Abstract
Background
Fascioliasis is a neglected tropical disease that affects poor people from poor and developing countries. In the world, it has been estimated that at least 2.6 million people are affected with this disease. The International agency for Research on Cancer, states that O. viverrini and C. sinensis, also liver flukes, are considered as definitive causes of cholangiocarcinoma. However, fascioliasis caused by F. hepatica has not been associated with cancer to date. There are not any known causative associations between this parasite and liver cancer (cholangiocarcinoma).
Methods
Chine Hamster Ovary (CHO) cells were treated with F. hepatica extracts and cell proliferation was assessed by using the indirect method for estimating cell number based on the mitochondrial activity with MTS cell proliferation reagent. We observed unexpected death of these cells when treated with F. hepatica extracts.
Results
We now hypothesize that this parasite could be used as a medically-important trematode pathogen in cancer therapy.
Keywords: Fasciola hepatica, mammalian cells, cholangiocarcinoma, O. viverrini, C. sinensis, Chine Hamster Ovary (CHO)
1. INTRODUCTION
Fascioliasis is a neglected tropical disease that affects poor people from poor and developing countries [1, 2]. In the world, it has been estimated that at least 2.6 million people are affected with this disease [3]. This parasitosis is very common. Almost one-third of world population has been diagnosed with this infection. Diagnosis is currently performed through the use of high-sensitive serological and parasitological tools. Although most patients are asymptomatic, patients with acute infection normally present fever and abdominal pain, while patients with chronic infection present biliary colic, cholecystitis and cholangitis [4]. Conversely, there is a limited number of studies to evaluate the natural history of patients affected with fascioliasis to assert chronic inflammation, liver fibrosis stages and carcinogenesis. Also, in patients who were treated there is a lack of studies relating to post-infectious liver damage. Therefore, the long- term effects of fascioliasis are unknown. According to the International Agency for Research on Cancer (IARC), two other liver flukes (O. viverrini and C. sinensis) have been recognized as definitive causes of cancer [5]. However, fascioliasis caused by F. hepatica has not been associated with cancer to date.
While performing several experiments with parasite extracts using cell lines, we observed unexpected death of these cells when treated with F. hepatica extracts. Therefore, to assert the anti-cancer potential of F. hepatica we now hypothesize that this parasite could be used as a medically-important trematode pathogen.
2. MATERIAL AND METHODS
2.1. F. hepatica and S. haematobium Extract Production
Fasciola hepatica worms were collected from the livers of infected bovines in slaughter houses.
S. haematobium adult worms were collected by perfusion of the hepatic portal system of golden hamsters and balb/c mice respectively, at 7 weeks after infection with 100 cercarie.
The worms were suspended in PBS and then sonicated. The protein extract was then ultracentrifuged and the protein concentration was estimated using a micro BCA protein assay reagent kit [6, 7]
2.2. Animals and Experimental Infections
Ten golden hamsters were experimentally infected with 100 cercariae of S. haematobium. Hamsters were infected by member’s extremities. The control animals consisted of 10 littermates. The cercariae were obtained by shedding of snails infected with miracidia.
2.3. Cell Lines
CHO cells were cultured and maintained at 37 °C in a 5% CO2 humidified atmosphere in CHO medium (Sigma) with 10% FBS and 1% penicillin/streptomycin (Sigma). Cells were passaged every 5 days. Before treatments cells were serum-starved for 16 h [6, 7]
2.4. Proliferation Assay
The CellTiter 96 AQ non-radioactive cell proliferation assay (Promega) was used to assess cell viability. The assay is composed of the tetrazolium compound MTS and an electron coupling reagent, PMS. MTS is reduced by viable cells to formazan, which can be measured with a spectrophotometer based on the amount of absorbance at 490 nm. Formazan production is time-dependent and proportional to the number of viable cells. CHO cells were cultured in 0.1 ml CHO media in 96-well flat-bottomed plates. Cultures were seeded at 1 000 cells/well and allowed to attach overnight. After the indicated time of incubation with the appropriate medium, 20 microl reagent was added per well, and cells were incubated for 1 h before measuring absorbance at 490 nm. Background absorbance from the control wells was subtracted. Studies were performed in triplicate for each experimental condition [6, 7].
2.5. Trypan Blue Exclusion Assay
The trypan blue exclusion method was used to assess cell viability. CHO cells were plated onto 24-well plates at approximately 1000 cells per well and incubated for 24 h. The cells were treated with F. hepatica and S. haematobium extracts at 50 microg/ml for 24 h. On days 0–4 after treatment, the cells were harvested by trypsinization and counted under a microscope after trypan blue staining. Three independent experiments were carried out based on the following formula: cell viability % = number of cells in drug treatment group/number of cells in control group 100%.
2.6. Statistical Analysis
All experiments were done in triplicate and Standard Deviation was calculated for all experiments.
3. RESULTS
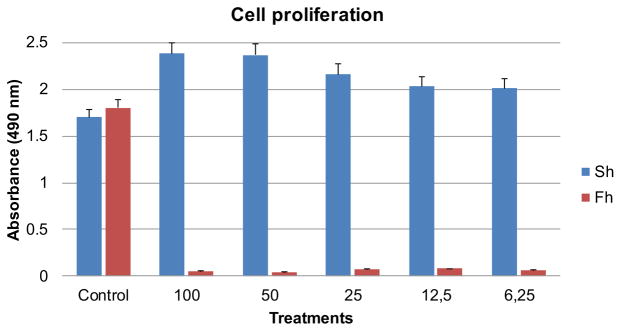
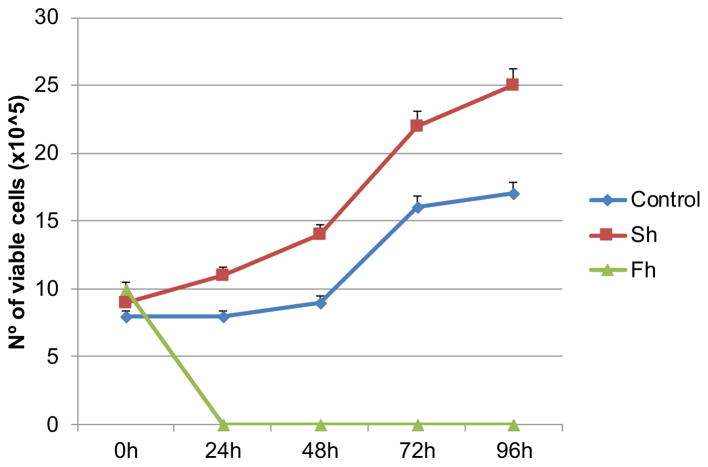
To begin investigating the effect of F. hepatica on cell viability and proliferation, CHO cells were seeded on 96-well plates, starved overnight, treated with increasing concentrations of F. hepatica extracts for 24 h, cultivated for 24, 48 and 72 h and then analyzed by MTS assay (Fig. 1). We compared the effects of F. hepatica extracts with S. haematobium extracts that increase proliferation of these cells as our group has demonstrated previously [6, 7]. The growth curve shows that treated cells show no growth while control cells and cells treated with S. haematobium extracts proliferate significantly. To evaluate the effect of F. hepatica on cell viability we used a trypan blue exclusion assay. We also used S. haematobium extracts to compare these results. Cell numbers were assessed by direct daily counting (Fig. 2).
Fig. 1.
Cell proliferation assay of Fasciola hepatica and Schistosoma haematobium extracts-treated cells. The growth curve shows that treated cells with F. hepatica showed no growth while cells treated with S. haematobium proliferated significantly faster and more than control cells. Experiments were done in triplicate and the bars show standard deviation.
Fig. 2.
Trypan exclusion assay of control and F. hepatica and S. haematobium-treated cells. We used the concentration of 50 microg/ml of F. hepatica and S. haematobium extracts. We confirmed the induced cell death of CHO cells when treated with F. hepatica extracts and increase in cell viability when treated with S. haematobium extracts by trypan exclusion assay. Cell numbers were assessed by direct daily counting. The experiments were done in triplicate and the curve shows standard deviation.
4. DISCUSSION
Although brief we believe that our results could prompt other researchers to investigate if this parasite hinders cancer, focusing on phenotypic effects of this parasite extracts on informative cancer cell lines, e.g., cholangiocarcinoma cell lines. Therefore, this work addresses the possible anti-cancer role of F. hepatica extracts. Fascioliasis is a food borne disease caused by infection with a fluke termed Fasciola hepatica. There are not any known causative associations between this parasite and liver cancer (cholangiocarcinoma).
While performing several experiments with parasite extracts using cell lines, we observed unexpected death of these cells when treated with F. hepatica extracts. Our results clearly show that extracts of F. hepatica induce cell death of CHO cells in culture. This effect seems to be specific since extracts of S. haematobium produce the opposite effect increasing proliferation of CHO cells.
Although there are not any reports in the literature addressing the effect of F. hepatica extracts on proliferation of mammalian cells in culture, the induced cell death in culture by F. hepatica is not surprising. Tsocheva et al. [8] isolated biologically active substances (BAS) from the tissues of Fasciola hepatica. These authors found a marked inhibiting effect of the BAS on hepatoma MC29 cell line proliferation and a slight inhibiting effect of the BAS on myeloma cell culture proliferation [8]. Several authors have attempted and succeeded in demonstrating the suppressive effects of Fasciola excretory/secretory extracts on lympho-proliferation. For example, Sharaf et al. [9] demonstrated that these extracts had significant suppressive effects on lympho-proliferation, up to 74%. Also, other team of researchers [10] demonstrated that rats infected with F. hepatica significantly decreased proliferation of spleen mononuclear cells. These authors hypothesize that a mechanism to avoid an immune response during the first stages of liver penetration could explain the suppression observed in spleen proliferative responses.
In the light of the results presented here, we demonstrate for the first time that F. hepatica extract induces cell death of mammalian cells. Further studies are necessary to identify and characterize this effect. We propose in the future to investigate if this parasite hinders cancer, focusing on phenotypic effects of this parasite extracts on informative cancer cell lines, e.g., cholangiocarcinoma cell lines. Therefore, to assert the anti-cancer potential of F. hepatica we now hypothesize that this parasite could be used as a medically-important tramatode pathogen.
Acknowledgments
We thank Dr. Antonio Castro for providing F. hepatica worms. This work was financed by FEDER - Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 - Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT - Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Inovação in the framework of the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274), and by UID/BIM/04293/2013. Fernandes R. is support by SAICTPOL/24325/2016 and SAICT-POL/24358/2016.
Footnotes
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This article does not contain any studies with human participants or animals performed by any of the authors.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
This article does not contain any individual person’s data in any form. “Not applicable” in this section.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
Send Orders for Reprints to reprints@benthamscience.ae
References
- 1.Botelho MC, Almeida F, Richter J, Sarmento A. Commentary: Human Liver Flukes. Front Public Health. 2018;6:122. doi: 10.3389/fpubh.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mas-Coma S, Valero MA, Bargues MD. Fascioliasis. Adv Exp Med Biol. 2014;766:77–114. doi: 10.1007/978-1-4939-0915-5_4. [DOI] [PubMed] [Google Scholar]
- 3.Fürst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: A systematic review and meta-analysis. Lancet Infect Dis. 2012;12(3):210–221. doi: 10.1016/S1473-3099(11)70294-8. [DOI] [PubMed] [Google Scholar]
- 4.Machicado C, Machicado JD, Maco V, Terashima A, Marcos LA. Association of Fasciola hepatica Infection with Liver Fibrosis, Cirrhosis, and Cancer: A Systematic Review. PLoS Negl Trop Dis. 2016;10(9):e0004962. doi: 10.1371/journal.pntd.0004962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IARC Biological agents. Volume 100 B A review of human carcinogens IARC monographs on the evaluation of carcinogenic risks to humans/WHO. IARC. 2012;100(Pt B):1–441. [PMC free article] [PubMed] [Google Scholar]
- 6.Botelho M, Ferreira AC, Oliveira MJ, Domingues A, Machado JC, da Costa JM. Schistosoma haematobium total antigen induces increased proliferation, migration and invasion, and decreases apoptosis of normal epithelial cells. Int J Parasitol. 2009;39(10):1083–1091. doi: 10.1016/j.ijpara.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Botelho MC, Vale N, Gouveia MJ, Rinaldi G, Santos J, Santos LL, Gomes P, Brindley PJ, Correia da Costa JM. Tumour-like phenotypes in urothelial cells after exposure to antigens from eggs of Schistosoma haematobium: An oestrogen-DNA adducts mediated pathway? Int J Parasitol. 2013;43(1):17–26. doi: 10.1016/j.ijpara.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Tsocheva N, Toshkova R, Filchev A. Inhibition of proliferation of tumour cell cultures by biologically active substances isolated from the tissues ofFasciola hepatica and Fasciola hepatica-infected rat liver. Folia Parasitol. 1992;39(4):387–390. [PubMed] [Google Scholar]
- 9.Sharaf OF, Amir EM, Hawash YA. Lympho-proliferative responses to various fasciola hepatica worm’s antigens: An in vitro study. J Egypt Soc Parasitol. 2016;46(1):217–222. doi: 10.12816/0026167. [DOI] [PubMed] [Google Scholar]
- 10.Cervi L, Rossi G, Cejas H, Masih DT. Fasciola hepatica-induced immune suppression of spleen mononuclear cell proliferation: Role of nitric oxide. Clin Immunol Immunopathol. 1998;87(2):145–154. doi: 10.1006/clin.1997.4499. [DOI] [PubMed] [Google Scholar]