Abstract
Purpose
To determine whether cotargeting poly (ADP-ribose) polymerase-1 plus androgen receptor is superior to androgen receptor inhibition in metastatic castration-resistant prostate cancer (mCRPC) and whether ETS fusions predict response.
Patients and Methods
Patients underwent metastatic site biopsy and were stratified by ETS status and randomly assigned to abiraterone plus prednisone without (arm A) or with veliparib (arm B). Primary objectives were: confirmed prostate-specific antigen (PSA) response rate (RR) and whether ETS fusions predicted response. Secondary objectives were: safety, measurable disease RR (mRR), progression-free survival (PFS), and molecular biomarker analysis. A total of 148 patients were randomly assigned to detect a 20% PSA RR improvement.
Results
A total of 148 patients with mCRPC were randomly assigned: arm A, n = 72; arm B, n = 76. There were no differences in PSA RR (63.9% v 72.4%; P = .27), mRR (45.0% v 52.2%; P = .51), or median PFS (10.1 v 11 months; P = .99). ETS fusions did not predict response. Exploratory analysis of tumor sequencing (80 patients) revealed: 41 patients (51%) were ETS positive, 20 (25%) had DNA-damage repair defect (DRD), 41 (51%) had AR amplification or copy gain, 34 (43%) had PTEN mutation, 33 (41%) had TP53 mutation, 39 (49%) had PIK3CA pathway activation, and 12 (15%) had WNT pathway alteration. Patients with DRD had significantly higher PSA RR (90% v 56.7%; P = .007) and mRR (87.5% v 38.6%; P = .001), PSA decline ≥ 90% (75% v 25%; P = .001), and longer median PFS (14.5 v 8.1 months; P = .025) versus those with wild-type tumors. Median PFS was longer in patients with normal PTEN (13.5 v 6.7 months; P = .02), TP53 (13.5 v 7.7 months; P = .01), and PIK3CA (13.8 v 8.3 months; P = .03) versus those with mutation or activation. In multivariable analysis adjusting for clinical covariates, DRD association with PFS remained significant.
Conclusion
Veliparib and ETS status did not affect response. Exploratory analysis identified a novel DRD association with mCRPC outcomes.
INTRODUCTION
Despite a high response rate (RR) to androgen deprivation (AD), a majority of patients with metastatic prostate cancer (PCa) will experience progression to castration resistance. Advances in understanding the biology and progression mechanisms to castration resistance led to development and approval of novel androgen receptor (AR) –targeted therapies: abiraterone acetate plus prednisone (AAP) and enzalutamide.1,2 Both prolong survival in metastatic castration-resistant prostate cancer (mCRPC) irrespective of prior docetaxel.3,4 However, many patients exhibit de novo resistance to both therapies, and resistance invariably occurs in responders, warranting a search for better treatments.5-8
Several studies have shown that AR regulates components of DNA-repair pathways, and conversely, several enzymes involved in DNA repair can modulate AR activity.9-15 An important example is poly (ADP-ribose) polymerase-1 (PARP1), an enzyme with essential roles in recognition and repair of single-strand DNA breaks through base excision repair process.16 Several cancers, including CRPC, exhibit increased PARP1 expression and/or activity.16-19 Compelling data implicate PARP1 in mediation of DNA-repair responses to alkylators, cellular survival in BRCA-deficient cells, and AR-mediated PCa cell proliferation.16,20-23 Specifically, preclinical studies using PARP1 inhibitors (eg, veliparib, olaparib) in PCa showed that PARP1 activity was required for maximal AR function.16 In vivo, PARP1 inhibition with veliparib was as effective as castration in preventing tumor growth, and even greater inhibition was achieved with combination veliparib and castration.16
Canonic ETS gene fusions (androgen-responsive promoters driving ETS transcription factor overexpression) are present in > 50% of patients with PCa. ERG, the predominant ETS gene fusion product, physically interacts with PARP1.24-26 PARP1 is required for full ERG activity and its downstream oncogenic functions. ERG-positive xenografts are preferentially sensitive to PARP1 inhibitors.26
On the basis of these data, we hypothesized that in patients with mCRPC, cotargeting AR and PARP1 would result in a better RR than AAP and the combination would be most effective in patients with ETS fusion–positive tumors.
PATIENTS AND METHODS
Patients
Eligible patients had mCRPC, Eastern Cooperative Oncology Group performance status of 0 to 2, testosterone < 50 ng/dL, normal organ function, no prior exposure to AAP, and up to two prior chemotherapy regimens. Complete eligibility criteria are outlined in the Study Protocol. All patients provided written informed consent per institutional and federal guidelines.
Study Design, Treatment, and End Points
This was a biomarker-stratified and randomized phase II multicenter trial (Fig 1). The primary objectives were to evaluate whether AAP plus veliparib is superior to AAP, as reflected by prostate-specific antigen (PSA) RR (≥ 50% decline), and whether ETS gene fusion predicts response. Other end points included measurable disease RR (mRR), progression-free survival (PFS), toxicities, and exploratory tumor molecular analysis.
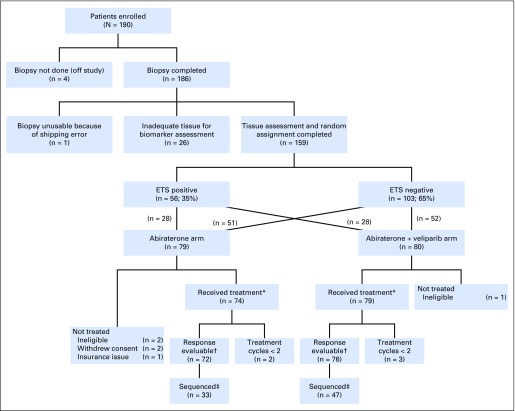
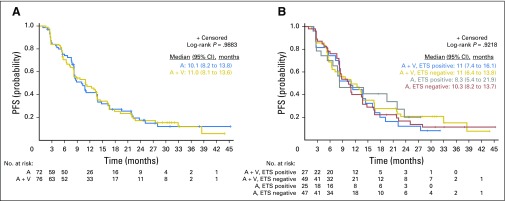
Fig 1.
CONSORT diagram. (*) Safety evaluable. (†) Defined as having received two cycles of therapy or removed because of toxicity; five patients who received < two cycles did so by patient choice. (‡) Sequencing completed for all patients with sufficient extra tissue from biopsy required for sequencing.
All patients underwent metastatic disease biopsy (unless metastatic archival tissue was available). ETS status was determined by immunohistochemistry (IHC) for ERG and in situ hybridization (ISH) –based assays for ETV1 fusions,27,28 conducted in a College of American Pathologists/Clinical Laboratory Improvement Amendments–accredited laboratory. The study was activated before AAP approval in prechemotherapy setting. Eligible patients were stratified by prior ketoconazole and ETS fusion status (positive or negative) and randomly assigned to AA 1,000 mg per day plus prednisone 5 mg twice per day (arm A) or AAP plus veliparib 300 mg twice per day (arm B), for days 1 to 28. Arm B patients underwent lead-in treatment with AAP, followed on day 8 by veliparib, in cycle 1 only. Treatment was continued until radiographic/clinical disease progression, intercurrent illness, unacceptable adverse events (AEs), withdrawal of consent, or death.
Assessments
Patients underwent baseline disease assessments and then every 12 weeks with bone scan, computed tomography or magnetic resonance imaging of abdomen/pelvis, and x-ray or computed tomography of chest for the first year. For patients who have completed ≥ 1 year of therapy, imaging can be done every 4 months, and for patients who have completed ≥ 2 years of therapy, imaging can be done every 6 months. Irrespective of duration on therapy, imaging can be done sooner than the specified intervals as clinically indicated. PSA was assessed at baseline and on day 1 of each cycle. AEs were graded according to Common Terminology Criteria for Adverse Events (version 4.0).
Tumor Sequencing
Extra tumor tissue for sequencing was available for 87 patients; 80 of 87 were response evaluable (four patients received < two cycles of treatment, three patients were never treated, one was ineligible, and two withdrew consent). Their baseline characteristics are detailed in Appendix Tables A1 and A2 (online only). Flash-frozen biopsies were processed for genomic DNA and total RNA isolation using Qiagen AllPrep Kit (Hilden, Germany) and then underwent targeted exon sequencing and capture transcriptome analysis at University of Michigan (Ann Arbor, MI), as previously detailed.29,30
Statistical Analysis
Biomarker-stratified design31 was used to determine a PSA RR difference between arms A and B and between arms by ETS fusion (positive v negative strata). The trial was designed to accrue 148 response-evaluable patients randomly assigned at a one-to-one ratio to arms A and B to provide 80% power at a one-sided 5% significance level to detect an improvement of 20% in PSA RR between arms with a χ2 test of proportions, assuming a PSA RR of 30% in arm A (based on data available at time of study design).1 Response-evaluable patients were those receiving at least two therapy cycles or those removed from study because of toxicity before completing two cycles. The PSA RR difference between treatment groups by ETS fusion status was an interaction test with a significance threshold of .15 from a logistic model (trial design details provided in protocol).
The primary outcome of confirmed PSA RR (complete or partial response) was analyzed with χ2 tests to test differences between treatment arms and differences within a biomarker stratum between treatment arms. Confirmed PSA RR was modeled to test ETS fusion status as prognostic using a logistic model with ETS status as the only covariate and as predictive using logistic models testing interaction of treatment arm and ETS. Similar models were used for mRR. Both prognostic and predictive models were used in the exploratory analyses for each sequencing biomarker including DNA-damage repair defect (DRD). Secondary end point PFS was reported using Kaplan-Meier methods and associated log-rank tests. Exploratory analysis for prognostic biomarkers with association with PFS was reported using product-limit estimates and log-rank tests. Cox models were used to test biomarkers as predictive of PFS with models including an interaction of treatment arm and biomarker status. An unplanned analysis using a multivariable Cox model for PFS was used to explore biomarker associations with PFS after controlling for clinical covariates by adding the biomarker to the model including the clinical covariates. Each biomarker was modeled separately. All analyses were completed using SAS software (version 9.4; SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
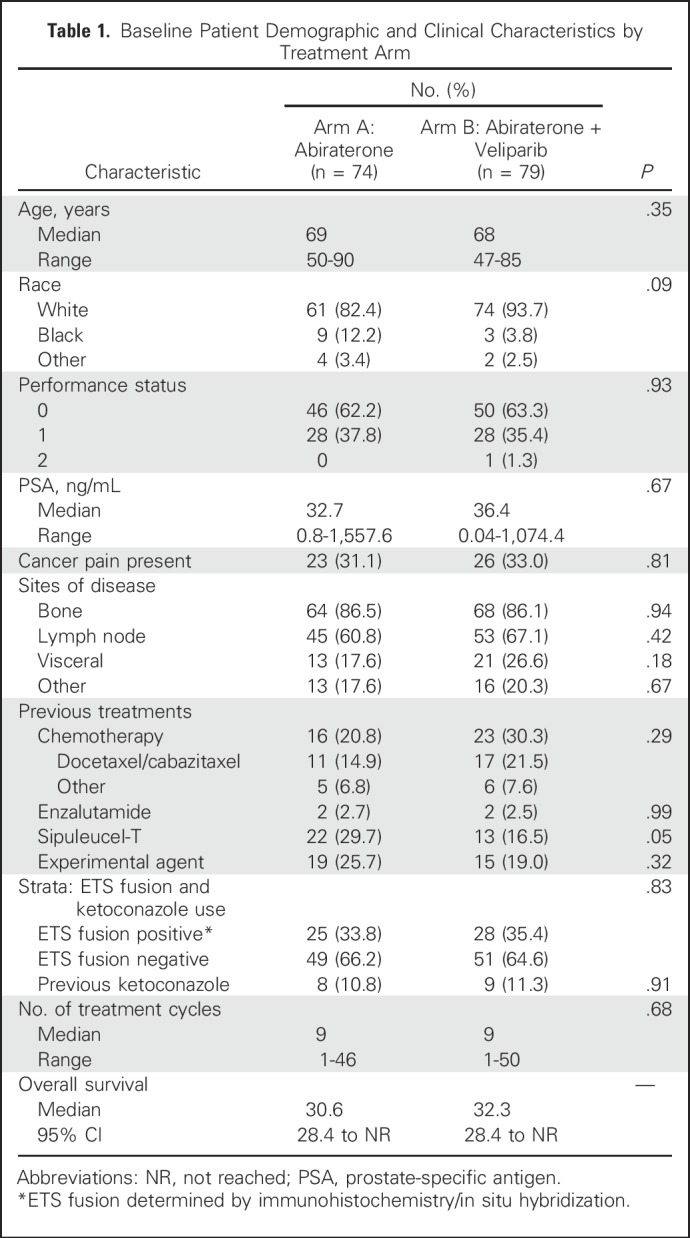
From May 2012 through December 2015, 190 patients with mCRPC were enrolled at 12 centers (Table 1); 185 eligible patients underwent metastatic biopsy (soft tissue, n = 89; bone, n = 96); 159 patients (86%) had adequate tissue; 35% were ETS positive; 153 patients (white, 88%; black, 8%; median age, 68 years; median PSA, 35.4 ng/mL) were randomly assigned to arm A (AAP; n = 74) or arm B (AAP + veliparib; n = 79; Fig 1).
Table 1.
Baseline Patient Demographic and Clinical Characteristics by Treatment Arm
Safety
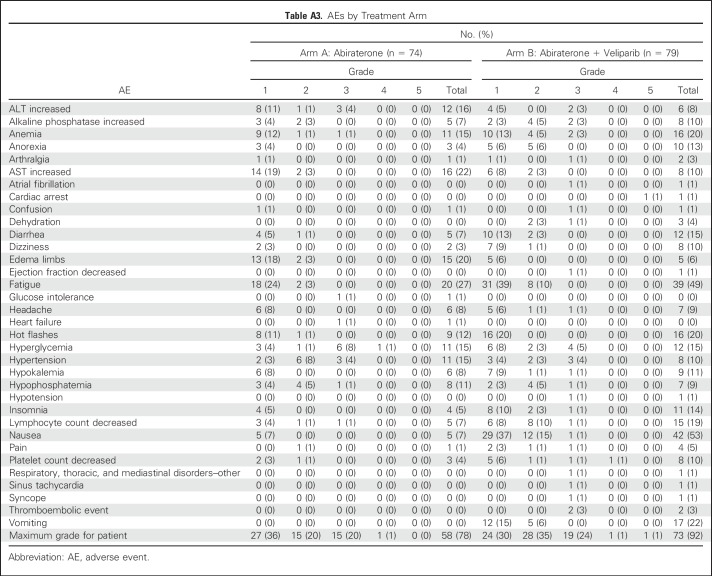
Because of bothersome low-grade AEs, veliparib dose was reduced to 200 mg twice per day for cycle 1, and if tolerated, dose was escalated to 300 mg twice per day for subsequent cycles. Distribution of grade ≥ 3 AEs irrespective of attribution was similar between arms. Overall, therapy was well tolerated (Appendix Table A3, online only); hyperglycemia was the only high-grade treatment-related AE that occurred in > 5% of patients in either arm (arm A, 9%; arm B, 5%). In arm A, 20% of patients (n = 15) had grade 3 treatment-related AEs, and one patient had grade 4 hyperglycemia. In arm B, 24% of patients (n = 19) had grade 3 treatment-related AEs, one patient had grade 4 thrombocytopenia, and one patient had grade 5 cardiac arrest possibly treatment related. Any-grade AEs that were significantly more frequent (P < .05) in arm B versus arm A were fatigue, lymphopenia, nausea, and vomiting; edema occurred more frequently in arm A than arm B.
Efficacy
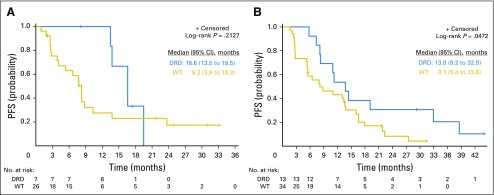
Of the 153 randomly assigned and treated patients, 148 were response evaluable; five (3%) were not evaluable (four patients chose to stop treatment within one cycle, and one had > 4-week treatment delay). There was no statistically significant difference between arms in confirmed PSA RR (arm A, 63.9%; arm B, 72.4%; P = .27), mRR (arm A, 45.0%; arm B, 52.2%; P = .51), or median PFS (arm A, 10.1 months; arm B, 11 months; P = .99; Table 2; Appendix Fig A1A). Furthermore, ETS fusion status did not predict PSA, mRR, or PFS (Appendix Fig A1B).
Table 2.
Detailed PSA and Measurable Response Outcomes by Treatment Arm and ETS Gene Fusion Status
DRD and Additional Prognostic Biomarkers
For 87 patients, extra biopsy tumor tissue was analyzed by next-generation sequencing; 80 of 87 were treated and response evaluable (arm A, n = 33; arm B, n = 47). Sequenced patients’ characteristics compared with those of patients who did not have tumor sequencing and their baseline characteristics by treatment arm are listed in Appendix Tables A1 and A2. Overall, the groups were fairly comparable, except for site of disease (bone v soft tissue [eg, lymph node, visceral disease]), which affected the site of biopsy: in the sequenced cohort, a majority (75%) were soft tissue biopsies, whereas in the nonsequenced population, a majority (70%) had bone biopsies. This is not surprising, considering tumor yield is known to be better with soft tissue biopsy. The tumor yield likely affected the difference between the two groups in the proportion of patients with ETS-positive tumors, which was higher in the sequenced group.
ETS fusion status was also analyzed by sequencing to evaluate concordance with the IHC/ISH methods used. Agreement between methods was observed for 72 (90%) of 80 patients (Appendix Table A4, online only); 41 patients (51.3%) were ETS positive by sequencing.
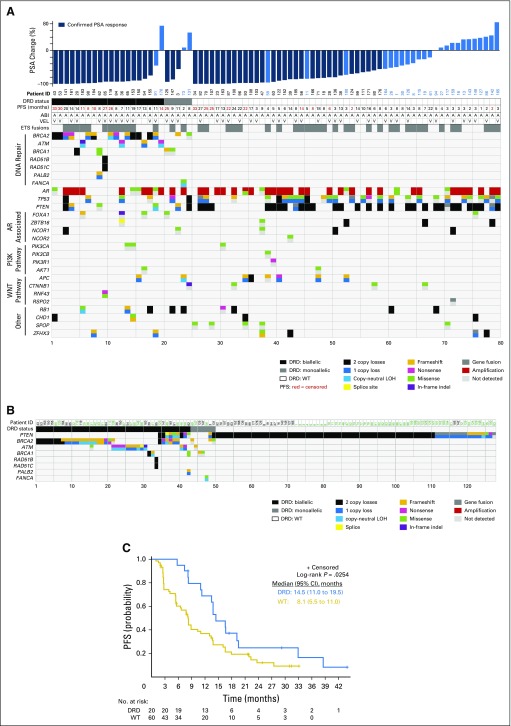
Sequencing classified patients into three categories of DNA-repair status (Fig 2A): wild type (WT; n = 55 [68.75%]), biallelic DRD (n = 20 [25%]), and monoallelic DRD (n = 5 [6.25%]). Patients with DRD had alterations in BRCA1, BRCA2, ATM, FANCA, PALB2, RAD51B, or RAD51C, with BRCA2 being the most frequently detected (Fig 2A). Notably, these genes represent major players in the homologous recombination (HR) pathway, which functions along with the nonhomologous end-joining (NHEJ) pathway to repair DNA double-strand breaks.15 Additional genes of interest were also significantly altered, including AR (n= 41 [51%]), TP53 (n = 33 [41%]), PTEN (n = 34 [42.5%]), and PIK3CA (n = 39 [49%]). Alterations were also annotated for AR-related genes and the WNT pathway.
Fig 2.
Landscape of molecular alterations, DNA-repair status, and survival in this cohort of patients with metastatic castration-resistant prostate cancer (mCRPC). (A) Next-generation sequencing of tumor tissues identified alterations in different genes for each patient (n = 80) as depicted in the matrix, called by each allele. Three groups of patients were determined based on DNA-damage repair defect (DRD) status, represented at the top by black (biallelic DRD), gray (monoallelic DRD), or white boxes (wild-type [WT] DRD). Above this, maximum percent decreases in prostate-specific antigen (PSA) levels throughout treatment are graphed for each patient, and those with confirmed PSA responses are noted with dark blue bars. Progression-free survival (PFS; months), treatment (abiraterone [A/ABI], veliparib [V/VEL]), and ETS fusion status are also indicated at the top of the matrix for each patient. (B) Matrix of DRD status associated with PTEN alterations. Patients along the top in black correspond to patients in this study, and patients in green represent cases from an additional mCRPC cohort.29 (C) PFS curves are shown for patients with WT/monoallelic or biallelic DRD status. LOH, loss of heterozygosity.
Outcome analysis combined WT and monoallelic DRD patients, because the DRD status of the latter group is considered nondeleterious, and compared them with biallelic DRD patients. Prognostic covariates (metastatic site, performance status, PSA, pain, and prior therapies) were similar between DRD and WT groups except for prior sipuleucel-T therapy (DRD, n = 1 [5%] v WT, n = 16 [29%]).
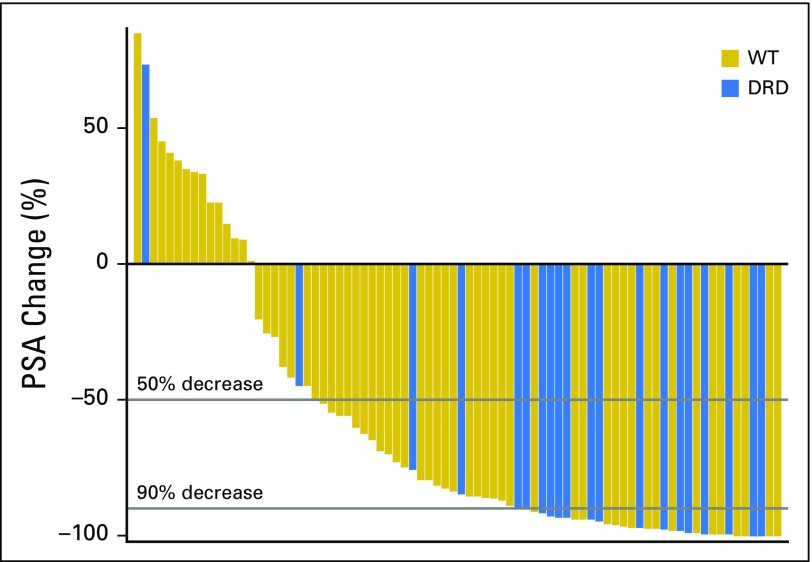
Unexpectedly, we uncovered a novel, significant association between DRD and overall outcome/response. Patients with DRD tumors had significantly higher confirmed PSA RR (90% v 56.7%; P = .007; Table 3; Fig 2A), PSA decline of ≥ 90% (75% v 25%; P = .001; Appendix Fig A2, online only), mRR (87.5% v 38.6%; P = .001; Table 3), and median PFS (14.5 v 8.1 months; P = .025; Fig 2C; depicted by treatment arm in Fig 3) compared with patients with WT tumors.
Table 3.
PSA and Measurable Disease Response Rate by DNA Repair Status
Fig 3.
Survival by DNA-repair status and treatment arm. Progression-free survival (PFS) curves are shown by treatment arm for patients with wild-type (WT)/monoallelic or biallelic DNA-damage repair defect (DRD), as determined by next-generation sequencing of tumors shown in Figure 2 of the main manuscript: (A) abiraterone/prednisone; (B) abiraterone/prednisone plus veliparib.
Analysis of Clinical and Molecular Variables
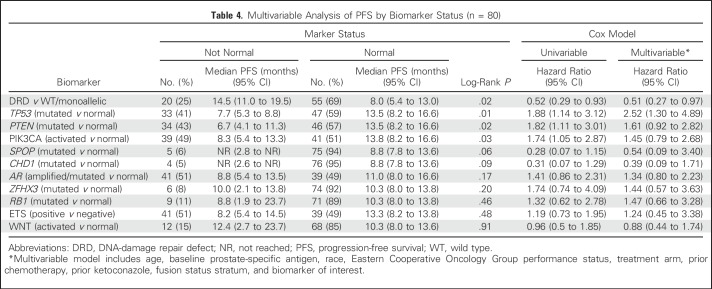
Exploratory biomarker analysis revealed three additional biomarkers associated with longer median PFS (Appendix Tables A5 and A6, online only). Significantly better overall outcomes were identified in patients with normal PTEN (13.5 v 6.7 months in those with mutation; P = .02), normal TP53 (13.5 v 7.7 months in those with mutation; P = .01), or nonactivated PIK3CA pathway (13.8 v 8.3 months in those with activation; P = .03; Appendix Table A5, online only). Multivariable analysis including clinical and biomarker variables individually revealed DRD and TP53 as biomarkers separately associated with PFS after controlling for clinical covariates (Table 4). We also noted that mutation or loss of PTEN seemed to be almost mutually exclusive with DRD (Fig 2A).
Table 4.
Multivariable Analysis of PFS by Biomarker Status (n = 80)
Exceptional Responders
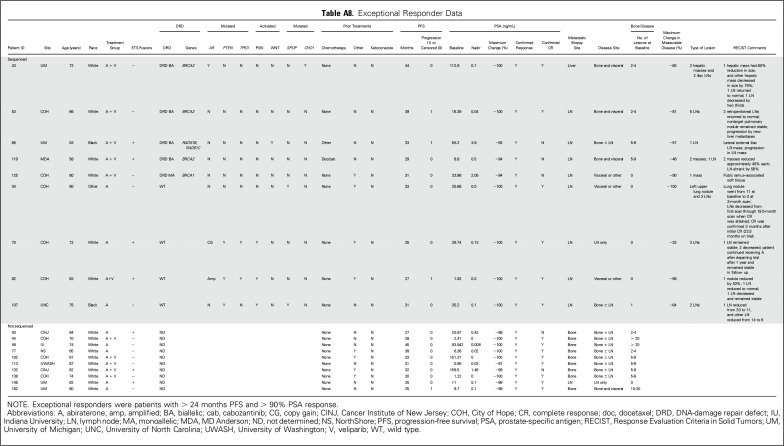
Several patients had exceptional and durable responses to therapy. These were patients in either arm with PFS > 24 months and PSA decline > 90%. On the basis of these criteria, 19 exceptional responders (arm A, n = 8; arm B, n = 11) were identified; their characteristics are listed in Appendix Table A7 (online only). Nine of 19 had tumor sequencing: four had biallelic DRD, one had monoallelic DRD, and four had WT tumors (Fig 2A; Appendix Table A8, online only).
DISCUSSION
This prospective metastatic tissue–based biomarker-stratified trial stratified patients with mCRPC by ETS fusion status and then randomly assigned them to AAP with or without veliparib. Preclinical data suggested that targeting PARP1 would synergize with AR inhibition, and ETS fusion–positive tumors would be preferentially sensitive to PARP1 inhibition.13,16,26 However, the addition of veliparib did not affect response, nor did ETS status predict response. There was no difference between arms in the rate of exceptional responders (AAP, n = 8; AAP + veliparib, n = 11). ETS fusion concordance between IHC/ISH and sequencing was 90%. ERG/ETS failure to predict response may have been a result of the high prevalence of defects in DRD genes (approximately 25% of patients), which was not known at time of study design.
Exploratory metastatic tissue sequencing analysis uncovered a novel finding. Several patients had alterations in genes involved in DNA repair, particularly those implicated in the HR pathway; DRD was significantly associated with better response and PFS irrespective of treatment arm. Prior studies demonstrated mechanistic connections between AR and DNA repair in prostate cancer models,9-15 but ours is the first report, to our knowledge, to show the association of DRD with outcome in patients with mCRPC treated with AAP with higher PSA RR, mRR, and PFS compared with patients with WT tumors. However, additional studies are needed for confirmation, because this trial was not designed specifically to test DRD predictive power with AR-targeted therapy. A recent trial in mCRPC showed that DRD was associated with high RRs to a different PARP1 inhibitor (olaparib), but there was no control arm of an AR-targeted agent.32
AR plays a major role in promoting double-strand DNA break repair.12,13 As such, AR blockade alone would be expected to compromise DNA repair. This concept is supported by our data, wherein tumors defective in DNA repair were sensitized to AAP. Given this recently realized redundancy in function (eg, capacity of both PARP1 inhibitors and AR blockade to suppress DNA repair), it is not unexpected that PARP1 inhibitors did not add to AR blockade.
Our results also raise the question of how DNA repair alterations may be associated with better outcomes with AR-targeted therapy. Recent studies have shown that AR directly regulates genes involved in DNA-damage responses that allow prostate cancer cells to enhance DNA repair, decrease DNA damage, and continue cycling.10,12,13,15 Conversely, castration or treatment with antiandrogens leads to decreased expression of DNA-repair enzymes and therefore increased DNA damage and decreased cellular survival; in particular, inhibition of AR signaling has been shown to inhibit the expression of genes primarily involved in the NHEJ pathway of double-strand DNA break repair.11-14 Disruption of NHEJ in the context of an underlying HR defect (the alterations identified in this trial cohort) could induce a synthetic lethality via disruption of both of the major repair pathways for double-stranded DNA breaks, thus explaining why patients with HR deficiencies fare better with AAP treatment. Furthermore, as described in this report, AR directly increases DNA-damage response effectors, and in turn, many DNA-damage response proteins directly modulate AR activity, including BRCA1 and BRCA2, two HR factors altered in several of the DRD patients.33,34 Without functional BRCA1 or BRCA2 cofactors, it can be hypothesized that these patients may have had altered AR transcriptional activity compared with WT patients. Further analysis of the sequencing data herein uncovered a positive association with outcome for patients with normal expression of PTEN and TP53 or nonactivated PIK3CA pathway. However, multivariable analysis including clinical and biomarker variables individually revealed DRD and TP53 as biomarkers separately associated with PFS after controlling for clinical covariates. Expanded analysis of DRD and PTEN from an additional mCRPC cohort29 demonstrated that DRD patients had significantly less aberrations in PTEN, whereas patients with WT DNA repair all had PTEN loss or aberration. The mutual exclusivity between DRD and PTEN could further explain why patients with WT DNA repair had worse outcome with therapy. PTEN loss has been associated with more aggressive prostate cancers, and preclinical models have suggested that PTEN loss/PIK3CA pathway activation can alter AR transcriptional activity and lead to hormonal therapy resistance.35,36 Directly related to our results, in retrospective analyses of patients with mCRPC receiving AAP in the postdocetaxel setting, PTEN loss was associated with shorter overall survival from time of initiation of AAP treatment.37
In contrast to findings presented here, a recent study proposed that patients with germline DRD have decreased time from androgen-deprivation therapy initiation to castration resistance and worse outcome with first-line hormonal therapy once CRPC develops.38 Important differences in these two studies are evident and could account for discrepancies. In our trial, DRD was determined by metastatic tumor tissue sequencing, the gold standard for detecting alterations. The contrasting study did not analyze tumor tissue but rather defined DRD through targeted germline sequencing. The WT DNA repair patients with whom the DRD patients were compared were only classified as such through germline sequencing, thus not accounting for those WT germline patients who may have acquired somatic DRD events. Indeed, the authors proceeded to sequence cell-free DNA, but only from those patients who were first determined to have germline DRD; these patients totaled 21 in comparison with the 80 tumors sequenced in our study. Finally, the patients with mCRPC in the previous study were treated with enzalutamide or AAP; in contrast, patients in our trial all received AAP.
There are several limitations in our study. The analyses of the biomarkers from sequencing were unplanned and exploratory and included a convenient sample of 80 of 148 patients who had extra biopsy tissue. The sequenced cohort included more soft tissue biopsies compared with patients who were not sequenced. This is not surprising, considering the known fact that the tissue yield is better from soft tissue metastases. The tumor tissue yield also likely affected the difference in the rate of ETS-positive tumors between the two cohorts. The multivariable modeling included many covariates for the sample size, so caution should be taken when interpreting these results. Additionally, there was not a correction for multiple comparisons in this study. Additional validation is needed for the exploratory findings.
In conclusion, this metastatic tissue biomarker-stratified, randomized trial in mCRPC showed that the approach is feasible. Despite robust preclinical supporting evidence, the addition of veliparib to AAP did not affect response, nor did ETS fusion predict response. Nonetheless, exploratory analysis led to the novel and unexpected finding that DRD was associated with improved outcomes with AAP treatment, possibly through induction of a synthetic lethality in the context of HR defects. Interestingly, DRD was also generally associated with normal PTEN status. Normal PTEN, normal TP53, and nonactivated PIK3CA signaling were significantly associated with improved outcome overall. These hypothesis-generating observations are being evaluated in a follow-up DRD-preselected randomized trial (AAP v olaparib v combination). These results highlight the complexity of mCRPC, importance of the totality of the biologic context, and need for informative clinical trial designs.
ACKNOWLEDGMENT
We thank Stephanie Ellison for assistance in formatting and writing this manuscript as well as the Mi-Oncoseq clinical sequencing laboratory for sequence analysis of samples in this study. We are especially grateful to all the patients who participated in this investigational study. We would also like to thank the Michigan Center for Translational Pathology.
Appendix
Fig A1.
Progression-free survival (PFS) in patients with metastatic castration-resistant prostate cancer treated with abiraterone (A) or abiraterone plus veliparib (A + V) and stratified by ETS gene fusion status. (A) PFS curves are shown for all 148 response-evaluable patients treated with abiraterone/prednisone alone (n = 72) or in combination with veliparib (n = 76). (B) PFS curves are shown for each treatment arm stratified by ETS gene fusion status (determined by immunohistochemistry or in situ hybridization).
Fig A2.
Depth of prostate-specific antigen (PSA) decline by DNA-damage repair defect (DRD) status. WT, wild type.
Table A1.
Demographic and Clinical Characteristics of Patients by Tumor Sequencing Status
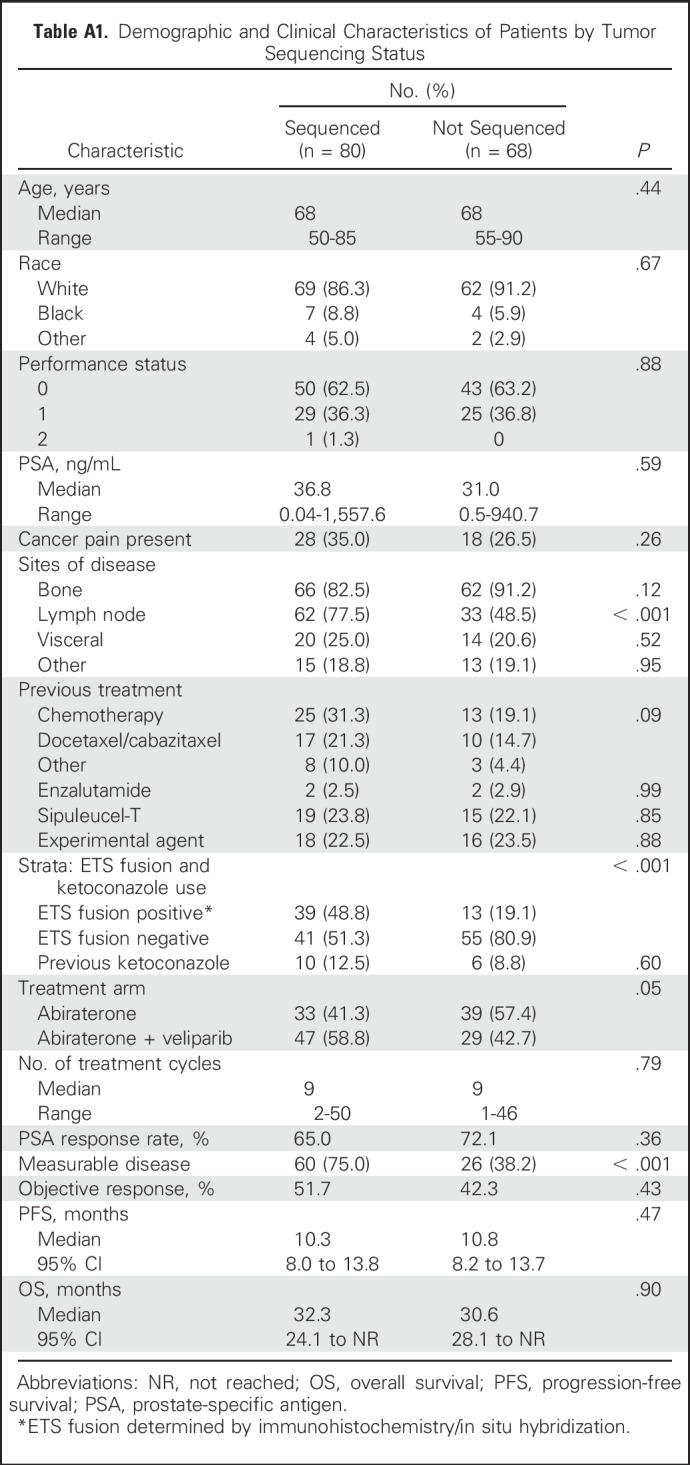
Table A2.
Baseline Covariates Among Patients With Tumor Sequencing
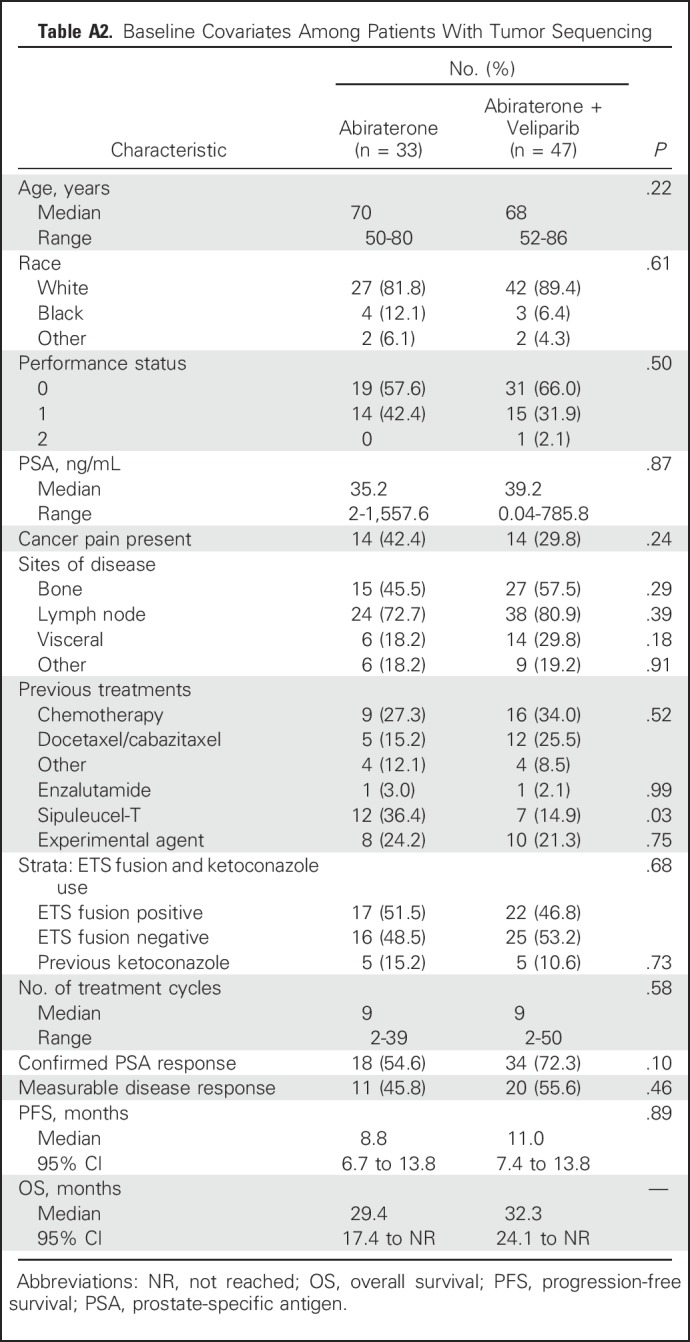
Table A3.
AEs by Treatment Arm
Table A4.
ETS Gene Fusion Agreement Between Trial Methods and Sequencing
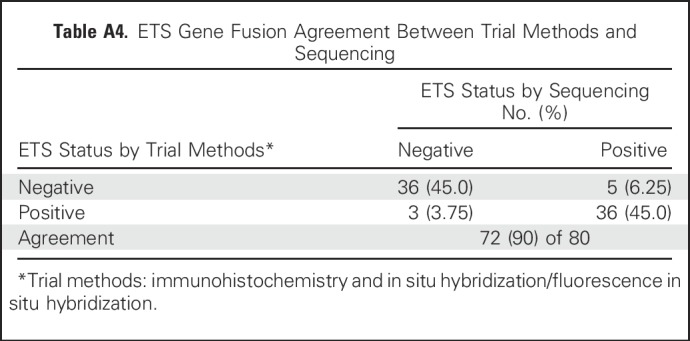
Table A5.
Biomarker Prognostic Analysis: Overall PSA Response, Measurable Disease Response, and PFS by Biomarker Status
Table A6.
Biomarker Predictive Analysis: Overall PSA Response, Measurable Disease Response, and PFS by Biomarker Status and Treatment Arm
Table A7.
Multivariable Analysis of PFS
Table A8.
Exceptional Responder Data
Footnotes
Supported by Grants No. N01-CM-2011-00071C from the National Cancer Institute (NCI), SU2C-AACR-DT0712 from Stand up to Cancer and American Association for Cancer Research, P50CA186786 from NCI, PC080189 from the US Department of Defense, and the Prostate Cancer Foundation.
Presented at the 52nd Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, June 3-7, 2016, and 53rd ASCO Annual Meeting, Chicago, IL, June 2-6, 2017.
Clinical trial information: NCT01576172.
See accompanying article on page 1017
AUTHOR CONTRIBUTIONS
Conception and design: Maha Hussain, Stephanie Daignault-Newton, Karen E. Knudsen, Walter M. Stadler, Felix Y. Feng, Arul M. Chinnaiyan
Financial support: Maha Hussain, Walter M. Stadler, Arul M. Chinnaiyan
Administrative support: Maha Hussain, Walter M. Stadler
Provision of study materials or patients: Maha Hussain, Przemyslaw W. Twardowski, Costantine Albany, Mark N. Stein, Kathleen A. Cooney, Bruce Montgomery, Emmanuel S. Antonarakis, Daniel H. Shevrin, Paul G. Corn, Young E. Whang, David C. Smith, and Megan V. Caram, Walter M. Stadler
Collection and assembly of data: Maha Hussain, Przemyslaw W. Twardowski, Costantine Albany, Mark N. Stein, Javed Siddiqui, Robert J. Lonigro, Xuhong Cao, Scott A. Tomlins, Rohit Mehra, Bruce Montgomery, Emmanuel S. Antonarakis, Daniel H. Shevrin, Paul G. Corn, Young E. Whang, David C. Smith, Megan V. Caram, Walter M. Stadler
Data analysis and interpretation: Maha Hussain, Stephanie Daignault-Newton, Lakshmi P Kunju, Yi-Mi Wu, Dan Robinson, Emmanuel S. Antonarakis, Felix Y. Feng, Arul M. Chinnaiyan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Targeting Androgen Receptor and DNA Repair in Metastatic Castration-Resistant Prostate Cancer: Results From NCI 9012
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Maha Hussain
Honoraria: Sanofi, Onclive
Research Funding: Genentech (Inst), Pfizer (Inst), Prostate Cancer Clinical Trials Consortium (Inst), Bayer HealthCare Pharmaceuticals (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Systems and methods for tissue imaging, 3676 Our File: Serial No.: UM-14437/US-1/PRO 60/923,385 UM-14437/US-2/ORD 12/101,753US 8,185,186 (US patent No.), Systems and methods for tissue imaging (issued patent) EP 08745653.9 (EP application No.), Systems and methods for tissue imaging (pending) CA 2683805 (Canadian application No.), Systems and methods for tissue imaging (pending) US 13/362,500 (US application No.), Systems and methods for tissue imaging (continuation application of US 8,185,186); Method of treating cancer docket No: serial No.: 224990/10-016P2/311733 61/481/671, application filed on February 5, 2011; Dual inhibition of MET and VEGF for the treatment of castration resistant prostate cancer and osteoblastic bone metastases, applicant/proprietor Exelexis, application No./patent No. 11764665.4- 1464, application No./patent No. 11764656.2-1464, application filed on September 26, 2011
Travel, Accommodations, Expenses: Sanofi
Stephanie Daignault-Newton
Consulting or Advisory Role: Augmenix
Research Funding: Pfizer (Inst), Bayer HealthCare Pharmaceuticals (Inst)
Przemyslaw W. Twardowski
No relationship to disclose
Costantine Albany
Stock or Other Ownership: Advaxis
Honoraria: Sanofi
Consulting or Advisory Role: Seattle Genetics, AstraZeneca/MedImmune
Speakers’ Bureau: Bayer HealthCare Pharmaceuticals, Sanofi
Research Funding: Astex Pharmaceuticals (Inst), Seattle Genetics (Inst), Tesaro (Inst), Bristol-Myers Squibb (Inst)
Mark N. Stein
Research Funding: Oncoceutics (Inst), Merck Sharp & Dohme (Inst), AbbVie (Inst), Janssen Oncology (Inst), Medivation (Inst), Astellas Pharma (Inst), Advaxis (Inst), Bavarian Nordic (Inst), Suzhou Kintor Pharmaceuticals (Inst)
Lakshmi P. Kunju
No relationship to disclose
Javed Siddiqui
No relationship to disclose
Yi-Mi Wu
No relationship to disclose
Dan Robinson
No relationship to disclose
Robert J. Lonigro
No relationship to disclose
Xuhong Cao
Consulting or Advisory Role: Tempus
Patents, Royalties, Other Intellectual Property: Tempus
Scott A. Tomlins
Leadership: Strata Oncology
Consulting or Advisory Role: Ventana Medical Systems, AbbVie, Janssen Pharmaceuticals, Astellas Pharma, Medivation, Roche, Strata Oncology, Sanofi, Almac Diagnostics
Research Funding: Thermo Fisher Scientific (Inst), Compendia Bioscience (Inst), Life Technologies (Inst), Astellas Pharma (Inst), Medivation (Inst), GenomeDx (Inst)
Patents, Royalties, Other Intellectual Property: coauthor on patent issued to the University of Michigan on ETS gene fusions inprostate cancer; diagnostic field of use has been licensed to Hologic/Gen-Probe, which has sublicensed some rights to Ventana Medical Systems/Roche
Travel, Accommodations, Expenses: Thermo Fisher Scientific, Strata Oncology
Rohit Mehra
Patents, Royalties, Other Intellectual Property: Holgic
Kathleen A. Cooney
Patents, Royalties, Other Intellectual Property: patent on discovery of HOXB13 as prostate cancer susceptibility gene (Inst)
Bruce Montgomery
Research Funding: ESSA, Medivation, Janssen Oncology, Astellas Pharma, Clovis Oncology
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma
Consulting or Advisory Role: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma
Research Funding: Janssen Biotech (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Aragon Pharmaceuticals (Inst), Exelixis (Inst), Millennium Pharmaceuticals (Inst), Genentech (Inst), Novartis (Inst), Astellas Pharma (Inst), Tokai Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
Daniel H. Shevrin
Honoraria: Sanofi
Speakers’ Bureau: Bayer HealthCare Pharmaceuticals
Paul G. Corn
No relationship to disclose
Young E. Whang
Research Funding: Astellas Pharma, Medivation, Inovio Pharmaceuticals, Tokai Pharmaceuticals, Innocrin Pharma
David C. Smith
Research Funding: Agensys (Inst), Atterocor (Inst), Millendo Therapeutics (Inst), Bayer HealthCare Pharmaceuticals (Inst), Boston Biomedical (Inst), Celgene (Inst), Exelixis (Inst), ImClone Systems (Inst), Incyte (Inst), Eli Lilly (Inst), MedImmune (Inst), Millennium Pharmaceuticals (Inst), Novartis (Inst), Oncogenex (Inst), OncoMed (Inst), Seattle Genetics (Inst), TEVA Pharmaceuticals Industries (Inst), Tekmira (Inst), Bristol-Myers Squibb (Inst), Medarex (Inst), ESSA (Inst), Genentech (Inst), Medivation (Inst), Astellas Pharma (Inst), Takeda Pharmaceuticals (Inst)
Megan V. Caram
No relationship to disclose
Karen E. Knudsen
Stock or Other Ownership: Novartis, Pfizer
Honoraria: Celgene, CellCentric, Sanofi, Janssen Pharmaceuticals
Consulting or Advisory Role: CellCentric
Research Funding: Celgene
Walter M. Stadler
Honoraria: CVS Caremark, Sotio, AstraZeneca, Bristol-Myers Squibb, Roche, Genentech
Consulting or Advisory Role: CVS Caremark, Sotio, AstraZeneca, Bristol-Myers Squibb
Research Funding: Active Biotech (Inst), Bayer HealthCare Pharmaceuticals (Inst), Bristol-Myers Squibb (Inst), Boehringer Ingelheim (Inst), Dendreon (Inst), Exelixis (Inst), Novartis (Inst), Genentech (Inst), Roche (Inst), GlaxoSmithKline (Inst), Medivation (Inst), Pfizer (Inst), Merck (Inst), Millennium Pharmaceuticals (Inst), Janssen Pharmaceuticals (Inst), Johnson & Johnson (Inst)
Other relationship: UpToDate, American Cancer Society
Felix Y. Feng
Leadership: PFS Genomics
Stock or Other Ownership: PFS Genomics
Consulting or Advisory Role: Medivation, Astellas Pharma, GenomeDx, Celgene, Dendreon, EMD Serono, Janssen Oncology, Ferring, Bayer HealthCare Pharmaceuticals, Clovis Oncology
Research Funding: Varian Medical Systems, Celgene
Patents, Royalties, Other Intellectual Property: Helped develop a molecular signature to predict radiation resistance in breast cancer, and this signature was patented by the University of Michigan, my employer; it is in the process of being licensed to PFS Genomics, a company that I helped found (Inst)
Arul M. Chinnaiyan
No relationship to disclose
REFERENCES
- 1.de Bono JS, Logothetis CJ, Molina A, et al. : Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995-2005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scher HI, Fizazi K, Saad F, et al. : Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187-1197, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Ryan CJ, Smith MR, de Bono JS, et al. : Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138-148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beer TM, Armstrong AJ, Rathkopf DE, et al. : Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424-433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonarakis ES, Lu C, Wang H, et al. : AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 371:1028-1038, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mostaghel EA, Marck BT, Plymate SR, et al. : Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: Induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res 17:5913-5925, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson PA, Arora VK, Sawyers CL: Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer 15:701-711, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korpal M, Korn JM, Gao X, et al. : An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov 3:1030-1043, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Haffner MC, Aryee MJ, Toubaji A, et al. : Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet 42:668-675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ta HQ, Gioeli D: The convergence of DNA damage checkpoint pathways and androgen receptor signaling in prostate cancer. Endocr Relat Cancer 21:R395-R407, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarish FL, Schultz N, Tanoglidi A, et al. : Castration radiosensitizes prostate cancer tissue by impairing DNA double-strand break repair. Sci Transl Med 7:312re11, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Goodwin JF, Schiewer MJ, Dean JL, et al. : A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov 3:1254-1271, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polkinghorn WR, Parker JS, Lee MX, et al. : Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 3:1245-1253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Ubaidi FL, Schultz N, Loseva O, et al. : Castration therapy results in decreased Ku70 levels in prostate cancer. Clin Cancer Res 19:1547-1556, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Schiewer MJ, Knudsen KE: Linking DNA damage and hormone signaling pathways in cancer. Trends Endocrinol Metab 27:216-225, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiewer MJ, Goodwin JF, Han S, et al. : Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov 2:1134-1149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirai K, Ueda K, Hayaishi O: Aberration of poly(adenosine diphosphate-ribose) metabolism in human colon adenomatous polyps and cancers. Cancer Res 43:3441-3446, 1983 [PubMed] [Google Scholar]
- 18.Fukushima M, Kuzuya K, Ota K, et al. : Poly(ADP-ribose) synthesis in human cervical cancer cell-diagnostic cytological usefulness. Cancer Lett 14:227-236, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Tomoda T, Kurashige T, Moriki T, et al. : Enhanced expression of poly(ADP-ribose) synthetase gene in malignant lymphoma. Am J Hematol 37:223-227, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Farmer H, McCabe N, Lord CJ, et al. : Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434:917-921, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Bryant HE, Schultz N, Thomas HD, et al. : Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434:913-917, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Palma JP, Wang Y-C, Rodriguez LE, et al. : ABT-888 confers broad in vivo activity in combination with temozolomide in diverse tumors. Clin Cancer Res 15:7277-7290, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Donawho CK, Luo Y, Luo Y, et al. : ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 13:2728-2737, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM: Recurrent gene fusions in prostate cancer. Nat Rev Cancer 8:497-511, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomlins SA, Rhodes DR, Perner S, et al. : Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310:644-648, 2005 [DOI] [PubMed] [Google Scholar]
- 26. doi: 10.1016/j.ccr.2011.04.010. Brenner JC, Ateeq B, Li Y, et al: Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell 19:664-678, 2011 [Erratum: Cancer Cell 23:557, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunju LP, Carskadon S, Siddiqui J, et al. : Novel RNA hybridization method for the in situ detection of ETV1, ETV4, and ETV5 gene fusions in prostate cancer. Appl Immunohistochem Mol Morphol 22:e32-e40, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomlins SA, Palanisamy N, Siddiqui J, et al. : Antibody-based detection of ERG rearrangements in prostate core biopsies, including diagnostically challenging cases: ERG staining in prostate core biopsies. Arch Pathol Lab Med 136:935-946, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. doi: 10.1016/j.cell.2015.05.001. Robinson D, Van Allen EM, Wu YM, et al: Integrative clinical genomics of advanced prostate cancer. Cell 161:1215-1228, 2015 [Erratum: Cell 162:454, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDaniel AS, Hovelson DH, Cani AK, et al. : Genomic profiling of penile squamous cell carcinoma reveals new opportunities for targeted therapy. Cancer Res 75:5219-5227, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Freidlin B, McShane LM, Korn EL: Randomized clinical trials with biomarkers: Design issues. J Natl Cancer Inst 102:152-160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mateo J, Carreira S, Sandhu S, et al. : DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med 373:1697-1708, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park JJ, Irvine RA, Buchanan G, et al. : Breast cancer susceptibility gene 1 (BRCAI) is a coactivator of the androgen receptor. Cancer Res 60:5946-5949, 2000 [PubMed] [Google Scholar]
- 34.Shin S, Verma IM: BRCA2 cooperates with histone acetyltransferases in androgen receptor-mediated transcription. Proc Natl Acad Sci USA 100:7201-7206, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiao J, Wang S, Qiao R, et al. : Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res 67:6083-6091, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Carver BS, Chapinski C, Wongvipat J, et al. : Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19:575-586, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferraldeschi R, Nava Rodrigues D, Riisnaes R, et al. : PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol 67:795-802, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Annala M, Struss WJ, Warner EW, et al. : Treatment outcomes and tumor loss of heterozygosity in germline DNA repair-deficient prostate cancer. Eur Urol 72:34-42, 2017 [DOI] [PubMed] [Google Scholar]