Abstract
The lifestyle and feeding habits of nematodes are highly diverse. Several species of Pristionchus (Nematoda: Diplogastridae), including Pristionchus pacificus, have been reported to be necromenic, i.e. to associate with beetles in their dauer diapause stage and wait until the death of their host to resume development and feed on microbes in the decomposing beetle corpse. We review the literature and suggest that the association of Pristionchus to beetles may be phoretic and not necessarily necromenic. The view that Pristionchus nematodes have a necromenic lifestyle is based on studies that have sought Pristionchus only by sampling live beetles. By surveying for nematode genera in different types of rotting vegetal matter, we found Pristionchus spp. at a similar high frequency as Caenorhabditis, often in large numbers and in feeding stages. Thus, these Pristionchus species may feed in decomposing vegetal matter. In addition, we report that one species of Panagrellus (Nematoda: Panagrolaimidae), Panagrellus redivivoides, is found in rotting fruits but not in rotting stems, with a likely association with Drosophila fruitflies. Based on our sampling and the observed distribution of feeding and dauer stages, we propose a life cycle for Pristionchus nematodes and Panagrellus redivivoides that is similar to that of C. elegans, whereby they feed on the microbial blooms on decomposing vegetal matter and are transported between food patches by coleopterans for Pristionchus spp., fruitflies for Panagrellus redivivoides and isopods and terrestrial molluscs for C. elegans.
Introduction
The lifestyle and feeding habits of members of the nematode phylum are highly diverse. A single species may express diverse life cycles through production of free-living and host-associated stages. Many species of the nematode genus Pristionchus (Nematoda: Diplogastridae), including the most studied Pristionchus pacificus, have been recently considered to be associated with beetles in a necromenic lifestyle, i.e. to associate with beetles in the nematode dauer diapause stage and wait until the death of the beetle to resume development and feed on microbes in the decomposing beetle corpse [1]. The necromenic lifestyle of Pristionchus nematodes now appears as a qualifier for the species, as in the "necromenic nematode P. pacificus" [2,3]. Pristionchus have been considered to share some attributes of parasitic nematodes and to have a very different lifestyle from the free-living nematode C. elegans [4].
Yet, to our knowledge, carcasses of naturally dead beetles have not been looked for and examined to assess whether Pristionchus feeds on them. Recent field studies have looked for Pristionchus in live beetles, and to a lesser extent in soil [5–8]. We detail below in three points what has been documented about the association of Pristionchus with beetles.
Many Pristionchus species including P. pacificus can be found on beetles [6,8–14]. (Note that a subclade of Pristionchus species has recently been found in fresh tropical figs in association with their pollinating wasps [15], in the same way that some Caenorhabditis species are found in other specific substrates [16]—we will not consider this fig subclade here).
Pristionchus can be found on live beetles in the dauer stage [12], based on collecting 114 live Geotrupes stercorosus beetles in a forest near Tübingen, Germany. The authors found a total of 17 Pristionchus individuals, 71 Koerneria (a genus close to Pristionchus), 466 other diplogastrids, 3927 Pelodera (Rhabditidae) and 508 parasitic Spirurida. Pelodera dauer larvae were present in 56 out of 114 Geotrupes beetles, with up to hundreds of Pelodera dauer larvae per beetle. Pristionchus was comparatively rarer: eight of 114 beetles (7%) yielded one Pristionchus individual, one carried two individuals and one had seven individuals, all in the dauer stage. P. pacificus is rare in Europe [9]. These beetle-associated Pristionchus were P. sp. 6, entomophagus, and lheritieri. In addition, a survey of 4242 beetles from Germany, France, Spain, and Switzerland again suggested that Pristionchus spp. are found in the dauer stage on beetles [9].
Finally, the data supporting the conclusion that Pristionchus non-dauer stages feed on carcasses of beetles come from experiments where beetles were killed on a Petri dish containing E. coli in the laboratory (e.g., [9], and for P. pacificus: [6]). When a beetle is killed on a Petri dish, some of the bacterial species that are inside the gut or on the surface of the animal will proliferate using the beetle or the Petri dish as food and Pristionchus can then feed on them or E. coli (cf. Fig 1 in [8]). In these experiments, Pristionchus appeared only after 7–10 days, suggesting that they took time to exit the dauer stage [9]. The delay may be explained by the fact that some beetles secrete chemicals that prevent their associated nematode species from exiting the dauer stage [17]. Because this delay is longer than the generation time of Pristionchus in this environment (3–4 days), it is not possible to assess either the number of individuals or their developmental stage on the beetle. Similarly, if the beetles are killed by the experimenter and then placed in a soil environment, Pristionchus will proliferate in the vicinity of the dead beetle only after 7 days [18]. These data suggest that Pristionchus may adopt a necromenic lifestyle by consuming the progeny of bacteria that were on the living host beetle, but they do not address whether this is an event occurring in the wild, and if so, how frequent it is for Pristionchus. The claim of a necromenic relationship does not take into account the possibility that the nematodes may disembark from their insect host well before it dies, should they encounter some food source. Necromeny may take place occasionally, but beetle corpses may be a very minor source of food for Pristionchus.
By contrast, older literature on Pristionchus species had associated them with a number of substrates. Sudhaus and Fürst van Lieven [19] reviewed the systematic work on species in this genus and summarized the habitat as "mostly decaying plants, associated with insects". Specifically, Pristionchus species were described from soil, humus, compost, moss, "diseased" stem, coffee berry, rotting bulbs of Allium vineale, damaged roots of coffee and garlic, around roots of several species, rotten potatoes, rotten wood, and decomposing fungi. Additionally, Pristionchus species were described to be associated with a wide range of insects: termites (two species), Ostrinia (Lepidoptera), "dead insects", and finally beetles (three species). The spectrum of habitats and associated insects thus appears much wider than the recent literature indicates.
It is interesting to compare the recent history of research on Pristionchus ecology with what is known of the ecology of the model organism Caenorhabditis elegans and other Caenorhabditis species. The history of collecting Caenorhabditis has been different, as are the conclusions. Caenorhabditis nematodes have been found to be rare in soil, but abundant in rotting vegetal matter, such as fruits and soft plant stems. In addition, C. elegans, C. briggsae and C. remanei have been found on terrestrial molluscs, isopods and millipedes [20–27]. In laboratory experiments, the nematodes were shown to be capable of climbing on or off these invertebrates [20,21,27–30]. Perhaps anecdotally, C. nouraguensis were found on cockroaches in a tropical forest and C. tropicalis once on a beetle [16]. Some species, such as C. japonica, have an apparently specific carrier insect [31–34]. These associations are considered to be mostly phoretic or may correspond to occasional "facultative necromeny" [35]. The possibility of phoresis or facultative necromeny has not been considered for Pristionchus spp.
We here provide data on nematode genera found in rotting vegetal matter, focusing on the genera Pristionchus and Panagrellus. By surveying different types of rotting vegetal and fungal substrates, we found diverse Pristionchus spp. at a similar frequency as Caenorhabditis, often in high numbers and in non-dauer feeding stages in rotting fruits and stems. In addition, we report that a single species of Panagrellus (Nematoda: Panagrolaimidae), which we identify as Panagrellus redivivoides, is found in rotting fruits but not in rotting stems and appears to be associated with Drosophila fruitflies.
Materials and methods
Sample handling and nematode genus identification
Collected samples were placed onto standard C. elegans Normal Growth Medium agar plates [36] previously seeded with Escherichia coli strain OP50 in the center of the plate. The samples were spread around the bacterial lawn. 1–2 ml water or M9 solution were added to humidify the samples [37].
For surveys in S1 Table, all plates were examined regularly under the dissecting microscope: in the most stringent surveys (Orsay, Santeuil), observations were made several times within the first hours, once or twice on the next two days, and at least on days 4 and 7. Nematodes that crawled out of the sample were identified to the genus or family level by morphological criteria, under the dissecting microscope with trans-illumination and sometimes further by Nomarski microscopy [37]. Caenorhabditis, Pristionchus, Panagrellus, Oscheius, Mesorhabditis species were identified as described in [26,37] (see pictures and drawings therein; and also [38,39] for identification keys). Our previous developmental evolution work provided us with experience on these various genera [40–48] and all strains then tested by rDNA sequencing confirmed correct identification to the genus level (S2 Table). In addition, Caenorhabditis were systematically identified to the species level as indicated in [26,37,49]. Given a generation time for these nematodes of about 3–5 days at 20°C, the number of individuals and developmental stages were noted for samples that were less than 48 hours from collection time.
These surveys were primarily aimed at studying Caenorhabditis populations, as reported in [22,25,26,49]. Caenorhabditis individuals tend to rapidly exit the sample to colonize the E. coli lawn, as do Pristionchus, Panagrellus and most other rhabditids. Some genera are more difficult to survey, either because they have a small body size, are present in small numbers, move slowly out of the sample or are less easy to recognize. The three genera Caenorhabditis, Pristionchus and Panagrellus that are highlighted in S1 Table, are those that may have been least missed in the samples. However, the number of samples that contained Pristionchus or Panagrellus spp. is likely an underestimate as they were less carefully looked for than Caenorhabditis.
Diplogastrids are easy to recognize by their short buccal cavity bearing strong teeth, the absence of a grinder in the basal bulb of the pharynx, the color pattern of the body stemming from the shape of female gonad arms generally bending back diagonally towards the vulva, and the pore appearance of the vulva. Pristionchus are easy to distinguish from other diplogastrids as adults, with their characteristic dumpyish body shape and cuticle with strong longitudinal ridges (Fig 1A). We also systematically checked that our morphological criteria corresponded to the Pristionchus genus by SSU rDNA sequencing of a subset of them (JU isolates, S2A Table).
Fig 1. Rotting fruit and stem samples containing Pristionchus.
(A) Pristionchus adult female or hermaphrodite from rotting apple O824 from Orsay. The cuticle with longitudinal ridges and the pore-shaped vulva (arrowhead) are characteristic of Pristionchus. Bar: 100 μm. (B) Apple O1194 in Orsay orchard, France with large (>1,000 individuals) populations including feeding individuals of Pristionchus sp. and Caenorhabditis elegans. (C) Pear CZ12 in Prague, Czech Republic with Pristionchus and Panagrellus. (D) Olives F8 near Firenze, Italy with Pristionchus sp. (E) Arum stem B09-6 in a wood near Le Blanc, France with Pristionchus and Caenorhabditis. (F) Stem S156 in a wood near Santeuil, France with a feeding population of several thousand Pristionchus individuals. (G) Banana pseudostem S9 in Palermo Botanical Garden, Italy, yielding Pristionchus and C. elegans.
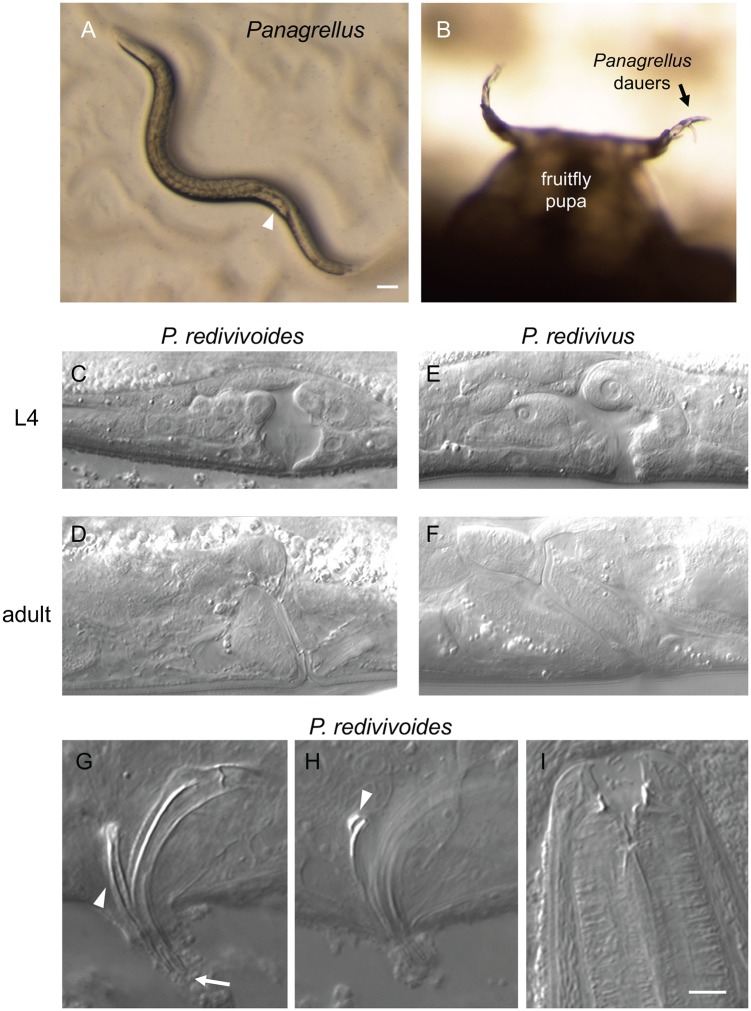
Panagrellus species are recognized by their large body size, light brown gut color (like Caenorhabditis), posterior vulva and viviparous reproduction (Fig 2A). Young first-stage larvae exit through the female vulva and gravid females may contain several dozens of embryos in their uterus. We also sequenced SSU rDNA for some of them (S2B Table).
Fig 2. Panagrellus in rotting fruits.
(A) Panagrellus adult female from rotting apple O801 from Orsay. The quite posterior vulva (arrowhead) and late-stage embryos accumulating in front of it are characteristic of Panagrellus. Bar: 100 μm. (B) Panagrellus dauers nictating on a Drosophila pupa in rotting pear B11-22 from Le Blanc. (C-F) Nomarski micrographs of L4 and adult vulvae of female Panagrellus redivivoides JU1476 (L4) and JU1798 (adult) and Panagrellus redivivus PS1163. Anterior is to the left, dorsal to the top for all panels, thus the uterus is on the left and the post-vulval sac is on the right in C-F. Most Panagrellus spp. display an anteriorly tilted vulva as shown for P. redivivus, while that of P. redivivoides is almost perpendicular to the ventral cuticle. (G) Spicule morphology of P. redivivoides (here strain JU1476). The forked ventral end of the spicules is indicated by an arrow, the gubernaculum by an arrowhead. (H) When in the proper focal plane, the dorsal side of the gubernaculum ends in the manner of a hook (arrowhead) (strain JU385). (I) Adult female mouth (here JU1055). Same scale for G-I. Bar: 5 μm.
Genera of other rhabditids can be Pelodera, Auanema, Rhabditella, Pellioditis, etc. In contrast to Caenorhabditis (or Panagrellus), their gut color is generally greyish/black, rather than brownish. Our notes are too scarce to distinguish them here and we placed them in a single category, with the exception of the ones for which we report 18S sequences.
Oscheius are very commonly found in rotting vegetal matter or soil and can be recognized by their greyish gut color, their lack of middle pharyngeal bulb, their thin female tail and their long and inflated rectum. The occurrence of Oscheius is likely underestimated as these animals are less striking, rarely reach large population sizes and develop more slowly than the above. They are here almost exclusively represented by small-size hermaphroditic Oscheius of the Tipulae and sometimes Dolichura subgroups (by contrast to large Oscheius of the Insectivora group; [45,50]).
Panagrolaimus are long and quite thin nematodes, with an only very slightly posterior vulva.
Mesorhabditis species are easier to find after one to two days on the plate and they may remain in the vicinity of the sample. They are recognized by their posterior vulva and dark body color. In our surveys, they were particularly looked for and isolated in 2015–17.
Animals of the Protorhabditis/Prodontorhabditis/Diploscapter clade [45,51] are small and tend to burrow in the agar and leave trails therein. Populations develop slowly and they can be missed.
Rhabditophanes has a black gut color and characteristically lay almost round eggs, instead of oval-shape ones. They were not systematically surveyed.
Bunonema individuals are easy to identify by their very small, spindle-shaped body, and a left-right asymmetric cuticle. They grow slowly and can easily be missed if their population is overwhelmed by other genera.
The "Other" category contains nematodes that are generally not bacterial eaters but fungi-eaters such as aphelenchs or parasitic nematodes that do not grow in our culture conditions.
Culture and freezing
Strains were derived from single individuals in selfing species, or from a mated female or a male-female pair in male-female species, and cultured on standard C. elegans Normal Growth Medium agar plates. Pristionchus species were frozen with the C. elegans freezing protocol [36], sometimes adding 1 mM CaCl2 to the freezing solution. This protocol does not allow for a good retrieval of Pristionchus and Panagrellus nematodes. We recently adopted a DMSO-Dextran protocol that allows for better recovery (courtesy of Andre Pires da Silva), whereby a pellet of nematodes in M9 solution is resuspended in 7 ml of a mix of 1 g Dextran (Sigma D9260-500G), 1 ml DMSO and autoclaved H2O to 10 ml. The nematodes are incubated for 10 min at room temperature before being place in the -80°C freezer in styrofoam boxes.
Mating tests
For Pristionchus, we crossed 5 hermaphrodites from the P. pacificus JU1102 strain [11] with 5 males of our new isolates and monitored the proportion of males in the progeny as an evidence of crossing. For two of them, BRC20259 and BR20261, we further checked whether these F1 males were fertile by crossing them to hermaphrodites of either parental strain. All crosses were positive. Two replicates of a control with JU1102 animals only did not yield a high percentage of males.
For Panagrellus, we crossed 3–5 L4 females with 3–5 males and monitored first and second generation progeny. A control with 5 L4 females of the tester strain JU385 without males did not yield any progeny (2 replicates).
PCR and sequence analysis of rDNA
In some surveys indicated in S2 Table, the nematodes were assigned to a genus using a molecular tag. The small subunit (SSU, 18S) of ribosomal DNA of Pristionchus isolates was amplified using primers SSU18A (5'-AAAGATTAAGCCATGCATG-3') and SSU26R (CATTCTTGGCAAATGCTTTCG), and sequenced using SSU18A or SSU9R (AGCTGGAATTACCGCGGCTG), as in [5–9,52]; alternatively, as indicated in S2 Table, primers RHAB1350F (5’-TACAATGGAAGGCAGCAGGC) and RHAB1868R (5’-CCTCTGACTTTCGTTCTTGATTAA) were used.
The large subunit (LSU, 28S) of ribosomal DNA of Panagrellus isolates was amplified using primers D2A (ACAAGTACCGTGGGGAAAGTTG) and D3B (TCGGAAGGAACCAGCTACTA) as in [53].
The sequences were trimmed by visual inspection of the chromatograms, leaving the first four nucleotides of the downstream primer when present at the end of the sequence. A ‘N’ corresponds either to a low-quality sequence (including the possibility of a gap, especially when the same nucleotide occurred at consecutive positions) or a putative polymorphism of rDNA repeats. The sequences are available at Genbank with accession # (SUBMITTED SUB4276706 (18S) and SUB4277191 (28S)).
The sequences were run through NCBI Blast (April-May 2018) with default parameters. In case of the apparent polymorphism in our Panagrellus redivivoides 28S sequences (AGTTGATCGGGTGTTGGCTTCGGY; cf. S2B Table, column P), the highest peak was used in the blast analysis. Differences with the closest sequence in the database were manually checked.
Data analysis
The abundance index is defined on a Log10 scale as in [26]. An index of 1 corresponds to one to 10 individuals, 2 for 11 to 100, 3 for 102 to 103, 4 for 103 to 104, and 5 for > 104.
Statistical analysis was performed in R version 3.4.1 [54].
Results
Sampling for Caenorhabditis
We sampled rotting vegetal matter, mostly fruits and stems from herbaceous plants (Fig 1), and occasionally flowers, leaves, and wood as well as a few other sample types, such as fungi, soil, humus mixes and occasionally larger invertebrates (S1 and S2 Tables). Our primary goal was to isolate Caenorhabditis spp. Around 20 years ago, we first had sampled soil and found some Pristionchus (see S2A Table, first lines) but as we could not find Caenorhabditis we then focused on richer rotting vegetal matter [25]. In 2015–2017, we also sought to isolate Mesorhabditis spp. and thus included samples of humus and rotting leaves where species of this genus are easy to find. We present here all samples for which we systematically noted the different nematode genera. Some locations (in France) were sampled on many occasions while others have been sampled once.
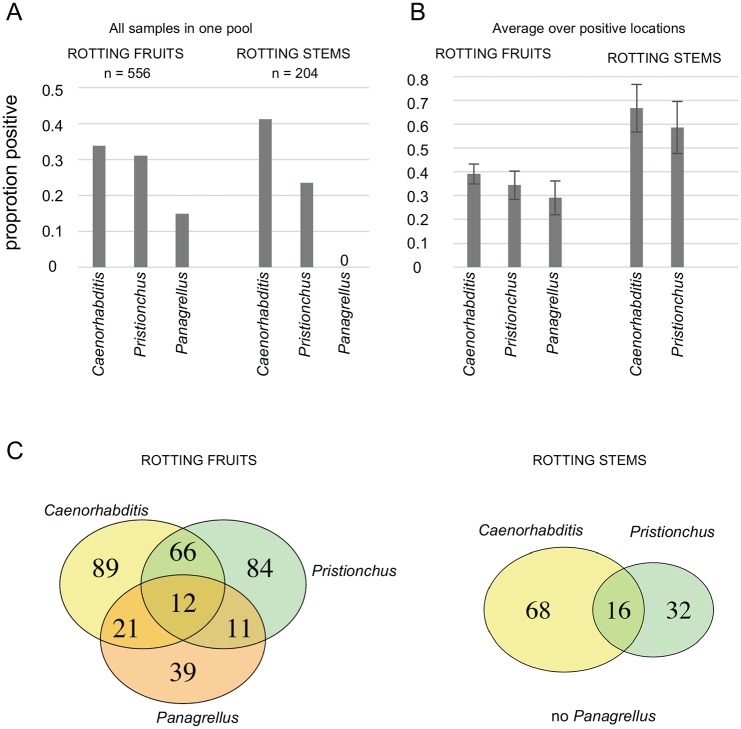
Caenorhabditis was found in 34% of rotting fruit samples (n = 556) and 41% of stem samples (n = 204) (Fig 3, Table 1). As noted previously [26], we found them in these various samples either in a feeding stage or in the dauer diapause stage.
Fig 3. Proportions of rotting fruit and stem samples containing Caenorhabditis, Pristionchus or Panagrellus.
(A) Proportions of samples positive for each genus, pooling all samples from rotting fruits or stems from 25 locations. (B) Proportions of samples positive for each genus, averaging over positive locations. Bars: standard error over locations. Caenorhabditis, Pristionchus are found in both types of sample, while Panagrellus are found in rotting fruits only. (C) Venn diagrams for the three genera in decomposing fruits (left) and stems (right).
Table 1. Occurrence of Caenorhabditis, Pristionchus and Panagrellus nematodes in rotting fruits and stems from various locations around the world.
Note that while Caenorhabditis was thoroughly searched for, the number of positive samples for Pristionchus and Panagrellus are underestimates, both because they may have been overlooked and because we may not have kept notes on batches of samples without Caenorhabditis. Detailed data for each sample are in S1 Table, with locations in France first in alphabetical order, then non-French locations.
| Location | Total # rotting fruits | # fruits with Caenorh. | # fruits with Prist. | # fruits with Panag. | Total # rotting stems | # stems with Caenorh. | # stems with Prist. | # stems with Panag. |
|---|---|---|---|---|---|---|---|---|
| Crouy-sur-Ourcq, FR | 4 | 0 | 0 | 0 | 14 | 10 | 3 | 0 |
| Le Blanc & Indre | 62 | 18 | 9 | 24 | 17 | 2 | 7 | 0 |
| Longueville | 9 | 1 | 3 | 0 | 2 | 1 | 2 | 0 |
| Orsay | 316 | 124 | 120 | 37 | 1 | 1 | 1 | 0 |
| Plougasnou & Finistère | 5 | 2 | 1 | 0 | 31 | 7 | 2 | 0 |
| Santeuil & Vexin | 39 | 8 | 2 | 5 | 89 | 55 | 17 | 0 |
| Vaucluse & Simiane | 28 | 7 | 11 | 7 | 0 | 0 | 0 | 0 |
| Bangalore, India | 7 | 4 | 1 | 0 | 1 | 1 | 0 | 0 |
| Barcelona, SP | 5 | 2 | 1 | 0 | 0 | 0 | 0 | 0 |
| Oku, Cameroon | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Cologne, DE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cambridge, UK | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Czech Republic | 17 | 6 | 4 | 9 | 3 | 3 | 1 | 0 |
| Heidelberg, DE | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Kazakhstan | 15 | 0 | 0 | 0 | 15 | 0 | 5 | 0 |
| Los Angeles, USA | 3 | 2 | 1 | 0 | 1 | 0 | 0 | 0 |
| Norway | 1 | 0 | 0 | 0 | 7 | 0 | 4 | 0 |
| New Zealand | 6 | 3 | 1 | 0 | 2 | 0 | 0 | 0 |
| Potsdam, DE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tuscany, IT | 12 | 1 | 11 | 0 | 2 | 1 | 2 | 0 |
| Vienna, Austria | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| São Tomé | 3 | 1 | 1 | 1 | 3 | 2 | 0 | 0 |
| Shanghai, China | 7 | 3 | 4 | 0 | 9 | 0 | 3 | 0 |
| Sicily, IT | 11 | 3 | 1 | 0 | 1 | 1 | 1 | 0 |
| U Warwick, UK | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Yerevan, Armenia | 3 | 1 | 2 | 0 | 0 | 0 | 0 | 0 |
| total | 556 | 188 | 173 | 83 | 204 | 84 | 48 | 0 |
| proportion | 0.34 | 0.31 | 0.15 | 0.41 | 0.24 | 0.00 |
Among species of the Elegans supergroup [25], C. elegans, C. briggsae, C. remanei are commonly found in temperate areas. We also found C. elegans in samples from the Oku mountains (Cameroon) and South Yunnan (China) and C. elegans, C. briggsae and C. tropicalis in samples from São Tomé. Among the Caenorhabditis species outside the Elegans supergroup, we found C. virilis in rotting apples (Orsay, also [25]) and in droppings from a small mammal having eaten fruits (Longueville), in both cases close to Paris, France. C. portoensis was found in several places in Western Europe in rotting fruits: in apples in Portugal [25] and France, and in oranges in Sicily. Finally, C. monodelphis [55] was found in one rotting wood sample including a tree fungus, from Oslo, Norway.
Pristionchus is commonly found in rotting vegetal matter
Pristionchus was found in 31% of rotting fruit samples (n = 556) and 24% of stem samples (n = 204; Table 1), thus in a comparable proportion to Caenorhabditis, especially considering that the numbers for Pristionchus are underestimates (especially in the large set of Santeuil stem samples, where Pristionchus was not always distinguished from other diplogastrids; S1 Table). Pristionchus is thus commonly found in both rotting fruit and stem samples.
In addition to rotting fruits and stems, we noted the presence of Pristionchus in other types of decomposed vegetal matter (S1 and S2 Tables): soil/humus and leaf litter (over 20 samples), compost, flowers, iris and hyacinth bulbs, cacti, leaves, moss, and wood. We also found Pristionchus in fungi (on trees or on the ground), a Geophilus myriapod, a dead bee, a dead Helix aspersa snail (S1 and S2 Tables), and droppings from a small mammal. This list is quite similar to the samples where we found Caenorhabditis. The only exception may be that C. elegans and C. briggsae were found in live snails, slugs and isopods (here and [21,22,27,30]) and we never found Pristionchus in such samples.
Pristionchus was found in most geographic locations (23/26 locations; while Caenorhabditis was found in 21/26 locations). The three locations where Pristionchus was not found were those sampled for Mesorhabditis spp. (mostly humus/rotting leaves samples), where Caenorhabditis was also not found. The two locations where Pristionchus but not Caenorhabditis was found were at Northern latitudes (Lofoten Islands) and on mountains with a continental climate and temperatures below freezing in winter (ca. 1500–2000 meters altitude near Almaty, Kazakhstan). Conversely, it would be interesting to establish whether Pristionchus may be less common than Caenorhabditis in equatorial regions (as could be suggested by the low frequency of Pristionchus in samples from São Tomé and Bangalore; S1 Table).
Within a single sample, the census size of Pristionchus appeared comparable to Caenorhabditis, with populations ranging from 1-few animals to over 1,000 (over 10,000 for Caenorhabditis) in one sample (S1 Table; for example samples shown in Fig 1B and 1F). Pristionchus feeding stages were noted to be present alongside dauer larvae in both rotting fruits and stems (Orsay, Santeuil). Pristionchus was the predominant species in terms of numbers in some samples (S1 Table), while in others Caenorhabditis, Oscheius, other rhabditids, or Panagrellus were most abundant.
A variety of Pristionchus species were found, as indicated by our 18S sequencing (S2 Table), consistent with previous reports [7,8,11,56]. Among them, we found P. pacificus, as verified by crosses (S2 Table). Some of our 18S sequences do not match those of any Pristionchus species in the databases and may be new species. Conversely, some Pristionchus species may not be distinguished by this short fragment. Importantly, the sampled diversity covers all groups of Pristionchus species in [56], except the more basal 'Elegans' group. Indeed, we found 18S best hits in the Pacificus group to P. pacificus, P. arcanus, P. japonicus and P. quartusdecimus, in the Maupasi group to P. atlanticus, in the Lheritieri group to P. uniformis and P. entomophagus, and in the Triformis group to P. triformis and P. hoplostomus. In addition, in a rotting bulb we found a putative representative of the fresh fig clade [15] with a sequence resembling that of Pristionchus sp. 35.
Panagrellus redivivoides is found in rotting fruits but not rotting stems
Panagrellus nematodes were found in 15% of rotting fruit samples (n = 556; confidence interval using a binomial distribution 12–18%) and 0% of the rotting stem samples (n = 204; confidence interval 0–2%) (Table 1). Thus, in our sampled substrate types, Panagrellus is specifically enriched in fruits versus stems (Fisher exact test rejecting homogeneity, p = 10−12). Rotting fruits contain bacteria and fungi and we observed that Panagrellus adults were often attracted to fungi (yeasts) on the culture plates.
Another invertebrate commonly found on these rotting fruits but not rotting stems is the fruit fly Drosophila (various species). We sampled live Drosophila in the field and on two occasions found Panagrellus (S2 Table and [26]). We also found Panagrellus in a Drosophila willistoni culture (S2 Table). We observed Panagrellus dauer larvae climbing on Drosophila pupae in our samples and waving, especially on the pupal appendages (Fig 2B). It is thus likely that fruitflies constitute vectors for Panagrellus between rotting fruit food patches.
We performed pairwise mating tests between Panagrellus fruit isolates from various places in Europe, Canary Islands, Armenia and North America, and to our surprise, found that all tested combinations were compatible, thus representing a single biological species. Morphologically, the vulva slit appeared almost perpendicular to the ventral side of the females (Fig 2C and 2D, S2B Table). This feature is characteristic of a single species of Panagrellus, called P. redivivoides [57,58]. In all other species such as P. redivivus, the vulva is strongly bent towards the anterior side of the animal (Fig 2E and 2F). Other morphological features of our isolates also matched the original and subsequent redescription of P. redivivoides [57,59,60], including the shape of the spicules and the gubernaculum dorsal end bearing a hook in lateral view (Fig 2G and 2H).
This Panagrellus species was collected from diverse fruit samples (apples, pears, peach, grapes, plums, cherries, tomatoes, walnuts, figs), but never from other substrates (except once in compost containing rotting fruits). Among the substrates we sampled, P. redivivoides thus appears specific to rotting fruits. It does not appear in every orchard or region where we have sampled, for example it was not found in Utah, while it was found in Oregon and Washington states (S2B Table). We recently found in China a new Panagrellus isolate (JU3343) in a compost heap of unclear composition but containing no fruits. As an exception that proves the rule, this isolate corresponds to a different Panagrellus species both through mating tests and morphology (S2B Table).
We amplified and sequenced 18S and 28S rDNA fragments for the Panagrellus isolates, which yielded the same sequence for all our rotting fruit Panagrellus isolates from eight locations spanning four continents, confirming our crossing tests. DNA sequence tags of P. redivivoides were not included in the Stock and Nadler article [59] that aimed to associate DNA sequence tags to Panagrellus morphological species. Two later articles with new Panagrellus sp. isolates [53,61] yielded closely related sequences but the authors did not attempt to identify the species morphologically or through mating tests. We found that the faster evolving 28S rDNA fragment was most similar but not identical to that of Panagrellus sp. MC2014 KM489128 (isolated from an aberrant specimen of the red palm weevil Rhynchophorus ferrugineus in [61]). The sequence of the 18S rDNA fragment is also similar but not identical to Panagrellus sp. MC2014 and to a newly described species Panagrellus levitatus [62] (see Discussion).
Other nematodes
Other nematode genera were found in rotting fruits and stems. Oscheius (species of the Dolichura group including the common Oscheius tipulae) was present in all types of samples. Although it was found frequently in soil [63], we had never observed feeding stages until we started collecting rotting fruits and stems (S1 Table). Other rhabditids included Rhabditella, Auanema, Pelodera, etc. Mesorhabditis was often present in small numbers, and also found in humus and rotting leaves in forests. Panagrolaimus was also found in soil but often present in rotting fruits and stems, even if quite dry. Rhabditophanes was particularly observed in cold weathers and climates. Fruit samples also commonly contained fungi-eating nematodes such as aphelenchs.
Co-occurrence
Many of the different nematodes co-occurred in the same sample (Table 1, S2C Table). Fig 3C summarizes the co-occurrence of the three genera Caenorhabditis, Pristionchus and Panagrellus in fruits and stems. A weak positive correlation was found in rotting fruits for the co-occurrence of Pristionchus and Caenorhabditis (correlation coefficient r = 0.16 [0.08–0.24 confidence interval], p = 0.0001). For example, in fruits, 41.0% of the samples with Caenorhabditis (n = 188, for a total of 556 fruits) also had Pristionchus and conversely, 44.5% of the samples with Pristionchus (n = 173) contain Caenorhabditis. No other correlations were found significant (note however that sample sizes were smaller in stems and for Panagrellus).
Seasonal pattern in the Orsay orchard
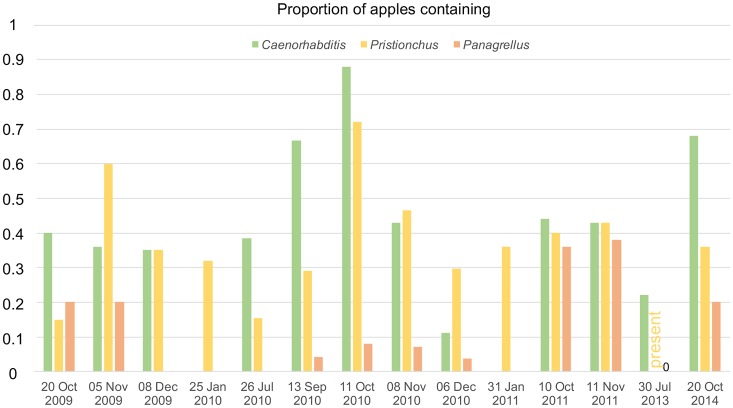
Fig 4 shows the abundance of the three genera over time in apples of the Orsay orchard. Pristionchus was present in rotting apples on every tested date of the year, spanning July to January. Caenorhabditis was not found on the two January dates (see further data in [26]) and Panagrellus was not found in July (twice in different years) nor on three dates in December and January.
Fig 4. Seasonal pattern of presence of Caenorhabditis, Pristionchus or Panagrellus in apples of the Orsay orchard.
This figure shows the proportion of positive apples for Caenorhabditis, Pristionchus or Panagrellus at different dates of sampling. Pristionchus was noted present in a few apples on the 30 July 2013 timepoint but the number of positive apples was not scored. n = 20–28 apples per date.
Discussion
Not only fond of beetles: Pristionchus also lives in rotting vegetal matter
Our results demonstrate that Pristionchus nematodes are found in rotting vegetal matter frequently and abundantly, in both dauer and feeding stages. This includes P. pacificus and other "beetle-associated" Pristionchus species, such as P. uniformis, P. entomophagus, P. triformis, P. quartusdecimus or P. atlanticus [8,9,12,56,64], or closely related species (S2 Table). From these data, we conclude that these Pristionchus species feed in rotting vegetal matter and are thus not exclusively necromenic, as previously reported by only sampling beetles. These decomposing invertebrates are likely not their main source of food compared to the microbial blooms and other prey nematodes that Pristionchus may encounter in rotting vegetal substrates.
From our literature review (see Introduction), Pristionchus have not been shown to be feeding in the wild on naturally decomposing beetle corpses. Thus, although it cannot be ruled out that Pristionchus may be necromenic on beetles in some instances, the relationship of Pristionchus species with beetles may be similar to the relationship of Caenorhabditis species with isopods or terrestrial molluscs. Indeed, when any of the carriers of Caenorhabditis are killed on a Petri dish as Pristionchus-bearing beetles have been, Caenorhabditis will exit the dauer stage and start reproducing. (Note that on live slugs and snails, other developmental stages of C. elegans are also found [26,27]). Evidence for a possible C. elegans necromenic behavior is anecdotal: non-dauer C. elegans were found once on a naturally dead Helix snail (Table 1 in [22]). Note that we found Pristionchus spp. on a live Geophilus myriapod, a dead snail and a dead honeybee (S2 Table; nematode developmental stage unknown), and some Pristionchus species also have been described on invertebrates other than beetles (e.g. P. entomophagus on a pamphilid wasp [65]). It is thus unclear for each Pristionchus species whether beetle species are the only specific associates. The present survey focused on rotting vegetal matter and a few other substrates and thus provides a narrow window on Pristionchus ecology, yet still considerably enlarging the sample diversity compared to only collecting beetles.
Each Pristionchus species (and possibly population) needs to be considered separately. Our survey found a variety of Pristionchus species covering several clades within the genus (S2A Table), with a biogeography consistent with that in previous reports in beetles [64]. Most of our sampling work was in Europe, where P. pacificus is rare compared to other Pristionchus spp. [8,9,11,66], but we found P. pacificus in decomposing vegetal matter in Asia (S2 Table).
Consequences for the life cycle of Pristionchus
A representation of the P. pacificus life cycle has recently been proposed [17], based on the beetle association and laboratory findings. In this model, adult P. pacificus (and possibly dauers) are attracted to the oriental beetle Exomala orientalis. On the beetle, eggs and dauers are produced and developmentally arrest due to a beetle chemical studied in the article. Upon beetle death, P. pacificus exit the dauer stage and as adults may be attracted to another beetle. Alternatively, they adopt a "free" life cycle (likely meaning that they do not go through a dauer stage) and eventually may reenter the dauer stage or be attracted to a new beetle as adults. Presumably, the free life cycle is on the dead beetle.
Two features of this proposed life cycle are surprising: i. the stage that is attracted to the beetle is the adult nematode (which has not been observed so far on wild-caught beetles); ii. once on the beetle, the adult nematode is able to produce dauer progeny. This new view of the nematode life cycle was probably derived from the fact that most behavioral studies of chemotaxis towards beetles used Pristionchus adults [6,17,67,68], with the exception of [69,70], which studied dauer nictation.
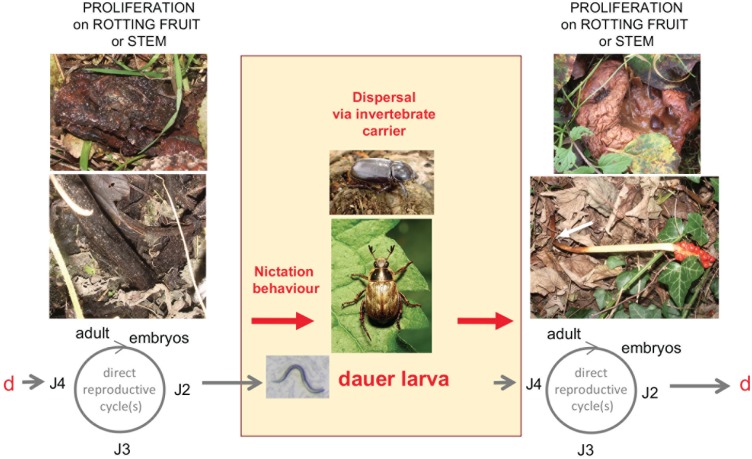
We propose in Fig 5 an alternative life cycle for P. pacificus and other Pristionchus species that may be found associated with various beetles and other insects. This life cycle is similar to that proposed for C. elegans [30,71]. In our model, rotting vegetal matter is the feeding ground for Pristionchus spp., whose populations expand until the food is exhausted whereupon the young larvae enter the dauer stage. Beetles may transport dauer larvae between these patchy plant food sources. In this life cycle scenario, the association of dauer larvae with the larger invertebrate is phoretic. Necromeny may be occasional, but its occurrence remains to be demonstrated.
Fig 5. Proposed life cycle of Pristionchus spp. in their natural habitat.
P. pacificus and other Pristionchus species proliferate in various types of rotting plant material, such as fruits. Dauer larvae are the stress-resistant, alternative third juvenile stage. (The first larval stage occurs within the embryo [97]). Dauer larvae may actively disperse to colonize new food sources. Alternatively, their nictation behaviour—standing on their tail and waving individually or in group—may allow them to attach and disperse via carriers, such as Exomala orientalis or Oryctes borbonicus for P. pacificus, until a new food source is encountered, where development resumes. J2–J4, juvenile stages; d, dauer larva. Modified after [71] drawn for C. elegans. Apples, stems and P. pacificus JU1102 dauer: Pictures by MAF. Bottom beetle: Exomala orientalis, by Katja Schulz via Wikimedia Commons CC BY-SA 2.0, https://commons.wikimedia.org/wiki/File%3AOriental_Beetle_-_Flickr_treegrow_(1).jpg. Top beetle: Oryctes borbonicus, by Jjargoud—Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=5063614.
In addition to bacteria and fungi, P. pacificus and other Pristionchus spp. may feed on other nematodes as a food source in decomposing vegetal matter. The predatory behavior of many Pristionchus species towards other nematodes is rendered possible by the plasticity of development of the mouth form [15,72,73]. From our data, it is clear that many other nematodes co-occur with Pristionchus species in rotting vegetal matter, including but not restricted to Caenorhabditis and Panagrellus species (S1 Table). These other nematodes may compete for bacterial food or serve as prey for Pristionchus. We found a weak positive correlation for the co-occurrence of Pristionchus and Caenorhabditis in rotting fruits. Rather than a specific attraction of Pristionchus to Caenorhabditis-containing substrates (or conversely), we propose that this correlation may be simply explained by the degree of decomposition, humidity or microbial fauna in the fruits, which needs to be such as to sustain these nematode species.
C. elegans was shown to avoid sulfolipids secreted by Pristionchus pacificus [74]. Due to geographical sampling biases for each genus, we have so far not detected the co-occurrence of C. elegans and P. pacificus in the same sample. It would be interesting to sample further in areas where both C. elegans and P. pacificus were found, such as Southern California, Hawaii, South Africa or La Réunion [11,75]. C. elegans and P. pacificus were chosen as the two most studied species of each genus, and it is not known whether the sulfolipids and the reaction to them are specific for these two species. It is possible that the avoidance behavior of C. elegans has evolved as a response to several Pristionchus species, and that conversely, several Caenorhabditis species may show avoidance to Pristionchus pacificus or other Pristionchus species.
To explain the presence of Pristionchus spp. dauers phoretic on beetles, it must be assumed that the beetles visit the kinds of habitats where we found Pristionchus populations, i.e. rotting fruits and stems. Are the beetles that have been associated with Pristionchus spp. likely to visit fruits or a forest floor with rotting stems? Detailed studies would be needed to answer this important question, taking into account the seasonality of the beetle’s life cycle itself. Geotrupes stercorosus was found to be the most reliable beetle source of Pristionchus nematodes in Europe by [9]. Indeed, Geotrupes adults may feed on forest litter or fungi [76] and lay eggs in nests they provide with forest litter [77]. The P. pacificus-carrying Exomala orientalis adults emerge from soil [78] and adult females may feed on flowers [79,80]. Indeed many of the scarab beetle species associated with Pristionchus were found to feed as adults on soft, high-quality diets and the micro-organisms therein [77] and many of the American tropical "dung beetle" species were found to feed on mature and rotting fruits [81]. Understanding the relationship between the rotting vegetal matter and the beetles is required to understand the biology of Pristionchus spp. and test our hypothesized life cycle (Fig 5). This includes answering the currently open question of whether Pristionchus is present on larval beetles.
Panagrellus in rotting fruits: A specific habitat for Panagrellus redivivoides?
The Panagrellus genus was previously found on a variety of substrates. The ‘sour paste nematode’ Panagrellus redivivus is used in many studies because of its ease of culture in the laboratory [82–89]. This species used to be commonly found in glues used to hang wallpaper and bind books [82,90]. Other Panagrellus species have been found in the slime flux or cankers of trees caused by bacterial or fungal diseases, in association with bark beetles or their frass, inside pitcher plants and, as a human associate, in beer mats and spoiled cider [59,60].
Taxonomic characterization of different Panagrellus species can be found in [38,59,60,91], with molecular data in [59]. The vagina of all but one of the Panagrellus species is tilted anteriorly towards the uterus, while a vagina that extends perpendicularly to the ventral cuticle of the female is specific to Panagrellus redivivoides [38,59,91]. P. redivivoides did not yet have a molecular tag to anchor the morphological description, which we provide here.
Our mating tests show that all the Panagrellus we isolated on rotting fruits or Drosophila belong to a single biological species, with a morphology matching Panagrellus redivivoides [57,58]. Its 28S rDNA sequence is identical to that of a Panagrellus sp. found in decomposing pomegranate in Italy [53] and both 28S and 18S sequences are similar but not identical to that of a Panagrellus found in a weevil in [61]. A new species of Panagrellus has been very recently described from a culture of Drosophila melanogaster and called P. levitatus [62]. This species presents similar but not identical 18S rDNA sequences to those we found (S2 Table) and has a weakly tilted vulva. The only morphological character of this isolate that is supposed to differ from P. redivivoides is the presence of circumcloacal papillae in P. levitatus, with a reference to [91] for their "absence" in P. redivivoides (to our knowledge only an absence of information). Further work would be required to make sure that there are two different species. Because of differences in rDNA sequence with P. levitatus, abundance on several continents and priority, we identify the biological species we found in rotting fruits as Panagrellus redivivoides.
Concerning phoretic associations, P. redivivoides may be associated with Drosophila fruit flies, in contrast to other Panagrellus sp. that appear associated with bark beetles. The fruit fly association relies on several reports. Early on, using Drosophila traps made of potato puree, Aubertot [92] found "Panagrellus silusiae", now synonymized with P. redivivus [90,93] (yet the species determination of Aubertot is unclear, especially since the species P. redivivoides was not described yet). P. redivivoides was found several times in laboratory cultures that were visited by fruitflies, including in the original description of the species by Goodey [57], which reports the arrival of the species in a banana maize-meal cider used to trap Drosophila flies. Lees [82] set up experiments to test the association of what he called "P. silusiae" (again, the species identification is unclear) and Drosophila funebris and showed that the latter could transport the former to new Petri dishes. "Panagrellus zymosiphilus", now synonymized with P. redivivoides [91] was also found twice in Drosophila cultures, the second time on Drosophila obscuroides recently isolated from nature [94,95]. "P. zymosiphilus" was also found in grapes, presumably brought by a Drosophila [96]. We added one data point to this association with Drosophila cultures with JU385, isolated in a Drosophila willistoni culture (S2B Table). In addition, Drosophila larvae are very often found in the rotting fruits we sampled and we could isolate Panagrellus redivivoides on Drosophila caught outside in two locations, Orsay and Le Blanc (reported in [26]), the latter yielding JU1055 (S2B Table).
We suggest the possibility that one species, P. redivivoides, is the most commonly associated with rotting fruits and Drosophila larvae, a distinguishing ecological feature compared to most Panagrellus species. Its life cycle may be similar to that depicted for C. elegans [71] or Pristionchus (Fig 5), but with Drosophila fruitflies carrying the dauer larvae from fruit to fruit.
In summary, based on our sampling and the observed distribution of feeding and dauer stages, we propose a life cycle for Pristionchus nematodes and Panagrellus redivivoides that is similar to that of many Caenorhabditis species, including C. elegans, whereby they feed on the microbial blooms on decomposing vegetal matter and are transported between these food patches by larger invertebrates, which may be beetles for Pristionchus spp., fruitflies for Panagrellus redivivoides and isopods and terrestrial molluscs for Caenorhabditis spp.
Supporting information
Each sheet corresponds to a sampling location and is designated by a letter: A-G, locations in France, H-Z locations outside France, in alphabetical order. Presence of a nematode genus is indicated by ‘x’. When the relative abundance of different genera has been scored, 1 indicates the most abundant, followed by 2, 3, etc. Caenorhabditis (green column), Pristionchus (yellow), Panagrellus (orange) are indicated first. The other groups are indicated roughly in order of ease of extraction from the sample and identification. On the right, in columns 'Caenorhabditis abundance log index’ and ‘Pristionchus abundance log index’, is indicated the abundance log index (see Methods): here 5 is highest and 1 lowest. ‘f’ indicates feeding stages, ‘d’ dauer larvae, in order of abundance, with the caveat that dauer larvae are more difficult to identify than feeding stages. Note that the care and timing with which the different nematodes were identified and monitored for number and stages differ among the different sampling dates and locations. Some data for Caenorhabditis in sheets D-F are from [26,49]. Pristionchus are best distinguished from other diplogastrids in the adult stage; thus, a '*' in the ‘other diplogastrid’ column indicates that these diplogastrids could have been Pristionchus. The Caenorhabditis species are abbreviated ‘Cel’ for C. elegans, ‘Cbr’ for C. briggsae, ‘Cre’ for C. remanei, ‘Ctr’ for C. tropicalis, ‘Cvi’ for C. virilis, ‘Cpo’ for C. portoensis, ‘Cmo’ for C. monodelphis. Latitude and longitude coordinates are indicated with the number of digits corresponding to the precision.
(XLSX)
Sheet A: Pristionchus. The table indicates the origin of the strains of Pristionchus (with JU or BRC standard strain names), and other samples that were noted to contain Pristionchus. The sample ID is a temporary ID for a given collection date. Other non-Pristionchus frozen strains from the same samples are indicated on the right. nd: not determined. Sheet B: Panagrellus. The table indicates the origin of Panagrellus strains. Sheet C: Other species of nematodes collected from rotten fruit and characterized by 18S rDNA sequencing.
(XLSX)
Acknowledgments
We thank members of our labs and all other sample collectors listed in S1 and S2 Tables, especially Jim Thomas. We thank Irini Topalidou for assistance with PCR and sequencing. We thank C. Braendle, S. Chalasani, K. Kiontke, B. Schlager and H. Teotónio for comments on the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files. The sequences are available at Genbank with accession numbers MH608216-608284 for 18S rDNA (all genera) and MH608291-608299 for 28S rDNA (Panagrellus).
Funding Statement
MAF and AR are supported by the Ecole Normale Superieure and the Centre National de la Recherche Scientifique. This work was supported by a National Science Foundation CAREER Award (MCB-1552101) to MA and the Ministry of Science and Technology, Taiwan and an Academia Sinica Career Development Grant to JW. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sommer RJ, McGaughran A (2013) The nematode Pristionchus pacificus as a model system for integrative studies in evolutionary biology. Mol Ecol 22: 2380–2393. 10.1111/mec.12286 [DOI] [PubMed] [Google Scholar]
- 2.Cinkornpumin JK, Hong RL (2011) RNAi mediated gene knockdown and transgenesis by microinjection in the necromenic nematode Pristionchus pacificus. J Vis Exp: e3270 10.3791/3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroetz SM, Srinivasan J, Yaghoobian J, Sternberg PW, Hong RL (2012) The cGMP signaling pathway affects feeding behavior in the necromenic nematode Pristionchus pacificus. PLoS One 7: e34464 10.1371/journal.pone.0034464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dieterich C, Clifton SW, Schuster LN, Chinwalla A, Delehaunty K, Dinkelacker I, et al. (2008) The Pristionchus pacificus genome provides a unique perspective on nematode lifestyle and parasitism. Nat Genet 40: 1193–1198. 10.1038/ng.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floyd R, Abebe E, Papert A, Blaxter M (2002) Molecular barcodes for soil nematode identification. Mol Ecol 11: 839–850. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann M, Mayer WE, Hong RL, Kienle S, Minasaki R, Sommer RJ (2007) The nematode Pristionchus pacificus (Nematoda: Diplogastridae) is associated with the oriental beetle Exomala orientalis (Coleoptera: Scarabaeidae) in Japan. Zoolog Sci 24: 883–889. 10.2108/zsj.24.883 [DOI] [PubMed] [Google Scholar]
- 7.Zauner H, Mayer WE, Herrmann M, Weller A, Erwig M, Sommer RJ (2007) Distinct patterns of genetic variation in Pristionchus pacificus and Caenorhabditis elegans, two partially selfing nematodes with cosmopolitan distribution. Molecular Ecology 16: 1267–1280. 10.1111/j.1365-294X.2006.03222.x [DOI] [PubMed] [Google Scholar]
- 8.D’Anna I, Sommer RJ (2011) Pristionchus uniformis, should I stay or should I go? Recent host range expansion in a European nematode. Ecol Evol 1: 468–478. 10.1002/ece3.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrmann M, Mayer WE, Sommer RJ (2006) Nematodes of the genus Pristionchus are closely associated with scarab beetles and the Colorado potato beetle in Western Europe. Zoology 109: 96–108. 10.1016/j.zool.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 10.Herrmann M, Mayer WE, Sommer RJ (2006) Sex, bugs and Haldane’s rule: the nematode genus Pristionchus in the United States. Front Zool 3: 14 10.1186/1742-9994-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann M, Kienle S, Rochat J, Mayer WE, Sommer RJ (2010) Haplotype diversity of the nematode Pristionchus pacificus on Réunion in the Indian Ocean suggests multiple independent invasions. Biol J Linn Soc 100: 170–179. [Google Scholar]
- 12.Weller AM, Mayer WE, Rae R, Sommer RJ (2010) Quantitative assessment of the nematode fauna present on Geotrupes dung beetles reveals species-rich communities with a heterogeneous distribution. J Parasitol 96: 525–531. 10.1645/GE-2319.1 [DOI] [PubMed] [Google Scholar]
- 13.Morgan K, McGaughran A, Villate L, Herrmann M, Witte H, Bartelmes G, et al. (2012) Multi locus analysis of Pristionchus pacificus on La Reunion Island reveals an evolutionary history shaped by multiple introductions, constrained dispersal events and rare out-crossing. Mol Ecol 21: 250–266. 10.1111/j.1365-294X.2011.05382.x [DOI] [PubMed] [Google Scholar]
- 14.Darsouei R, Karimi J, Shokoohi E (2014) Oscheius rugaoensis and Pristionchus maupasi, two new records of entomophilic nematodes from Iran. Russian Jorunal of Nematology 22: 141–155. [Google Scholar]
- 15.Susoy V, Herrmann M, Kanzaki N, Kruger M, Nguyen CN, Rodelsperger C, et al. (2016) Large-scale diversification without genetic isolation in nematode symbionts of figs. Sci Adv 2: e1501031 10.1126/sciadv.1501031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari C, Salle R, Callemeyn-Torre N, Jovelin R, Cutter AD, Braendle C (2017) Ephemeral-habitat colonization and neotropical species richness of Caenorhabditis nematodes. BMC Ecol 17: 43 10.1186/s12898-017-0150-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cinkornpumin JK, Wisidagama DR, Rapoport V, Go JL, Dieterich C, Wang X, et al. (2014) A host beetle pheromone regulates development and behavior in the nematode Pristionchus pacificus. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer JM, Baskaran P, Quast C, Susoy V, Rodelsperger C, Glockner FO, et al. (2017) Succession and dynamics of Pristionchus nematodes and their microbiome during decomposition of Oryctes borbonicus on La Reunion Island. Environ Microbiol 19: 1476–1489. 10.1111/1462-2920.13697 [DOI] [PubMed] [Google Scholar]
- 19.Sudhaus W, Fürst von Lieven A (2003) A phylogenetic classification and catalogue of the Diplogastridae (Secernentea, Nematoda). J Nem Morph Syst 6: 43–90. [Google Scholar]
- 20.Baird SE, Fitch DHA, Emmons SW (1994) Caenorhabditis vulgaris n.sp. (Nematoda: Rhabditidae): a necromenic associate of pill bugs and snails. Nematologica 40: 1–11. [Google Scholar]
- 21.Baird SE (1999) Natural and experimental associations of Caenorhabditis remanei with Trachelipus rathkii and other terrestrial isopods. Nematology 1: 471–475. [Google Scholar]
- 22.Barrière A, Félix M-A (2005) High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Curr Biol 15: 1176–1184. 10.1016/j.cub.2005.06.022 [DOI] [PubMed] [Google Scholar]
- 23.Caswell-Chen EP, Chen J, Lewis EE, Douhan GW, Nadler SA, Carey JR (2005) Revising the standard wisdom of C. elegans natural history: ecology of longevity. Sci Aging Knowl Environ 40: pe30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrière A, Félix M-A (2007) Temporal dynamics and linkage disequilibrium in natural C. elegans populations. Genetics 176: 999–1011. 10.1534/genetics.106.067223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiontke K, Félix M-A, Ailion M, Rockman MV, Braendle C, Pénigault J-B, et al. (2011) A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evol Biol 11: 339 10.1186/1471-2148-11-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Félix MA, Duveau F (2012) Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae. BMC Biol 10: 59 10.1186/1741-7007-10-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen C, Hermann RJ, Barg MC, Schalkowski R, Dirksen P, Barbosa C, et al. (2015) Travelling at a slug’s pace: possible invertebrate vectors of Caenorhabditis nematodes. BMC Ecol 15: 19 10.1186/s12898-015-0050-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H, Choi M-k, Lee D, Kim H-s, Hwang H, Kim H, et al. (2011) Nictation, a dispersal behavior of the nematode Caenorhabditis elegans, is regulated by IL2 neurons. Nature Neuroscience 15: 107–112. 10.1038/nn.2975 [DOI] [PubMed] [Google Scholar]
- 29.Lee D, Yang H, Kim J, Brady S, Zdraljevic S, Zamanian M, et al. (2017) The genetic basis of natural variation in a phoretic behavior. Nat Commun 8: 273 10.1038/s41467-017-00386-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulenburg H, Félix M-A (2017) The natural biotic environment of Caenorhabditis elegans. Genetics 206: 55–86. 10.1534/genetics.116.195511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okumura E, Tanaka R, Yoshiga T (2013) Species-specific recognition of the carrier insect by dauer larvae of the nematode Caenorhabditis japonica. J Exp Biol 216: 568–572. 10.1242/jeb.073593 [DOI] [PubMed] [Google Scholar]
- 32.Okumura E, Ishikawa Y, Tanaka R, Yoshiga T (2013) Propagation of Caenorhabditis japonica in the nest of its carrier insect, Parastrachia japonensis. Zoolog Sci 30: 174–177. 10.2108/zsj.30.174 [DOI] [PubMed] [Google Scholar]
- 33.Okumura E, Yoshiga T (2014) Host orientation using volatiles in the phoretic nematode Caenorhabditis japonica. J Exp Biol 217: 3197–3199. 10.1242/jeb.105353 [DOI] [PubMed] [Google Scholar]
- 34.Yoshiga T, Ishikawa Y, Tanaka R, Hironaka M, Okumura E (2013) Species-specific and female host-biased ectophoresy in the roundworm Caenorhabditis japonica. Naturwissenschaften 100: 205–208. 10.1007/s00114-013-1011-z [DOI] [PubMed] [Google Scholar]
- 35.Kiontke K, Sudhaus W (2006) Ecology of Caenorhabditis species In: WormBook (ed. The C. elegans Research Community), [http://www.wormbook.org/] 10.1895/wormbook.1.37.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stiernagle T (2006) Maintenance of C. elegans In: Wormbook (ed. The C. elegans Research Community), [http://www.wormbook.org/] 10.1895/wormbook.1.101.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrière A, Félix M-A (2014) Isolation of C. elegans and related nematodes In: WormBook (ed. The C. elegans Research Community), [http://www.wormbook.org/] 10.1895/wormbook.1.115.2 [DOI] [PubMed] [Google Scholar]
- 38.Andrássy I (1984) Klasse Nematoda. Stuttgart: Gustav Fischer Verlag; 509 p. [Google Scholar]
- 39.Sudhaus W (2011) A pictorial key to current genus groups of “Rhabditidae”. J Nematode Morphol Syst 14: 105–112. [Google Scholar]
- 40.Félix M-A, Hill RJ, Schwarz H, Sternberg PW, Sudhaus W, Sommer RJ (1999) Pristionchus pacificus, a nematode with only three juvenile stages, displays major heterochronic changes relative to C. elegans. Proc R Soc Lond B 266: 1617–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Félix M-A, De Ley P, Sommer RJ, Frisse L, Nadler SA, Thomas WK, et al. (2000) Evolution of vulva development in the Cephalobina (Nematoda). Dev Biol 221: 68–86. 10.1006/dbio.2000.9665 [DOI] [PubMed] [Google Scholar]
- 42.Félix M-A, Sternberg PW (1997) Two nested gonadal inductions of the vulva in nematodes. Development 124: 253–259. [DOI] [PubMed] [Google Scholar]
- 43.Delattre M, Félix M-A (2001) Evolution and development of a variable left-right asymmetry in nematodes: the handedness of P11/P12 migration. Dev Biol 232: 362–371. 10.1006/dbio.2001.0175 [DOI] [PubMed] [Google Scholar]
- 44.Félix M-A (2004) Alternative morphs and plasticity of vulval development in a rhabditid nematode species. Dev Genes Evol 214: 55–63. 10.1007/s00427-003-0376-y [DOI] [PubMed] [Google Scholar]
- 45.Kiontke K, Barrière A, Kolotuev I, Podbilewicz B, Sommer RJ, Fitch DHA, et al. (2007) Trends, stasis and drift in the evolution of nematode vulva development. Curr Biol 17: 1925–1937. 10.1016/j.cub.2007.10.061 [DOI] [PubMed] [Google Scholar]
- 46.Félix M-A, Vierstraete A, Vanfleteren J (2001) Three biological species related to Rhabditis (Oscheius) pseudodolichura Körner in Osche, 1952. J Nematol 33: 104–109. [PMC free article] [PubMed] [Google Scholar]
- 47.Delattre M, Félix M-A (2001) Polymorphism and evolution of vulval precursor cell lineages within two nematode genera, Caenorhabditis and Oscheius. Curr Biol 11: 631–643. [DOI] [PubMed] [Google Scholar]
- 48.Félix M-A, Sternberg PW (1996) Symmetry breakage in the development of one-armed gonads in nematodes. Development 122: 2129–2142. [DOI] [PubMed] [Google Scholar]
- 49.Richaud A, Zhang G, Lee D, Lee J, Félix M-A (2018) The local co-existence pattern of selfing genotypes in Caenorhabditis elegans natural metapopulations. Genetics 208: 807–821. 10.1534/genetics.117.300564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Félix M-A (2006) “Oscheius tipulae” In: WormBook (ed. The C. elegans Research Community), [http://www.wormbook.org/] 10.1895/wormbook.1.119.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fradin H, Kiontke K, Zegar C, Gutwein M, Lucas J, Kovtun M, et al. (2017) Genome architecture and evolution of a unichromosomal asexual nematode. Curr Biol 27: 2928–2939 e2926. 10.1016/j.cub.2017.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, et al. (1998) A molecular evolutionary framework for the phylum Nematoda. Nature 392: 71–75. 10.1038/32160 [DOI] [PubMed] [Google Scholar]
- 53.Fanelli E, Troccoli A, Vovlas N, Scarcia G, Mincuzzi A, Sanzani SM, et al. (2017) Occurrence of Sheraphelenchus sucus (Nematoda: Aphelenchoidinae) and Panagrellus sp. (Rhabditida: Panagrolaimidae) associated with decaying pomegranate fruit in Italy. J Nematol 49: 418–426. [PMC free article] [PubMed] [Google Scholar]
- 54.R Core Team (2015) R: A language and environment for statistical computing. In: Computing RFfS, editor. Vienna, Austria. [Google Scholar]
- 55.Slos D, Sudhaus W, Stevens L, Bert W, Blaxter M (2017) Caenorhabditis monodelphis n. sp.: defining the stem morphology and genomics of the genus Caenorhabditis. BMC Zoology 2: 4. [Google Scholar]
- 56.Ragsdale EJ, Kanzaki N, Röseler W, Herrmann M, Sommer RJ (2013) Three new species of Pristionchus (Nematoda: Diplogastridae) show morphological divergence through evolutionary intermediates of a novel feeding-structure polymorphism. Zool J Linn Soc 168: 671–698. [Google Scholar]
- 57.Goodey T (1943) On the systematic relationships of the vinegar eel-worm, Turbatrix aceti, and its congeners, with description of a new species. J Helminth 21: 1–9. [Google Scholar]
- 58.Goodey T (1945) A note on the subfamily Turbatricinae and the genus Turbator Goodey, 1943. Journ Helminth 21: 69–70. [Google Scholar]
- 59.Stock SP, Nadler SA (2006) Morphological and molecular characterization of Panagrellus spp. (Cephalobina: Panagrolaimidae): taxonomic status and phylogenetic relationships. Nematology 8: 921–938. [Google Scholar]
- 60.Abolafia J, Alizadeh M, Khakvar R (2016) Description of Panagrellus ulmi sp. n. (Rhabditida, Panagrolaimidae) from Iran, and comments on the species of the genus and its relatives. Zootaxa 4162: 245–267. 10.11646/zootaxa.4162.2.3 [DOI] [PubMed] [Google Scholar]
- 61.Camerota M, Mazza G, Carta LK, Paoli F, Torrini G, Benvenuti C, et al. (2016) Occurrence of Panagrellus (Rhabditida: Panagrolaimidae) nematodes in a morphologically aberrant adult specimen of Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). J Nematol 48: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ivanova E, Perfilieva K, Spiridonov S (2018) Panagrellus levitatus sp. n. (Rhabditida: Panagrlolaimidae), a nematode suppressing Drosophila melanogaster in laboratory cultures. Nematology 20: 285–297. [Google Scholar]
- 63.Baïlle D, Barrière A, Félix M-A (2008) Oscheius tipulae, a widespread hermaphroditic soil nematode, displays a higher genetic diversity and geographical structure than Caenorhabditis elegans. Mol Ecol 17: 1523–1534. 10.1111/j.1365-294X.2008.03697.x [DOI] [PubMed] [Google Scholar]
- 64.Kanzaki N, Ragsdale EJ, Herrmann M, Roseler W, Sommer RJ (2013) Two new species of Pristionchus (Nematoda: Diplogastridae) support the biogeographic importance of Japan for the evolution of the genus Pristionchus and the model system P. pacificus. Zoolog Sci 30: 680–692. 10.2108/zsj.30.680 [DOI] [PubMed] [Google Scholar]
- 65.Steiner G (1929) Diplogaster entomophaga n. sp., a new Diplogaster (Diplogasteridae, Nematoda) found on a Pamphilius stellatus (Christ) (Tenthredinidae, Hymenoptera). Zool Anz 80: 143–145. [Google Scholar]
- 66.Mayer WE, Herrmann M, Sommer RJ (2007) Phylogeny of the nematode genus Pristionchus and implications for biodiversity, biogeography and the evolution of hermaphroditism. BMC Evol Biol 7: 104 10.1186/1471-2148-7-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hong RL, Sommer RJ (2006) Chemoattraction in Pristionchus nematodes and implications for insect recognition. Curr Biol 16: 2359–2365. 10.1016/j.cub.2006.10.031 [DOI] [PubMed] [Google Scholar]
- 68.Hong RL, Svatos A, Herrmann M, Sommer RJ (2008) Species-specific recognition of beetle cues by the nematode Pristionchus maupasi. Evol Dev 10: 273–279. 10.1111/j.1525-142X.2008.00236.x [DOI] [PubMed] [Google Scholar]
- 69.Brown FD, D’Anna I, Sommer RJ (2011) Host-finding behaviour in the nematode Pristionchus pacificus. Proc Biol Sci 278: 3260–3269. 10.1098/rspb.2011.0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Penkov S, Ogawa A, Schmidt U, Tate D, Zagoriy V, Boland S, et al. (2014) A wax ester promotes collective host finding in the nematode Pristionchus pacificus. Nat Chem Biol 10: 281–285. 10.1038/nchembio.1460 [DOI] [PubMed] [Google Scholar]
- 71.Félix M-A, Braendle C (2010) The natural history of Caenorhabditis elegans. Curr Biol 20: R965–R969. 10.1016/j.cub.2010.09.050 [DOI] [PubMed] [Google Scholar]
- 72.Bento G, Ogawa A, Sommer RJ (2010) Co-option of the hormone-signalling module dafachronic acid-DAF-12 in nematode evolution. Nature 466: 494–497. 10.1038/nature09164 [DOI] [PubMed] [Google Scholar]
- 73.Serobyan V, Ragsdale EJ, Sommer RJ (2014) Adaptive value of a predatory mouth-form in a dimorphic nematode. Proc Biol Sci 281: 20141334 10.1098/rspb.2014.1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Z, Kariya MJ, Chute CD, Pribadi AK, Leinwand SG, Tong A, et al. (2018) Predator-secreted sulfolipids induce defensive responses in C. elegans. Nat Commun 9: 1128 10.1038/s41467-018-03333-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cook DE, Zdraljevic S, Tanny RE, Seo B, Riccardi DD, Noble LM, et al. (2016) The genetic basis of natural variation in Caenorhabditis elegans telomere length. Genetics 204: 371–383. 10.1534/genetics.116.191148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanski I (1991) North Temperate dung beetles In: Hanski I, Cambefort Y, editors. Dung beetle ecology. Princeton: Princeton University Press; pp. 75–96. [Google Scholar]
- 77.Cambefort Y (1991) From saprophagy to coprophagy In: Hanski I, Cambefort Y, editors. Dung beetle ecology. Princeton: Princeton University Press; pp. 22–35. [Google Scholar]
- 78.Facundo HY, Linn CEJ, Villano MG, Roelofs WL (1999) Emergence, mating and postmating behaviors of the Oriental Beetle (Coleoptera: Scarabaeidae). J Insect Behavior 12: 175–192. [Google Scholar]
- 79.Facundo HY, Villani MG, Linn CEJ, Roelofs WL (1999) Temporal and spatial distribution of the Oriental Beetle (Coleoptera: Scarabaeidae) in a golf course environment. Environmental Entomology 28: 14–21. [Google Scholar]
- 80.Choo HY, Lee DW, Park JW, Kaya HK, Smitley DR, Lee SM, et al. (2002) Life history and spatial distribution of Oriental Beetle (Coleoptera: Scarabaeidae) in golf courses in Korea. Journal of Economic Entomology 95: 72–80. 10.1603/0022-0493-95.1.72 [DOI] [PubMed] [Google Scholar]
- 81.Gill BD (1991) Dung beetles in Tropical American forests In: Hanski I, Cambefort Y, editors. Dung beetle ecology. Princeton: Princeton University Press; pp. 211–229. [Google Scholar]
- 82.Lees E (1953) An investigation into the method of dispersal of Panagrellus silusiae, with particular reference to its desiccation resistance. J Helminth 27: 95–103. [Google Scholar]
- 83.Hieb WF, Dougherty EC (1966) Evidence for tricarboxylic acid cycle in Panagrellus redivivus and changes observed under varying conditions of culture. Nematologica 12: 93. [Google Scholar]
- 84.Hechler HC (1970) Reproduction, chromosome number, and postembryonic development of Panagrellus redivivus (Nematoda: Cephalobidae). J Nematol 2: 355–361. [PMC free article] [PubMed] [Google Scholar]
- 85.Sternberg PW, Horvitz HR (1981) Gonadal cell lineages of the nematode Panagrellus redivivus and implications for evolution by the modification of cell lineage. Dev Biol 88: 147–166. [DOI] [PubMed] [Google Scholar]
- 86.Sternberg PW, Horvitz HR (1982) Postembryonic nongonadal cell lineages of the nematode Panagrellus redivivus: Description and comparison with those of Caenorhabditis elegans. Dev Biol 93: 181–205. [DOI] [PubMed] [Google Scholar]
- 87.Link CD, Graf-Whitsel J, Wood WB (1987) Isolation and characterization of a nematode transposable element from Panagrellus redivivus. Proc Natl Acad Sci 84: 5325–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choe A, Chuman T, von Reuss SH, Dossey AT, Yim JJ, Ajredini R, et al. (2012) Sex-specific mating pheromones in the nematode Panagrellus redivivus. Proc Natl Acad Sci U S A 109: 20949–20954. 10.1073/pnas.1218302109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Srinivasan J, Dillman AR, Macchietto MG, Heikkinen L, Lakso M, Fracchia KM, et al. (2013) The draft genome and transcriptome of Panagrellus redivivus are shaped by the harsh demands of a free-living lifestyle. Genetics 193: 1279–1295. 10.1534/genetics.112.148809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ferris H (2009) The beer mat nematode, Panagrellus redivivus: A study of the connectedness of scientific discovery. J Nematode Morphol Syst 12: 19–25. [Google Scholar]
- 91.Hechler HC (1971) Taxonomic notes on four species of Panagrellus Thorne (Nematoda: Cephalobidae). J Nematol 3: 227–237. [PMC free article] [PubMed] [Google Scholar]
- 92.Aubertot M (1925) Nématodes d’Alsace. Observations sur l’Anguillule de la bière (Anguillula silusiae de Man, 1914). Bull Ass philom Als Lorr 6: 333–342. [Google Scholar]
- 93.Rühm W (1956) Die Nematoden der Ipiden. Parasitol Schriftenreihe 6: 1–435. [Google Scholar]
- 94.Brunold E (1950) Über eine neue Nematodenart der Gattung Anguillula aus Drosophila-Nährböden. Vierteljahrsschrift der Natur Gesellschaft in Zuürich 95: 148–150. [Google Scholar]
- 95.Brunold E (1954) Zur Morphologie, Biologie und Baketerienfreien Züchtung des Nematoden Panagrellus zymosiphilus Brunold 1950. Z Morph u Ökol Tiere 42: 373–420. [Google Scholar]
- 96.Smith MT, Shann C, Batenburg-van der Vegte WH, Schmitt R, Wehrli E, Roeijmans HJ, et al. (1992) Botryozyma nematodophila gen. nov., spec. nov. (Candidaceae). Antonie Van Leeuwenhoek 61: 277–284. [DOI] [PubMed] [Google Scholar]
- 97.Fürst von Lieven A (2005) The embryonic moult in diplogastrids (Nematoda)—homology of developmental stages and heterochrony as a prerequisite for morphological diversity. Zool Anzeiger 244: 79–81. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each sheet corresponds to a sampling location and is designated by a letter: A-G, locations in France, H-Z locations outside France, in alphabetical order. Presence of a nematode genus is indicated by ‘x’. When the relative abundance of different genera has been scored, 1 indicates the most abundant, followed by 2, 3, etc. Caenorhabditis (green column), Pristionchus (yellow), Panagrellus (orange) are indicated first. The other groups are indicated roughly in order of ease of extraction from the sample and identification. On the right, in columns 'Caenorhabditis abundance log index’ and ‘Pristionchus abundance log index’, is indicated the abundance log index (see Methods): here 5 is highest and 1 lowest. ‘f’ indicates feeding stages, ‘d’ dauer larvae, in order of abundance, with the caveat that dauer larvae are more difficult to identify than feeding stages. Note that the care and timing with which the different nematodes were identified and monitored for number and stages differ among the different sampling dates and locations. Some data for Caenorhabditis in sheets D-F are from [26,49]. Pristionchus are best distinguished from other diplogastrids in the adult stage; thus, a '*' in the ‘other diplogastrid’ column indicates that these diplogastrids could have been Pristionchus. The Caenorhabditis species are abbreviated ‘Cel’ for C. elegans, ‘Cbr’ for C. briggsae, ‘Cre’ for C. remanei, ‘Ctr’ for C. tropicalis, ‘Cvi’ for C. virilis, ‘Cpo’ for C. portoensis, ‘Cmo’ for C. monodelphis. Latitude and longitude coordinates are indicated with the number of digits corresponding to the precision.
(XLSX)
Sheet A: Pristionchus. The table indicates the origin of the strains of Pristionchus (with JU or BRC standard strain names), and other samples that were noted to contain Pristionchus. The sample ID is a temporary ID for a given collection date. Other non-Pristionchus frozen strains from the same samples are indicated on the right. nd: not determined. Sheet B: Panagrellus. The table indicates the origin of Panagrellus strains. Sheet C: Other species of nematodes collected from rotten fruit and characterized by 18S rDNA sequencing.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. The sequences are available at Genbank with accession numbers MH608216-608284 for 18S rDNA (all genera) and MH608291-608299 for 28S rDNA (Panagrellus).