed by a rest block lasting 30 seconds and this sequence was repeated 4 times. The initial rest block lasted 24 seconds. Each task block lasted 30 seconds and was preceded by a 5-second display of the n-back condition (0, 1, or 2) to be completed for that block. Each task block presented a sequence of 20 letters with a blank screen between each letter. Each letter was shown for 0.5 seconds and each blank screen was shown for 1 second. During rest blocks, participants were presented with a black screen with three white asterisks.
fMRI data acquisition
Images were acquired on a Siemens 3T Trio MRI system (Erlangen, Germany). Scanning included a 533 volume whole-brain functional scan (gradient echo EPI; TE = 18 msec, TR = 1000 msec, flip angle = 70°, FOV = 20.0 cm × 20.0 cm, in-plane resolution of 3.125 mm × 3.125 mm, interleaved acquisition of 16 axial contiguous 5.0-mm slices) and a T1-weighted anatomical scan (MPRAGE; TE = 18 msec, TR = 1000 msec, Tl = 16 contiguous sagittal 5.0-mm thick slices). Field map information (to correct geometric distortions caused by static-field inhomogeneity) was also collected. E-prime software (version 1, www.pstnet.com; (Psychology Software Tools, 2012) running on a Windows computer connected to the MR scanner presented visual stimuli and recorded the time of each MR pulse, visual stimulus onset, and behavioral responses.
fMRI data preprocessing
Preprocessing and statistical analysis were conducted using FSL (v. 4.1.7, FMRIB, Oxford University, Oxford, U.K.). After brain extraction (BET) of the high-resolution MPRAGE image and gradient field map magnitude image, individual participant data were preprocessed using standardized preprocessing steps: head motion correction (using MCFLIRT), geometric distortion correction (using FUGUE), slice timing correction, spatial normalization/registration (12-parameter affine transformation to the MNI template), temporal high-pass filtering (cutoff = 120 sec), and spatial smoothing (FWHM=7 mm). The Montreal Neurological Institute (MNI) brain template was used to normalize brain images. Statistical analyses were then performed at the single-subject level (FEAT v. 5.98). Each subject’s scan was modeled with three EVs (explanatory variables; 0-back, 1-back, 2-back) convolved with a double gamma hemodynamic response function, and a temporal derivative. Resting blocks and the 5-second block indicating the n-back condition were not explicitly modeled. In addition to including six head motion parameters (three translational, three rotational) as confound EVs, we also identified outliers using “fsl_motion_outliers” which creates vectors of time points associated with excessive head motion using root mean squared error (RMSE). Following preprocessing, two types of statistical analysis were conducted: 1) a general linear model (GLM) analysis to examine fMRI signal magnitude changes as a function of n-back level, and 2) psychophysiological interaction (PPI) modeling (Friston et al., 1997) to examine functional connectivity changes as a function of working memory load.
GLM analysis
A group-level whole-brain voxel-wise analysis using mixed effects analysis (FLAME 1) was conducted. The main contrast of interest was the 2-back vs. baseline condition to isolate activation specific to the most demanding working memory load in this study. Statistical maps were thresholded using cluster correction (Z > 2.58, p = .05).
Psychophysiological interaction analysis
Results from the GLM analysis were used to define a seed region in the left dorsolateral prefrontal cortex (DLPFC), which is commonly activated in verbal working memory tasks. Using the GLM results to select a seed region ensured that the region was recruited for working memory in this sample. The DLPFC seed region was defined as a 10-mm sphere centered on the coordinates: −42, +24, +29. The time series for each subject was extracted in the seed region using ‘fslmeants’. The PPI analysis examined the interaction of the three EVs associated with the 0-, 1-, and 2-back conditions with the physiological regressor (the DLPFC time series), and the six head motion parameters and motion outlier EVs as nuisance variables.
Regions-of-interest analysis
Two regions-of interest (ROI) were the primary focus of this study: the anterior cingulate and the left inferior parietal cortex. These regions were chosen based on prior literature identifying them as key regions in working memory function (Baldo & Dronkers, 2006; Lenartowicz & McIntosh, 2005). ROIs were represented by 10-mm spheres centered on (0,+38,+8) for the anterior cingulate and on (−50, −32,+38) for the left inferior parietal cortex. The location of the spheres was based on anatomical criteria such that the Automated Anatomical Labeling (AAL; Tzourio-Mazoyer et al., 2002) atlas yielded labels for MNI coordinates that were consistent with “anterior cingulate cortex” and “inferior parietal cortex.” The two ROIs were used only for the PPI analysis. ‘featquery’ was used to extract the parameter estimates for each of the 3 interaction terms (0, 1, 2-back conditions × DLPFC physiological regressor) from the PPI analysis, with parameter estimates converted into percent signal change. Connectivity parameter estimates were analyzed using 3-way mixed ANOVA, consisting of group (PTSD, trauma-exposed control) as a between-subjects variable, drug (oxytocin, placebo) manipulated between-subjects, and working memory load (0-back, 1-back, 2-back) manipulated within subjects.
Analysis of working memory performance
The primary outcome variable for assessing n-back performance was the percent error for participants during the three n-back conditions. Due to a programming error, responses made after 500 milliseconds were not recorded in E-prime. This led to a high rate of missing values (M = 57%). However, a 3-way ANOVA revealed no effect of drug, F (1,33) = 1.89, p=.18) or subject group F (1,33) =. 22, p = .64) or the interaction of drug and subject group, F (1, 33) = .13, p = .72 on percent of missing responses. Because there is no way of knowing if those missing values were due to a late response not being recorded or a participant not making a response at all, these missing values were not counted as errors and only the responses recorded within the 500 millisecond time-window were used to calculate error rate.
Results
Demographics and clinical characteristics
Sample demographic and clinical characteristics are presented in Table 1. Participants in the PTSD group and those in the trauma-exposed control group were matched on age and education. As expected, participants in the PTSD group reported higher levels of childhood trauma, greater PTSD symptom frequency and severity, and lower stress coping ability. No differences in sex or race emerged.
Table 1.
Sample Demographic and Clinical Characteristics by Group
| Measure | PTSD Group Mean (SD) | Resilient Group Mean (SD) | t | p |
|---|---|---|---|---|
| CTQ Emotional Abuse | 15.58 (5.43) | 11.37 (5.89) | 2.29 | <.05 |
| CTQ Physical Abuse | 14.26 (5.43) | 9.63 (4.03) | 2.98 | <.01 |
| CTQ Sexual Abuse | 17.37 (7.37) | 11.42 (7.59) | 2.45 | <.05 |
| CTQ Emotional Neglect | 16.79 (5.01) | 11.63 (6.30) | 2.79 | <.01 |
| CTQ Physical Neglect | 12.72 (4.78) | 7.95 (3.10) | 3.62 | <.001 |
| CTQ Total | 76.05 (18.48) | 52.00 (21.81) | 3.67 | <.001 |
| PTSD Symptom Severity (PDS) | 25.14 (11.06) | 6.50 (6.34) | 5.47 | <.001 |
| PTSD Symptom Severity (CAPS) | 56.33 (24.58) | 22.50 (12.39) | 2.43 | 0.06 |
| Education (years post high school) | 3.11 (1.15) | 3.20 (.951) | 0.28 | 0.23 |
| Age (years) | 36.89 (9.69) | 37.90 (12.15) | 0.29 | 0.40 |
Note. Statistically significant at the p<0.05 level. PTSD=Posttraumatic Stress Disorder. DSM-IV PTSD diagnostic status was established using the MINI Neuropsychiatric Psychiatric Interview. CTQ=Childhood Trauma Questionnaire. PDS=Posttraumatic Diagnostic Scale.
Working memory performance
Figure 1 shows the effect of working memory load (0-back, 1-back, 2-back) on percentage of errors for each Group × Drug condition. The 3-way ANOVA revealed that the main effect of memory load was significant, F (2, 60) = 8.7, p = .0001 and participants in the PTSD group performed worse than participants in the control group, F (1, 30) = 5.07, p = .032. The Group × Memory load interaction was also significant, F (2, 60) = 3.39 p = 0.04. As shown in Figure 1, the memory load effect was more pronounced for participants in the PTSD group. To explore whether oxytocin improves performance in the PTSD group, as suggested in Figure 1, an independent samples t-test was conducted only for the 2-back condition comparing the PTSD and trauma-exposed groups in the oxytocin and placebo conditions separately. Results indicate that PTSD participants on placebo performed worse than trauma-exposed controls on placebo, t (13) = 2.54, p = .025, but PTSD participants on oxytocin did not perform differently than trauma-exposed controls on oxytocin in the 2-back condition, t (17) = .57, p = .58. The 3-way interaction was not significant, F (2, 32) = .13, p = .72.
Figure 1.

Percent error for each level of the N-back task by group and drug. The PTSD-placebo group performed worse in the 1-back and 2-back condition as compared to the control groups. Asterisk indicates that the PTSD group mean was different from the control group mean on placebo. Error bars are standard error of the mean.
Head motion
A 2-way ANOVA assessed absolute head motion (in mm) as a function of drug, group and the interaction. Head motion was not different between PTSD (M = .49, SD = .42) and trauma-exposed (M = .77, SD = .79) participants, F(1, 30) = 1.7, p = .197, nor was there a main effect of oxytocin (M = .64, SD = .60) versus placebo (M = .64, SD = .65), F(1, 30) = .01, p = .932. The Group × Drug interaction was not significant, F(1, 30) = 1.23, p .28.
GLM
The GLM analysis (Table 2) revealed extensive activation at a cluster-corrected threshold (Z = 3.1, p = .05) for each of the n-back conditions versus baseline. As shown in Figure 2, activation was more extensive for the 1-back and 2-back conditions, but all three conditions show activation in the inferior parietal cortex. One- and 2-back conditions show activation in the canonical working memory network, including DLPFC. The seed region selected for the PPI analysis is shown as a green sphere in Figure 2, which demonstrates that the left DLPFC is involved in this verbal working memory task, particularly for the 1-back and 2-back conditions. Activation for 2 v. 0 back did not survive the cluster-corrected threshold of Z = 3.1.
Table 2.
Results from the general linear model (GLM) voxel-wise whole brain analysis.
| N-back Condition | Region | Size (voxels) | Cluster p | Maximum Z−score | MNI x | MNI y | MNI z |
|---|---|---|---|---|---|---|---|
| 0 | Right cuneus | 1,413 | 1.19 E-07 | 4.39 | 12 | −68 | 18 |
| Right acumbens | 1,338 | 2.38 E-07 | 4.63 | 6 | 18 | −2 | |
| Left superior parietal | 1,139 | 1.31 E-06 | 4.71 | −38 | −46 | 60 | |
| Right precuneus | 917 | 1.01 E-05 | 4.51 | 12 | −76 | 52 | |
| Right superior frontal cortex | 692 | 9.55 E-05 | 4.62 | 28 | 54 | 24 | |
| Right paracingulate | 525 | 0.000595 | 4.44 | 2 | 28 | 46 | |
| 1 | Right superior frontal cortex | 15,365 | 6.52 E-41 | 5.26 | 28 | 54 | 24 |
| Left calcarine | 826 | 8.58 E-06 | 4.75 | −8 | −94 | 8 | |
| Right superior temporal cortex | 618 | 9.08 E-05 | 4.81 | 64 | −2 | −4 | |
| 2 | Paracingulate | 4,124 | 4.47 E-15 | 5.05 | 0 | 26 | 46 |
| Right precuneus | 2,527 | 6.90 E-11 | 4.84 | 2 | −70 | 32 | |
| Left inferior frontal cortex | 2,248 | 4.49 E-10 | 4.91 | −50 | 20 | −4 | |
| Right caudate | 1,742 | 1.66 E-08 | 4.83 | 8 | 18 | 0 | |
| White matter | 846 | 2.85 E-05 | 4.87 | 10 | −24 | 24 |
Note. Regions listed are associated with the maximum voxel within each cluster detected at a cluster corrected threshold of Z > 3.1, p = .05
Figure 2.
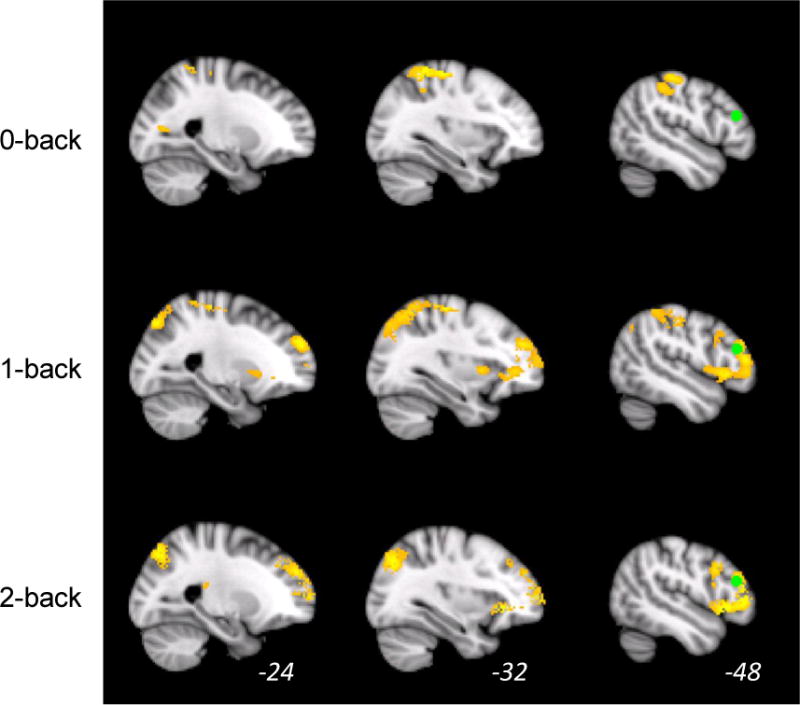
Activation is shown during each of the three levels of the N-back working memory task versus baseline. Activation is significant at a cluster corrected threshold of z > 2.33, p = .05. The green sphere is the region of interest in the left dorsolateral prefrontal cortex used as the seed region in the psychophysiological interaction analysis (−42, +48, +29).
Region of interest
As shown in Figure 3, left DLPFC-anterior cingulate connectivity is mostly negative (i.e., negatively correlated). However, oxytocin was associated with reduced negative connectivity and increased positive connectivity in the most difficult 2-back condition. Controls show a change from very negative values on placebo to less negative values on oxytocin, whereas PTSD subjects show a shift from negative to positive connectivity values in the 2-back condition. Supporting these trends, the main effect of drug was significant, F (1, 30) = 4.7, p = .04 with connectivity on oxytocin (M = -.01) being less negative than on placebo (M = -.59). In addition, oxytocin had the greatest effect in the most difficult 2-back condition among participants in the PTSD group. An independent-samples t-test confirmed that 2-back connectivity was indeed higher on oxytocin (M = .70) than on placebo (M = -.52) among participants in the PTSD group, t (14) = 2.7, p = .02. Although the 3-way interaction of drug, group, and n-back condition was not significant, the two-way Group × N-back condition interaction was significant, F (2, 60) = 4.29 p = .023. This interaction is mostly explained by the increased connectivity on oxytocin in the PTSD group for the 2-back condition. A Group × Drug ANOVA was conducted for the 2 v. 0 back parameter estimate. The main effect of group was significant, F(1, 30) = 5.5, p =.025, with the PTSD group showing positive connectivity (M = .43, SD = 1.14) and the trauma-exposed group showing negative connectivity (M = -.57, SD = 1.37). Neither the main effect of drug nor the Group × Drug interaction was significant for the 2 v. 0 back parameter estimate. Importantly, percent of missing values from the error data was not correlated with the PPI parameter estimate value for any n-back condition (0-back: rho = -.035, p = .84; 1-back: rho = .21, p = .23; 2-back: rho = .20, p = .25; 2 v 0 back: rho = .18, p = .31), which demonstrates that the PPI results are not related to the missing data. The ANOVA that assessed inferior parietal-DLPFC connectivity as a function of group, drug, and n-back condition revealed no main effects or interactions.
Figure 3.
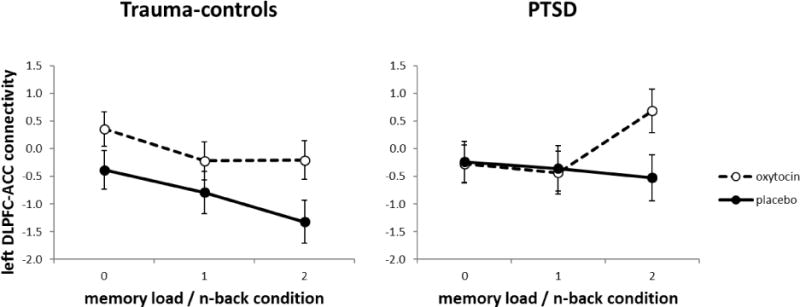
Left DLPFC – ACC connectivity as a function of n-back condition (0-, 1- and 2-back), drug (OT: oxytocin, PLC: placebo) and group (controls, PTSD). Connectivity is expressed as the parameter estimate converted to % signal change. Connectivity on placebo was significantly different than on oxytocin for the PTSD group. Error bars are standard error of the mean.
Discussion
Consistent with previous research, findings from this preliminary study indicate that individuals with PTSD associated with childhood trauma performed worse on the n-back working memory task compared to a trauma-exposed control group comprised of individuals who were exposed to potentially traumatic events during childhood but did not develop PTSD (Schuitevoerder et al., 2013; Vasterling et al., 2002). Findings also indicate that a single dose of intranasal oxytocin, as compared to placebo, was associated with improved performance in the most difficult working memory task condition among participants in the PTSD group. We found that the working memory task activated the DLPFC and inferior parietal cortex (as well as other regions) and that connectivity between the left DLPFC and anterior cingulate was modulated by oxytocin such that oxytocin increased connectivity during the n-back task. This increased connectivity was most pronounced among participants in the PTSD group. These findings provide novel information in support of oxytocin improving executive functioning deficits among individuals with PTSD. This is also the first study to our knowledge to make a direct comparison of working memory performance and executive control system connectivity between individuals with PTSD related to childhood trauma and trauma-exposed control participants.
Previous studies have examined the effects of oxytocin on executive functioning and social memory, but no studies have examined working memory performance specifically, nor have they done so among individuals with PTSD or trauma-exposed control participants. This study addressed another important gap in the literature by examining neural correlates of working memory performance in PTSD as well as oxytocin’s effects on this outcome. Given the salient clinical relevance of the construct of working memory in the distress and functional impairment associated with PTSD and in PTSD treatment, these findings indicate a rationale to conduct a larger clinical trial closely examining the associations between these constructs. Specifically, a robust theoretical rationale exists to translate oxytocin in the treatment of PTSD (Koch et al., 2014; Olff et al., 2010). Thus, it is important to extend the limited literature that has examined the ability of oxytocin to prevent PTSD onset (van Zuiden et al., 2016) and enhance psychotherapy in the treatment of PTSD (Flanagan et al., in press). Our study underscores the importance of examining working memory as a possible additional outcome of interest in the therapeutic translation of oxytocin for the treatment and prevention of PTSD.
While this study provided some insight into the neurobiological response to oxytocin in individuals with PTSD, the underlying mechanism of oxytocin in working memory and executive control system connectivity deficits remains unknown. One of the main findings was that oxytocin reduced 2-back errors in the PTSD group. As illustrated in Figure 3, neither control group demonstrated an increase in error rate between the 1-back and 2-back condition and the overall error rate was low. This suggests that when trauma-exposed control subjects made fast responses (within 500 milliseconds), those responses were usually correct. This outcome did not occur with the PTSD group; fast responding was more error prone in participants with PTSD. Nevertheless, oxytocin reduced rate-limited errors in the most difficult n-back condition in the PTSD group. However, given that only 43% of the performance data could be analyzed for accuracy but fMRI activation and connectivity reflected all the cognitive processing engaged for the n-back task, caution should be taken in relating the fMRI findings to working memory performance. The accuracy data reflects only those cognitive processes that occur during the time window of a trial when the letter was displayed. Nevertheless, we speculate that oxytocin may modulate working memory performance and underlying neural circuitry in two ways.
First, oxytocin may exert a direct effect on cognitive processing by modulating response in regions involved in the executive control aspects of working memory. Some studies report that the anterior cingulate is associated with monitoring performance and response during working memory (A. W. MacDonald, Cohen, Stenger, & Carter, 2000), and is likely involved in more complex executive function operations together with the DLPFC (Reuter-Lorenz et al., 2000). These findings support the speculation that oxytocin has a direct effect on cognitive processing by increasing connectivity with the DLPFC (as demonstrated in the present study). Oxytocin receptors have indeed been found in the anterior cingulate in humans (Boccia, Petrusz, Suzuki, Marson, & Pedersen, 2013). Also, some studies that have used resting state fMRI report negative connectivity between the anterior cingulate cortex and other brain regions in trauma exposed individuals (e.g., Cisler, 2017) or individuals with anxiety disorders on placebo (e.g., Dodhia et al., 2014). Of note, Dodhia et al (2014) reported that oxytocin enhanced resting state connectivity of the amygdala with the rostral, pregenual anterior cingulate/medial PFC. On placebo, this connectivity was negative but on oxytocin this connectivity shifted to positive, similar to DLPFC-anterior cingulate connectivity in the present study. However, increased coupling of the anterior cingulate with DLPFC response by oxytocin in the present study was not the “normative” response exhibited by trauma-exposed control participants in this sample, suggesting that recruitment of the anterior cingulate coupled with DLPFC may not necessarily reflect increased cognitive efficiency or performance monitoring. Trauma-exposed control participants in this study did not show the same coupling, yet they performed more the n-back tasks more efficiently compared to participants with PTSD.
Another possibility is that oxytocin’s effects occur in other brain circuits thereby exerting a more indirect influence on working memory performance. For example, oxytocin might modulate neural circuitry associated with stress-related arousal. A reduction in hyper-activation of arousal or limbic and para-limbic circuitry (such as the anterior cingulate) may allow for more efficient cognitive processing in working memory. In a recent meta-analysis, De la Vega et al. (2016) note that ventral aspects of the mPFC (which include the anterior cingulate ROI in the present study) are less involved in the cognitive aspects of working memory and more associated with affective processing and episodic memory. Also, Shaw et al. (2009) found that participants with PTSD recruited an additional working memory network including the anterior cingulate that they speculated was more related to hyperarousal and abnormal activity than to cognitive processing in PTSD. In the present study, it is possible that increased coupling between DLPFC and anterior cingulate was more related to cognitive control of arousal response than working-memory related cognitive control. Future studies can help differentiate cognitive control of arousal response versus cognitive control in working memory by comparing connectivity in working memory tasks to connectivity using experimental paradigms that target arousal-related behaviors, self-reported PTSD hyperarousal symptoms as well as trait characteristics such as impulsivity and aggression.
Limitations
Several limitations of the current study should be considered when interpreting these findings. The PDS replaced the CAPS assessment of PTSD symptom severity early in the study to reduce participant burden. While the MINI is reliable and valid assessment of psychiatric disorders, utilizing the CAPS to assess PTSD diagnosis and symptom severity might have been a more valuable approach. The inconsistency in assessment also limits the interpretation of the current findings and they should be replicated in future studies using consistent assessment approaches. Relatedly, trauma symptom severity might influence oxytocin response as the existing literature is in conflict regarding oxytocin’s effects on healthy versus more impaired individuals (Bartels, 2012). Future studies can improve on the current design by recruiting a larger sample size and matching participant groups on the severity of trauma exposure. The relatively small sample size prevented us from explicitly examining sex differences in oxytocin response (Ditzen et al., 2012; Hoge et al., 2014; Lynn, Hoge, Fischer, Barrett, & Simon, 2014; Rilling et al., 2014), although the similar distribution by sex within groups partially mitigates this concern. Larger studies can also examine moderators of oxytocin response overall or between participants in the PTSD group compared to the trauma-exposed control group.
Future studies can also improve on the current design by utilizing a crossover design rather than the cross-sectional design employed here. It is possible that individuals may experience more stress on the first day of scanning because of the novel scanning environment, which could in turn impact performance during the first scanner exposure. Finally, while a 24 IU dose of intranasal oxytocin is commonly employed, single doses commonly range up to 60 IU and multiple daily doses of oxytocin are regarded as safe and feasible in the psychiatric literature, including among individuals with PTSD (Pedersen et al., 2011; Pedersen et al., 2013; van Zuiden et al., 2016). A higher or repeated dose of oxytocin might have resulted in additional findings to explain the results gathered here.
Neuroimaging limitations
While the n-back task captured working memory performance, accuracy results are limited to the cognitive processes that occur in the initial time window of 500 msec. We note that we did not analyze reaction time data for this reason. Importantly, the limited response window applied to all subjects, and analysis of percent of missing data showed no systematic group or treatment condition effects. Nevertheless, future studies that examine working memory in PTSD and trauma-exposed individuals are needed to determine whether the modulation of working memory performance by oxytocin in PTSD is limited to only the early cognitive processing engaged in this n-back task.
Conclusions
Improving working memory and executive control deficits might be a target in the development of pharmacologic treatment of PTSD. The findings from the present study suggest that oxytocin may be a viable candidate to modulate this and other neural and behavioral correlates of PTSD. Further studies are necessary to understand the underlying mechanisms responsible for deficits in working memory, and to determine the potential of oxytocin to inform the development of future therapeutic agents for individuals with PTSD.
Public Significance Statements.
This preliminary study found that oxytocin, as compared to placebo, was associated with 1) improved working memory performance among individuals with PTSD, and 2) greater functional connectivity between the dorsolateral prefrontal cortex and anterior cingulate during a working memory task. Results from this study support emerging literature suggesting that oxytocin has promise as a potential pharmacologic in the treatment of PTSD, for which effective medications are scant.
Acknowledgments
This manuscript is the result of work supported, in part, by the National Institute on Mental Health (R21MH099619) and the National Institute on Alcohol Abuse and Alcoholism (K23AA023845; T32AA007474).
References
- Acheson D, Feifel D, de Wilde S, Mckinney R, Lohr J, Risbrough V. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology. 2013;229(1):199–208. doi: 10.1007/s00213-013-3099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Publishing, Inc; 1994. [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: Disengaging from trauma. Neuropharmacology. 2012;62(2):686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006;20(5):529–538. doi: 10.1037/0894-4105.20.5.529. [DOI] [PubMed] [Google Scholar]
- Barbey AK. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013;49(5):1195. doi: 10.1016/j.cortex.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A. Oxytocin and the social brain: beware the complexity. Neuropsychopharmacology. 2012;37(8):1795–1796. doi: 10.1038/npp.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Desmond D. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child abuse & neglect. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bethlehem RA, van Honk J, Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38(7):962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1002/jts.2490080106. [DOI] [PubMed] [Google Scholar]
- Boccia M, Petrusz P, Suzuki K, Marson L, Pedersen C. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience & Biobehavioral Reviews. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- Bomyea J, Lang JA. The role of executive functioning in PTSD and its treatment. Current Psychiatry Reviews. 2015;11(3):160–171. [Google Scholar]
- Bomyea J, Stein MB, Lang AJ. Interference control training for PTSD: A randomized controlled trial of a novel computer-based intervention. Journal of Anxiety Disorders. 2015;34:33–42. doi: 10.1016/j.janxdis.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, Beall SK, Van Voorhees E, Green KT. Altered Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes in Posttraumatic Stress Disorder. Neuropsychopharmacology. 2013;39(2):361–369. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kozlovsky N, Gidron Y, Matar MA, Zohar J. Hippocampal microinfusion of oxytocin attenuates the Behavioural response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. Journal of Neuroendocrinology. 2010;22(8):889–904. doi: 10.1111/j.1365-2826.2010.02003.x. [DOI] [PubMed] [Google Scholar]
- Cox RC, Ebesutani C, Olatunji BO. Linking sleep disturbance and maladaptive repetitive thought: The role of executive function. Cognitive Therapy and Research. 2016;40(1):107–117. [Google Scholar]
- de la Vega A, Chang LJ, Banich MT, Wager TD, Yarkoni T. Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. Journal of Neuroscience. 2016;36(24):6553–6562. doi: 10.1523/JNEUROSCI.4402-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Nater UM, Schaer M, La Marca R, Bodenmann G, Ehlert U, Heinrichs M. Sex-specific effects of intranasal oxytocin on autonomic nervous system and emotional responses to couple conflict. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodhia S, Hosanagar A, Fitzgerald DA, Labuschagne I, Wood AG, Nathan PJ, Phan KL. Modulation of Resting-State Amygdala-Frontal Functional Connectivity by Oxytocin in Generalized Social Anxiety Disorder. Neuropsychopharmacology. 2014;39:2061–2069. doi: 10.1038/npp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC. Oxytocin Attenuates Amygdala Responses to Emotional Faces Regardless of Valence. Biological Psychiatry. 2007;62(10):1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Eckstein M, Becker B, Scheele D, Scholz C, Preckel K, Schlaepfer TE, Hurlemann R. Oxytocin Facilitates the Extinction of Conditioned Fear in Humans. Biological Psychiatry. 2014;78(3):194–202. doi: 10.1016/j.biopsych.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Feifel D, MacDonald K, Cobb P, Minassian A. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophrenia research. 2012;139(1):207–210. doi: 10.1016/j.schres.2012.05.018. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, Benjamin L. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II) (Version 2.0) New York, NY: Biometrics Research Department, New York State; 1994. [Google Scholar]
- Flanagan JC, Baker NL, McRae AL, Brady KT, Moran-Santa Maria M. Effects of Adverse Childhood Experiences on the Association between Intranasal Oxytocin and Social Stress Reactivity among Individuals with Cocaine Dependence. Psychiatry Research. 2015;229(1–2):94–100. doi: 10.1016/j.psychres.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JC, Sippel LM, Wahlquist A, Moran-Santa Maria MM, Back SE. Augmenting Prolonged Exposure Therapy for PTSD with Intranasal Oxytocin: A Randomized, Placebo-Controlled Pilot Trial. Journal of Psychiatric Research. doi: 10.1016/j.jpsychires.2017.12.014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychological Assessment. 1997;9(4):445–451. doi: 10.1037/1040-3590.9.4.445. [DOI] [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cognitive, Affective, & Behavioral Neuroscience. 2001;1(2):137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Frijling JL, van Zuiden M, Koch S, Nawijn L, Goslings JC, Luitse JS, Denys D. Efficacy of oxytocin administration early after psychotrauma in preventing the development of PTSD: study protocol of a randomized controlled trial. BMC psychiatry. 2014;14(1):92. doi: 10.1186/1471-244X-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling JL, van Zuiden M, Koch S, Nawijn L, Veltman DJ, Olff M. Intranasal oxytocin affects amygdala functional connectivity after trauma script-driven imagery in distressed recently trauma-exposed individuals. Neuropsychopharmacology. 2016;41(5):1286–1296. doi: 10.1038/npp.2015.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373(9657):68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán YF, Tronson NC, Sato K, Mesic I, Guedea AL, Nishimori K, Radulovic J. Role of oxytocin receptors in modulation of fear by social memory. Psychopharmacology. 2014;231(10):2097–2105. doi: 10.1007/s00213-013-3356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland KY, Sadek JR, Keller JE, Castillo DT. Neurocognitive correlates of successful treatment of PTSD in female veterans. Journal of the International Neuropsychological Society. 2016;22(6):643–651. doi: 10.1017/S1355617716000424. [DOI] [PubMed] [Google Scholar]
- Hartley AA, Speer NK. Locating and Fractionating Working Memory Using Functional Neuroimaging: Storage, Maintenance, and Executive Functions. Microscopy Research and Technique. 2000;51:45–53. doi: 10.1002/1097-0029(20001001)51:1<45::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Highland KB, Costanzo M, Jovanovic T, Norrholm SD, Ndiongue R, Reinhardt B, Roy MJ. Biomarkers of post-deployment resilience among military service members. Neurobiology of stress. 2015;2:62–66. doi: 10.1016/j.ynstr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Anderson E, Lawson EA, Bui E, Fischer LE, Khadge SD, Simon NM. Gender moderates the effect of oxytocin on social judgments. Human Psychopharmacology: Clinical and Experimental. 2014;29(3):299–304. doi: 10.1002/hup.2402. [DOI] [PubMed] [Google Scholar]
- Honzel N, Justus T, Swick D. Posttraumatic stress disorder is associated with limited executive resources in a working memory task. Cognitive, Affective, & Behavioral Neuroscience. 2014;14(2):792–804. doi: 10.3758/s13415-013-0219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Stein DJ. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD) The International Journal of Neuropsychopharmacology. 2012;15(6):825–840. doi: 10.1017/S1461145711001209. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Cusack K, Forneris CA, Wilkins TM, Sonis J, Middleton JC, Brownley K. Psychological and pharmacological treatments for adults with posttraumatic stress disorder (PTSD) 2013 Retrieved from Rockville, MD. [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48(8):778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52(12):1048. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: salience processing and fear inhibition processes. Psychoneuroendocrinology. 2014;40:242–256. doi: 10.1016/j.psyneuen.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Koch S, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Intranasal oxytocin normalizes amygdala functional connectivity in posttraumatic stress disorder. Neuropsychopharmacology. 2016;41(8):2041–2051. doi: 10.1038/npp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz A, McIntosh AR. The Role of Anterior Cingulate Cortex in Working Memory is Shaped by Functional Connectivity. Journal of Cognitive Neuroscience. 2005;17(7):1026–1042. doi: 10.1162/0898929054475127. [DOI] [PubMed] [Google Scholar]
- Lynn SJ, Hoge EA, Fischer LE, Barrett LF, Simon NM. Gender Differences in oxytocin-associated disruption of decision bias during emotion perception. Journal of Cognitive Neuroscience. 2014;219(1):198–203. doi: 10.1016/j.psychres.2014.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacDonald K, MacDonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harvard Review of Psychiatry. 2010;18(1):1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Majer M, Nater UM, Lin JS, Capuron L, Reeves WC. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC neurology. 2010;10(1):61. doi: 10.1186/1471-2377-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale SL, Morissette SB, Rowland JA, Dolan SL. Sleep quality affects cognitive functioning in returning combat veterans beyond combat exposure, PTSD, and mild TBI history. Neuropsychology. 2017;31(1):93–104. doi: 10.1037/neu0000312. [DOI] [PubMed] [Google Scholar]
- McDermott TJ, Badura-Brack AS, Becker KM, Ryan TJ, Khanna MM, Heinrichs-Graham E, Wilson TW. Male veterans with PTSD exhibit aberrant neural dynamics during working memory processing: an MEG study. Journal of psychiatry & neuroscience. 2016;41(4):251. doi: 10.1503/jpn.150058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulou PG, Averbeck BB, Kalpakidou AK, Evans S, Bobin T, Kapur S, Shergill SS. The effects of a single dose of oxytocin on working memory in schizophrenia. Schizophrenia research. 2015;162(1–3):62. doi: 10.1016/j.schres.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missig G, Ayers LW, Schulkin J, Rosen JB. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm. Neuropsychopharmacology. 2010;35(13):2607–2616. doi: 10.1038/npp.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawijn L, van Zuiden M, Koch S, Frijling JL, Veltman DJ, Olff M. Intranasal oxytocin increases neural responses to social reward in post-traumatic stress disorder. Social Cognitive and Affective Neuroscience. 2016;12(2):212–223. doi: 10.1093/scan/nsw123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(Supp 1):18–28. [PubMed] [Google Scholar]
- Nievergelt CM, Maihofer AX, Mustapic M, Yurgil KA, Schork NJ, Miller MW, O’Connor DT. Genomic predictors of combat stress vulnerability and resilience in US Marines: a genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology. 2015;51:459–471. doi: 10.1016/j.psyneuen.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Witteveen A, Denys D. A psychobiological rationale for oxytocin in the treatment of posttraumatic stress disorder. CNS Spectrums. 2010;15(8):522–530. doi: 10.1017/S109285290000047X. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Penn DL. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophrenia research. 2011;132(1):50–53. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Garbutt JC. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcoholism: clinical and experimental research. 2013;37(3):484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Carpenter SL, Albright SE, Price LH, Carpenter LL. Exposure to childhood trauma is associated with altered n-back activation and performance in healthy adults: implications for a commonly used working memory task. Brain imaging and behavior. 2016;10(1):124–135. doi: 10.1007/s11682-015-9373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein MB, Malley JC, Rivers AJ, Johnson DC, Southwick SM. Risk and protective factors associated with suicidal ideation in veterans of Operations Enduring Freedom and Iraqi Freedom. J Affect Disord. 2010;123(1–3):102–107. doi: 10.1016/j.jad.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Polak AR. The role of executive function in posttraumatic stress disorder: A systematic review. Journal of Affective Disorders. 2012;141(1):11. doi: 10.1016/j.jad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Psychology Software Tools, I. E.-P. 2012 Retrieved from http://www.pstnet.com.
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12(1):174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, Patel R. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AA, Braver TS, Reynolds JR, Burgess GC, Yarkoni T, Gray JR. Individual differences in amygdala activity predict response speed during working memory. Journal of Neuroscience. 2006;26(40):10120–10128. doi: 10.1523/JNEUROSCI.2567-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Plota J, Stoffel-Wagner B, Maier W, Hurlemann R. Hormonal contraceptives suppress oxytocin-induced brain reward responses to the partner’s face. Social Cognitive and Affective Neuroscience. 2015;11(5):767–774. doi: 10.1093/scan/nsv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuitevoerder S, Rosen JW, Twamley EW, Ayers CR, Sones H, Lohr JB, Thorp SR. A meta-analysis of cognitive functioning in older adults with PTSD. Journal of Anxiety Disorders. 2013;27(6):550–558. doi: 10.1016/j.janxdis.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Scott JC, Harb G, Brownlow JA, Greene J, Gur RC, Ross RJ. Verbal memory functioning moderates psychotherapy treatment response for PTSD-Related nightmares. Behaviour research and therapy. 2017;91:24–32. doi: 10.1016/j.brat.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, Schweinsburg BC. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141:105–140. doi: 10.1037/a0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Metzler TJ, Gima KS, Bertenthal D, Maguen S, Marmar CR. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using department of veterans affairs health care, 2002–2008. Am J Public Health. 2009;99(9):1651–1658. doi: 10.2105/AJPH.2008.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw ME, Moores KA, Clark RC, McFarlane AC, Strother SC, Bryant RA, Taylor JD. Functional connectivity reveals inefficient working memory systems in post-traumatic stress disorder. Psychiatry Research: Neuroimaging. 2009;172(3):235–241. doi: 10.1016/j.pscychresns.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of clinical psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Sofuoglu M, Rosenheck R, Petrakis IL. Pharmacological treatment of comorbid PTSD and substance use disorder: recent progress. Addictive Behaviors. 2014;39(2):428–433. doi: 10.1016/j.addbeh.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research. 2013;47(10):1469–1478. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Management of Posttraumatic Stress Disorder Work Group. VA/DoD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder. 2017 Retrieved from Washington, D.C.: https://www.healthquality.va.gov/guidelines/MH/ptsd/
- Tian F, Yennu A, Smith-Osborne A, Gonzalez-Lima F, North CS, Liu H. Prefrontal responses to digit span memory phases in patients with post-traumatic stress disorder (PTSD): a functional near infrared spectroscopy study. Neuroimage: Clinical. 2014;4:808–819. doi: 10.1016/j.nicl.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van der Werff S, van den Berg SM, Pannekoek JN, Elzinga BM, Van Der Wee N. Neuroimaging Resilience to Stress: A Review. Frontiers in Behavioral Neuroscience. 2013;7(13):39. doi: 10.3389/fnbeh.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zuiden M, Frijling JL, Nawijn L, Koch S, Goslings JC, Luitse JS, Olff M. Intranasal oxytocin to prevent PTSD symptoms: a randomized controlled trial in emergency department patients. Biological Psychiatry. 2016;81(12):1030–1040. doi: 10.1016/j.biopsych.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16(1):5–14. doi: 10.1037/0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Walter KH, Palmieri PA, Gunstad J. More than symptom reduction: Changes in executive function over the course of PTSD treatment. Journal of Traumatic Stress. 2010;23(2):292–295. doi: 10.1002/jts.20506. [DOI] [PubMed] [Google Scholar]
- Woon FL, Farrer TJ, Braman CR, Mabey JK, Hedges DW. A meta-analysis of the relationship between symptom severity of Posttraumatic Stress Disorder and executive function. Cognitive neuropsychiatry. 2017;22(1):1–16. doi: 10.1080/13546805.2016.1255603. [DOI] [PubMed] [Google Scholar]


