Abstract
How species respond to shifting environmental conditions is a central question in ecology, especially because ecosystems are experiencing rapidly changing climatic conditions. However, predicting the responses of species interactions and community composition to changing conditions is often difficult. We examined the effects of rearing temperature and resource level on larval survival of two ecologically similar dragonflies, Erythemis collocata and Pachydiplax longipennis. Within high and low (26 and 21°C) temperatures, we crossed species and resource level and reared larvae individually. We predicted that warmer temperatures would reduce survival and increase growth rate, that higher resource availability would increase survival and growth rate, and that the two species would respond similarly. We found that increased temperature reduced survival for both species. There was also an interaction between temperature and species: E. collocata had higher survival at the lower temperature, but lower survival at the higher temperature when compared to P. longipennis. Resource level did not affect survival. In general, P. longipennis grew more than E. collocata, with no effects of temperature or resource level. These results suggest that these species respond dissimilarly to changing thermal conditions, that increased food availability cannot always compensate for the negative effects of higher temperatures, and that climate change may affect interactions between these two sympatric, ecologically similar species, with potential consequences for community composition.
Keywords: thermal performance, resource level, dragonfly, Pachydiplax longipennis, Erythemis collocate
Introduction
Environmental warming due to climate change can affect organisms in multiple ways, with consequences that have placed many species at risk and are threatening global biodiversity (Parmesan, 2006). Temperature can affect many aspects of individual performance, including growth, development, and survival. Ectotherms can be especially vulnerable to the effects of warming, because many of their physiological processes are regulated by environmental temperature (Deutsch et al., 2008; Gillooly, Charnov, West, Savage, & Brown, 2002), making an understanding of ectothermic responses to thermal conditions an important component of assessing the effects of climate change. One major response to increased temperatures in ectotherms is accelerated developmental rate, which can result in smaller body sizes at the adult stage (Callier & Nijhout, 2013; Ghosh, Testa, & Shingleton, 2013). Reduced adult body size can have consequences for fecundity, and may also affect dispersal ability, potentially limiting the ability of species to shift their ranges in response to climate change (McCauley & Mabry, 2011).
Although ectotherms in general are expected to be especially sensitive to warming, there can be considerable variation in responses by different species. For example, an early study manipulating temperature in a stream environment (Hogg & Williams, 1996) compared two dominant insect species in the same experimental treatments, and found that warming had similar effects on phenology, with adult emergence advanced in both species by approximately two weeks. However, the effects of warming on adult body size were widely divergent: in one species, adult body size was reduced by as much as 25%, while the other species experienced only a < 5% reduction in body size. Such differential responses can determine the “winners and losers” of climate change (Domisch, Jaehnig, & Haase, 2011; Rosset & Oertli, 2011), and assessing differential responses among species is essential for predicting future species interactions (Nilsson-Örtman, Stoks, & Johansson, 2014; Yang & Rudolf, 2010) and community structure (Singer, Travis, & Johst, 2013).
Because larval odonates (dragonflies and damselflies) play a central role in many freshwater food webs (McPeek, 1990) and their biphasic life-cycle links terrestrial and aquatic systems (Knight, McCoy, Chase, McCoy, & Holt, 2005), understanding the responses of larval odonates to climate change is critically important (Hassall & Thompson, 2008). An estimated 10% of odonates are at risk of global extinction, and climate change is expected to be a contributing factor in odonate extinctions (Clausnitzer et al., 2009). A range of other odonate responses to climate change have also been documented, including body size reductions (Forster, Hirst, & Atkinson, 2012), northerly range shifts (Flenner&Sahlén, 2008; Grewe, Hof, Dehling, Brandl, & Braendle, 2013; Hickling, Roy, Hill, & Thomas, 2005), and advancing phenologies (Dingemanse & Kalkman, 2008; Hassall, Thompson, French, & Harvey, 2007). Larval dragonflies exposed to experimental warming show a range of responses including increased mortality and advancing phenologies (McCauley, Hammond, Frances, & Mabry, 2015; Richter, Suhling, Mueller, & Kern, 2008; Suhling, Suhling, & Richter, 2015). Further, there is evidence of variation between odonate species in response to warming (Suhling & Suhling, 2013).
We determined how water temperature and resource level affected larval growth and survival in two abundant dragonfly species, Pachydiplax longipennis Burmeister and Erythemis collocata Hagen (Odonata: Anisoptera: Libellulidae). These species are ecologically similar and frequently co-occur in the same microhabitats within lentic water-bodies across their ranges. We examined responses of larvae of both of these species to two temperature treatments under two feeding regimes, because resource availability can also modify responses to environmental warming. For example, warmer conditions may allow larvae to better utilize available food resources, and higher food availability may also offset potential negative effects of higher temperatures by compensating for the costs of increased metabolic rates under warmer conditions. Differences between species in their responses to temperature and food level should have consequences for the persistence of these species and for their interactions in a changing thermal environment.
Materials and methods
Egg collection
We collected eggs from both dragonfly species at the Wantrup Wildlife Sanctuary (Napa County, California, USA, 38°35’58.07”N, 122°22’13.10”W) during 17–18 July 2013. We used aerial insect nets to catch mature females or mating pairs during or after copulation. Females were removed from the nets, their wings folded back, and their abdomens dipped in a small sampling cup filled with pond water until eggs were released (n = 6 clutches per species). Females were marked with permanent ink on one wing before release to prevent sampling eggs from the same female on multiple occasions. We observed each clutch the next morning to determine if eggs had tanned, an indication that fertilization had occurred. Fertilized clutches were then placed into a cooler and transported to the laboratory at New Mexico State University.
Survival/growth experiment
Upon arrival at the laboratory, we placed clutches from each species into two separate 189-l rearing tanks (Rubbermaid). Each tank was filled with approximately 100 l of aged tap water, 40 g of leaf litter (mostly Quercus spp.) collected from the Cibola National Forest, Socorro County, New Mexico, USA, an aliquot of zooplankton from the northern California field site, and 3 g of Zoo Med™ Natural Juvenile Bearded Dragon Food for nutrients (Zoo Med Laboratories Inc., San Luis Obispo, California, USA). These tanks were kept outdoors, covered with a 60% shade cloth to reduce temperatures and to eliminate colonization by other organisms. More water was added as needed. Each tank received an equal ab libitum amount (28.3 g) of washed and filtered Artemia franciscana (San Francisco strain brine shrimp, Brine Shrimp Direct, Ogden, UT, USA) three times a week for nine weeks post hatching.
In late September 2013, larvae of both species were placed into one of four experimental treatments in tanks inside the laboratory under indirect natural light. We first visually sorted larvae by size (mean approximately fifth stadium), and removed individuals at both tails of the size distribution. Average sized larva were then placed in an individually numbered perforated 470-ml plastic cup then placed inside a 190 l cattle tank filled with 95 l of aged tap water. This resulted in each cup being filled to approximately 250 ml. A schematic representation of our experimental design is in Figure 1. We used a total of six 190 l tanks, each equipped with two aquarium heaters (each potentially rated up to 340 l), a Hobo temperature logger, and two air supplies (each rated to 1100 l, equally divided between all six tanks). Three of the tanks were heated to approximately 21°C (mean ± SE: 20.98 ± 0.01, 20.76 ± 0.01, and 21.48 ± 0.01°C), and the other three tanks were heated to 26°C (mean ± SE: 25.91 ± 0.01, 25.86 ± 0.01, and 26.23 ± 0.01°C). The 26°C treatment represents a reasonable summer high temperature at our field collection site (McCauley et al., 2015), while the 21°C treatment represents a close approximation to the summer daily average. Both of these temperatures are well under the general upper lethal limits (ULL) documented for dragonflies (~ 42°C, Stewart, Close, Cook, & Davies, 2013), including P. longipennis (44.5–45.8°C; Garten & Gentry, 1976). Larvae received one of two resource treatments made from concentrated slurry with a ratio of 1 ml of filtered and patted dry live Artemia franciscana to 11 ml of freshwater. Individuals in the low resource treatment received 75 μl of the solution on Monday and Friday while individuals in the high resource treatment received 75 μl on Monday and 150 μl on Friday. Aged tap water was added to each tank as needed once a week. Within each tank, 12 individuals of each species were reared separately and received either the high or low resource treatment. Thus, we used a total of 288 larvae in the experiment (12 individuals reared separately of each species and resource treatment/tank, with three tanks/temperature see Figure 1).
Figure 1.
Experimental design. Twelve individuals of each species and resource treatment were reared separately within each 1901 tank. We used three tanks for each temperature, for a total of 36 individuals per treatment.
We assessed two response variables over the course of the experiment: survival and growth. Survival was measured on days 21, 39, and 48 by counting individuals (193 individuals survived to the end of the experiment). We also photographed each individual on days 21 and 48, and photographs were used to measure head width in ImageJ™ (http://imagej.nih.gov/ij/). We used the change in head width between days 21 and 48 to determine the growth of each individual.
Statistical methods
Due to variation between tank temperatures, we used nested analyses for the survival and growth responses with temperature (fixed effect) nested within tank (random effect). For both responses, we examined the effects of temperature, species, resource level, and all possible interactions. The survival analysis employed a Cox proportional hazard model with tanks clustered by temperature. For growth, we log-transformed the two measures of head width, then subtracted the day 21 measure from the day 48 measure. Head width data were non-normally distributed, so we rank-transformed data before analysis using nested analysis of variance (ANOVA).
Results
Individuals exposed to higher temperatures experienced higher mortality than did individuals exposed to lower temperatures, regardless of species (χ2 = 4.0, df = 1, p = 0.046; Figure 2, Table 1). There was also an interaction between temperature and species, with E. collocata responding more strongly to the difference in temperature than P. longipennis. Interestingly, E. collocata had higher survival than P. longipennis in the low temperature treatment, and lower survival in the high temperature treatment (χ2 = 4.7, df = 1, p = 0.030; Figure 2). This shift in survival for E. collocata was ~ 25% difference between the two temperatures. Mortality in P. longipennis increased only 10% between temperatures. Neither resource level nor species affected survival (all p > 0.18; Table 1). Pachydiplax longipennis grew more than E. collocata (F1,170 = 26.8, p = 0.007; mean log difference in mm ± SE, P. longipennis: 0.30 ± 0.02, E. collocata: 0.17 ± 0.01, log difference in mm; Figure 3). Neither temperature nor resource level significantly affected growth rates (all p > 0.21, Table 1).
Figure 2.
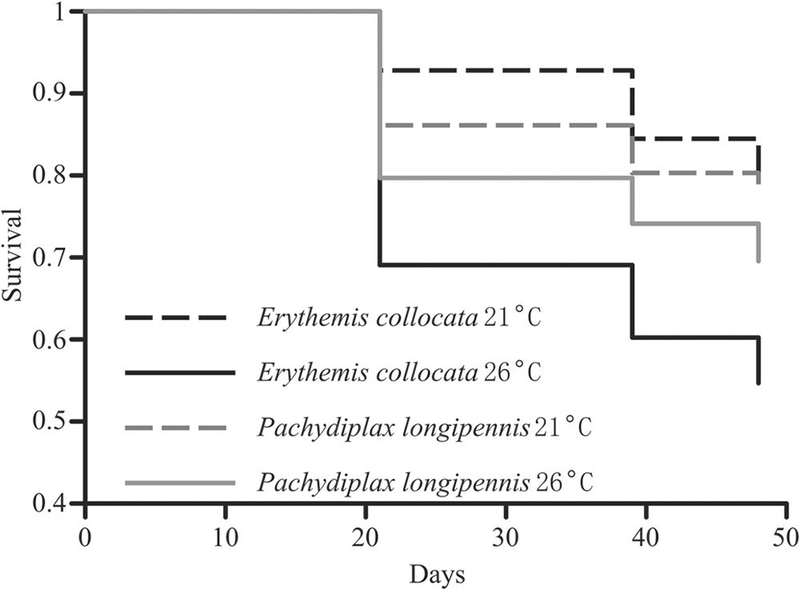
Survival probability of E. collocata (black lines) and P. longipennis (grey lines) individuals across high (26°C, solid lines) and low (21°C, dashed lines) treatments.
Table 1.
Statistical results for the survival and growth analysis. Both analyses used nested models to account for the differences between tanks, with temperature as a fixed effect nested within tank as a random effect.
| Survival |
Growth |
|||||
|---|---|---|---|---|---|---|
| df | Wald χ2 | p | df | F | p | |
| Species | 1 | 0.28 | 0.599 | 1, 170 | 26.8 | 0.007 |
| Temperature | 1 | 3.97 | 0.046 | 1, 170 | 2.2 | 0.211 |
| Species × temperature | 1 | 4.69 | 0.030 | 1, 170 | 0.2 | 0.706 |
| Resources | 1 | 0.86 | 0.354 | 1, 170 | 0.1 | 0.749 |
| Species × resources | 1 | 1.79 | 0.181 | 1, 170 | 0.6 | 0.468 |
| Temperature × resources | 1 | 0.59 | 0.441 | 1, 170 | 0.0 | 0.911 |
| Three-way interaction | 1 | 0.21 | 0.648 | 1, 170 | 0.0 | 0.900 |
Figure 3.
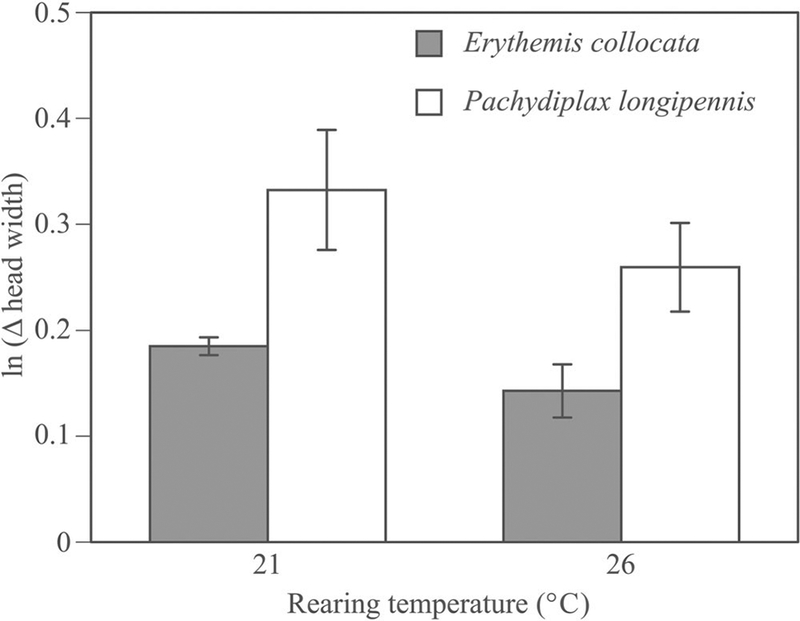
Erythemis collocata grew significantly more slowly than P. longipennis but there was no effect of either temperature or resource level (not shown) on growth rate.
Discussion
An increase in mortality with warmer temperatures, such as we found here, has been observed in many studies (Flenner, Richter, & Suhling, 2010; Folt, Chen, Moore, & Burnaford, 1999), including our own previous work (McCauley et al., 2015). In a field experiment in northern California with P. longipennis in outdoor tanks heated to + 2.5°C and + 5.0°C above the ambient temperature from December through July, we observed the expected decrease in survival and phenology due to temperature (McCauley et al., 2015). The temperatures used in the present study are realistic for conditions these larvae would experience in the field and track well with our previous study, with the lower temperature similar to the average ambient temperature in June, and the higher temperature approximately matching the daily highs.
A difference in mortality of 10–25% due to a 5°C change in rearing temperature can have consequences at the population level, as well as altering the magnitude of aquatic to terrestrial energy and nutrient transfers (Wesner, 2010) and other cross-ecosystem trophic connections (Knight et al., 2005). In northern California, where our larval dragonflies were collected, temperatures appear to have increased by ~ 2°C over the last 40 years (NOAA National Climatic Data Center, retrieved October 30, 2014, from http://www.ncdc.noaa.gov/), and climate models predict a similar magnitude of change in temperature in the future (+ 5.0°C over the next 100 years; Cayan et al., 2009). Moreover, in the absence of competition and other ecological interactions, the results of this study predict that one species should have a survival advantage over the other at lower temperatures, that switches to a survival disadvantage with an increase of a few degrees. This prediction creates an interesting scenario for testing not only the inherent physiological tolerances of these species and their consequences for population and community structure, but also the roles of disease, competition, and predation (including interactions such as intraguild predation) in altering these outcomes due to the combined effects of multiple stressors (i.e. Folt et al., 1999; Janssens & Stoks, 2013; Verbeck & Calosi, 2012).
We observed strong effects of temperature on mortality, but temperature did not affect growth rates in either species. Nor did temperature interact with resource levels to affect growth or mortality. It was surprising that increased resource availability did not offset the negative effects of temperature on mortality. We had expected that increased resources would mitigate the increased metabolic costs associated with higher temperatures and that larvae with access to more food would experience lower mortality. Our results suggest that either these larvae may be limited in their capacity to compensate for the metabolic costs associated with higher temperatures, or our feeding regime was inappropriate to reveal this effect either because of the level of resources or the length of the experiment. Low survival of Artemia may have contributed to this result, because they tend to survive for only a few hours in fresh water. Thus, the feeding rate may have been similar between the treatments within the time window when Artemia were alive and far below that needed for maximum growth. If we compare the treatment with the maximum growth rate for each species to the maximum growth rates of other Libellulidae at their thermal growth optimum (Suhling et al., 2015), we find both of the current species to have much lower growth rates than other similar species (maxima E. collocata 0.01 mm day−1 and P. longipennis 0.02 mm day−1 as compared to 0.03–0.05 mm day−1 for the slower growing Leucorrhinia dubia Vander Linden, Leucorrhinia rubicunda Linnaeus, and Orthetrum cancellatum Linneaus). Further work exploring the interaction between resource levels and temperature may reveal new insights into whether increasing resources mitigate the consequences of increased metabolic costs for ectotherms as temperatures rise (Nilsson-Örtman et al., 2014; Suhling et al., 2015).
Our results indicate that changes in temperature similar to the difference between daily average and daily high can affect freshwater insects such as dragonflies by altering survival, and that ecologically similar species may not respond in similar ways. They also suggest that the negative effects of temperature may not be compensated for by increasing resource levels. These differences suggest that the response of at least some ectotherms developing in warmer environments will likely affect population dynamics and community interactions, including connections between freshwater and terrestrial environments.
Acknowledgments
We thank Chris Hill, Marge Hill, and the Wantrup Wildlife Refuge of the Napa Land Trust for research support and access to research sites, and the field assistants who helped with egg collection. This work was conducted under permits from the California Department of Fish and Game (SC-9036) and the Napa Land Trust.
Funding
M. Y. Chavez was supported by the Maximizing Access to Research Careers program at NMSU (NIH GM07667–36). J. I. Hammond was supported by grant number K12GM088021 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. This work was supported by the US National Science Foundation [grant number DEB1245415].
References
- Callier V, & Nijhout HF (2013). Body size determination in insects: a review and synthesis of size- and brain-dependent and independent mechanisms. Biological Reviews, 88, 944–954. doi: 10.1111/brv.12033 [DOI] [PubMed] [Google Scholar]
- Cayan D, Tyree M, Dettinger M, Hidalgo H, Das T, Maurer E, Bromirski P, Graham N, & Flick R (2009). Climate change scenarios and sea level rise estimates for the California 2008 climate change scenarios assessment. Sacramento, CA: California Climate Change Center. [Google Scholar]
- Clausnitzer V, Kalkman VJ, Ram M, Collen B, Baillie JEM, Bedjanic M, Darwall WRT, Dijkstra KDB, Dow R, Hawking J, Karube H, Malikova E, Paulson D, Schuette K, Suhling F, Villanueva RJ, von Ellenrieder N, & Wilson K (2009). Odonata enter the biodiversity crisis debate: The first global assessment of an insect group. Biological Conservation, 142, 1864–1869. doi: 10.1016/j.biocon.2009.03.028 [DOI] [Google Scholar]
- Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, & Martin PR (2008). Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of the National Academy of Sciences, USA, 105, 6668–6672. doi: 10.1073/pnas.0709472105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, & Kalkman VJ (2008). Changing temperature regimes have advanced the phenology of Odonata in the Netherlands. Ecological Entomology, 33, 394–402. doi: 10.1111/j.1365-2311.2007.00982.x [DOI] [Google Scholar]
- Domisch S, Jaehnig SC, & Haase P (2011). Climate-change winners and losers: stream macroinvertebrates of a submontane region in Central Europe. Freshwater Biology, 56, 2009–2020. doi: 10.1111/j.1365-2427.2011.02631.x [DOI] [Google Scholar]
- Flenner I, Richter O, & Suhling F (2010). Rising temperature and development in dragonfly populations at different latitudes. Freshwater Biology, 55, 397–410. doi: 10.1111/j.1365-2427.2009.02289.x [DOI] [Google Scholar]
- Flenner I, & Sahlén G (2008). Dragonfly community re-organisation in boreal forest lakes: rapid species turnover driven by climate change? Insect Conservation and Diversity, 1, 169–179. doi: 10.1111/j.1752-4598.2008.00020.x [DOI] [Google Scholar]
- Folt CL, Chen CY, Moore MV, & Burnaford J (1999). Synergism and antagonism among multiple stressors. Limnology and Oceanography, 44, 864–877. doi: 10.4319/lo.1999.44.3_part_2.0864 [DOI] [Google Scholar]
- Forster J, Hirst AG, & Atkinson D (2012). Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proceedings of the National Academy of Sciences of the United States of America, 109, 19310–19314. doi: 10.1073/pnas.1210460109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten CT, & Gentry JB (1976). Thermal tolerance of dragonfly nymphs. 2. Comparison of nymphs from control and thermally altered environments. Physiological Zoology, 49, 206–213. [Google Scholar]
- Ghosh SM, Testa ND, & Shingleton AW (2013). Temperature-size rule is mediated by thermal plasticity of critical size in Drosophila melanogaster. Proceedings of the Royal Society B-Biological Sciences, 280. doi: 10.1098/rspb.2013.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly JF, Charnov EL, West GB, Savage VM, & Brown JH (2002). Effects of size and temperature on developmental time. Nature, 417, 70–73. doi: 10.1038/417070a [DOI] [PubMed] [Google Scholar]
- Grewe Y, Hof C, Dehling DM, Brandl R, & Braendle M, (2013). Recent range shifts of European dragonflies provide support for an inverse relationship between habitat predictability and dispersal. Global Ecology and Biogeography, 22, 403–409. doi: 10.1111/geb.12004 [DOI] [Google Scholar]
- Hassall C, & Thompson DJ (2008). The effects of environmental warming on Odonata: a review. International Journal of Odonatology, 11, 131–153. doi: 10.1080/13887890.2008.9748319 [DOI] [Google Scholar]
- Hassall C, Thompson DJ, French GC, & Harvey IF (2007). Historical changes in the phenology of British Odonata are related to climate. Global Change Biology, 13, 933–941. doi: 10.1111/j.1365-2486.2007.01318.x [DOI] [Google Scholar]
- Hickling R, Roy DB, Hill JK, & Thomas CD (2005). A northward shift of range margins in British Odonata. Global Change Biology, 11, 502–506. doi: 10.1111/j.1365-2486.2005.00904.x [DOI] [Google Scholar]
- Hogg ID, & Williams DD (1996). Response of stream invertebrates to a global-warming thermal regime: An ecosystem-level manipulation. Ecology, 77, 395–407. doi: 10.2307/2265617 [DOI] [Google Scholar]
- Janssens L, & Stoks R (2013). Fitness effects of chlorpyrifos in the damselfly Enallagma cyathigerum strongly depend upon temperature and food level and can bridge metamorphosis. Plos One, 8, e68107. doi: 10.1371/journal.pone.0068107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight TM, McCoy MW, Chase JM, McCoy KA, & Holt RD (2005). Trophic cascades across ecosystems. Nature, 437, 880–883. doi: 10.1038/nature03962 [DOI] [PubMed] [Google Scholar]
- McCauley SJ, Hammond JI, Frances DN, & Mabry KE (2015). Effects of experimental warming on survival, phenology and morphology of an aquatic insect (Odonata). Ecological Entomology, 40, 211–220. doi: 10.1111/een.12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley SJ, & Mabry KE (2011). Climate change, body size, and phenotype dependent dispersal. Trends in Ecology and Evolution, 26, 554–555. doi: 10.1016/j.tree.2011.06.017 [DOI] [PubMed] [Google Scholar]
- McPeek MA (1990). Determination of species composition in the Enallagma damselfly assemblages of permanent lakes. Ecology, 71, 83–98. doi: 10.2307/1940249 [DOI] [Google Scholar]
- Nilsson-Örtman V, Stoks R, & Johansson F (2014) Competitive interactions modify the temperature dependence of damselfly growthrates. Ecology, 95, 1394–1406. doi: 10.1890/13-0875.1 [DOI] [PubMed] [Google Scholar]
- NOAA National Climatic Data Center. Retrieved October 30, 2014, from http://www.ncdc.noaa.gov/cdo-web/datasets/GHCNDMS/stations/GHCND:USC00045360/detail
- Parmesan C (2006). Ecological and evolutionary responses to recent climate change. Annual Review of Ecology Evolution and Systematics, 37, 637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100 [DOI] [Google Scholar]
- Richter O, Suhling F, Mueller O, & Kern D (2008). A model for predicting the emergence of dragonflies in a changing climate. Freshwater Biology, 53, 1868–1880. doi: 10.1111/j.1365-2427.2008.02012.x [DOI] [Google Scholar]
- Rosset V, & Oertli B (2011). Freshwater biodiversity under climate warming pressure: Identifying the winners and losers in temperate standing waterbodies. Biological Conservation, 144, 2311–2319. doi: 10.1016/j.biocon.2011.06.009 [DOI] [Google Scholar]
- Singer A, Travis JMJ, & Johst K (2013). Interspecific interactions affect species and community responses to climate shifts. Oikos, 122, 358–366. doi: 10.1111/j.1600-0706.2012.20465.x [DOI] [Google Scholar]
- Stewart BA, Close PG, Cook PA, & Davies PM (2013). Upper thermal tolerances of key taxonomic groups of stream invertebrates. Hydrobiologia, 718, 131–140. doi: 10.1007/s10750-013-1611-9 [DOI] [Google Scholar]
- Suhling I, & Suhling F (2013). Thermal adaptation affects interactions between a range-expanding and a native odonate species. Freshwater Biology, 58, 705–714. doi: 10.1111/fwb.12074 [DOI] [Google Scholar]
- Suhling F, Suhling I, & Richter O (2015). Temperature response of growth of larval dragonflies - an overview. International Journal of Odonatology, 18, 15–30. doi: 10.1080/13887890.2014.928241 [DOI] [Google Scholar]
- Verberk W, & Calosi P (2012). Oxygen limits heat tolerance and drives heat hardening in the aquatic nymphs of the gill breathing damselfly Calopteryx virgo (Linnaeus, 1758). Journal of Thermal Biology, 37, 224–229. doi: 10.1016/j.jtherbio.2012.01.004 [DOI] [Google Scholar]
- Wesner JS (2010). Aquatic predation alters aterrestrial prey subsidy. Ecology, 91, 1435–1444. doi: 10.1890/09-1532.1 [DOI] [PubMed] [Google Scholar]
- Yang LH, & Rudolf VHW (2010). Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecology Letters, 13, 1–10. doi: 10.1111/j.1461-0248.2009.01402.x [DOI] [PubMed] [Google Scholar]


