Abstract
Commercially available physical activity monitors provide clinicians an opportunity to obtain oncology patient health measures to an unprecedented degree. These devices can provide objective and quantifiable measures of physical activity, which are not subject to errors or bias of self-reporting or shorter duration of formal testing. Prior work on so-called quantified-self data was based on older-generation, research-grade accelerometers, which laid the foundation for consumer-based physical activity monitoring devices to be validated as a feasible and reliable tool in patients with cancer. Physical activity monitors are being used in chronic conditions including chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, and obesity. Differing demographics, compounded with higher symptom and treatment burdens in patients with cancer, imply that additional work is needed to understand the unique strengths and weaknesses of physical activity monitors in this population. Oncology programs can systematically implement these tools into their workflows in an adaptable and iterative manner. Translating large amounts of data collected from an individual physical activity monitoring device into clinically relevant information requires sophisticated data compilation and reduction. In this article, we summarize the characteristics of older- and newer-generation physical activity monitors, review the validation of physical activity monitors with respect to health-related quality-of-life assessments, and describe the current role of these devices for the practicing oncologist. We also highlight the challenges and next steps needed for physical activity monitors to provide relevant information that can change the current state of oncology practice.
INTRODUCTION
The adoption of modern-day wearable monitors has contributed to the idea of the quantified self, where individuals can track and store measurable health parameters. This can provide oncologists an opportunity to obtain patient health measures, particularly related to physical activity, to a degree not previously possible. Commercially available monitors can measure multiple parameters, including steps taken, active minutes, pulse oximetry, and heart rate as well as variables related to sleep, posture, and gait. Accurate assessment of physical activity is essential in patients with cancer, and wearable physical activity monitors can help oncologists make management decisions and evaluate the suitability and effects of therapy. Commercially available physical activity monitors are being rapidly adopted by patients and researchers and have the potential to greatly improve our ability to quantify physical activity in patients with cancer. Prior work on quantified-self data was based on older-generation, research-grade accelerometers, which laid the foundation for consumer-based physical activity monitoring devices to be validated as a feasible and reliable tool in patients with cancer. Translation of what is known about the application of physical activity monitors in the nononcology world will need validation in patients with cancer, who have significant symptom burden, including fatigue, depression, stress, weight loss, anorexia, and neuropathy.1 Patients with cancer are also typically older and may be less technologically literate.
Clinical and research programs should prepare to systematically implement these devices into their workflows in an adaptable and iterative manner. In this article, we summarize the characteristics of older- and newer-generation physical activity monitors, review the validation of physical activity monitors with respect to health-related quality-of-life (QOL) assessments, describe the current role of physical activity monitors for the practicing oncologist, and highlight the next steps needed to integrate these devices into the modern oncologist–patient relationship.
OVERVIEW OF PHYSICAL ACTIVITY MONITORS
Previous-generation accelerometers were limited by their complexity, and their application was largely confined to the research setting. As technology becomes more ubiquitous, wearable devices are more commonly being used across all patient populations, warranting a formal study of their application in oncology. Wearable consumer-based physical activity monitors are small devices that are worn by attaching to the body or clothing. These devices provide objective and quantifiable measures of physical activity, which are not subject to errors or bias of self-reporting or shorter duration of formal testing. Although traditional pedometers are able to measure step count, they lack comprehensive representation of physical activity. Modern physical activity monitors provide measures beyond step count, such as variability, minutes and intensity of activity, energy expenditure, and posture (eg, hours spent sitting, standing, or lying down per day).2 Recent studies have shown that sedentary behavior can influence health significantly, and measures beyond just moderate and vigorous physical activity can affect health. Although the measured variable of interest can vary based on the primary objective being explored, the ability to differentiate even slight variations in activity with modern devices can be important and relevant.
These devices continue to rapidly evolve, making it imperative to harness their potential to provide useful information. Newer-generation physical activity monitors offer multiple technologic advantages over older-generation devices, including wireless updates with computers or smartphones, improved battery life and increased aesthetics, and ease of daily use and passive data collection. Validation studies comparing the accuracy of commercially available physical activity monitors with older-generation accelerometers have demonstrated high correlation in younger and older adults.3,4 Modern devices have shown high levels of accuracy in monitoring physical activity.5 Discrepancies between physical activity monitors occur as devices measure data using different technologies and process data using unique algorithms.6 Studies have suggested consumer-based physical activity monitors may report a higher number of steps per day, which reflects the sensitivity threshold of the specific devices.4,7,8 Steps are often measured using a three-axis accelerometer with specific algorithms designed to include real steps but exclude nonstep movements. Heart rate and sleep measurements are newer concepts that are being standardized.6,9 Depending on the objective of incorporating physical activity monitors into clinic or research processes, some devices may therefore be better suited than others, and understanding those nuances is critical before implementation.
SPECIFIC ROLE OF PHYSICAL ACTIVITY MONITORS IN ONCOLOGY
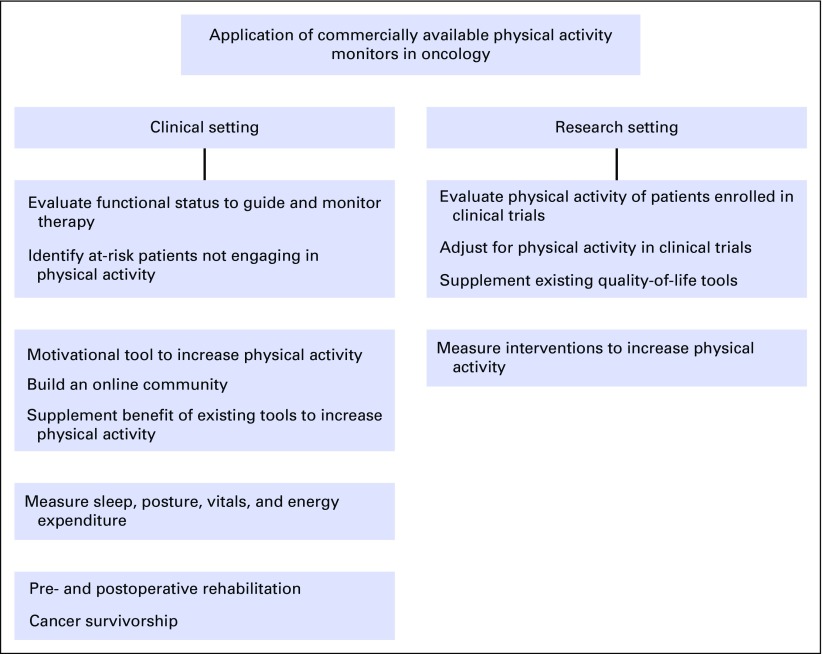
Oncology investigators and providers often use clinical and functional status measures to evaluate treatment and disease symptom burdens (Fig 1). Standardized scales are used to classify therapy- and disease-related toxicities by assigning grades based on clinical descriptions of severity, need, or level of required intervention or hospitalization and the patient’s ability to perform activities of daily living.10 The strength of these grading systems is in their ability to accurately classify adverse events that rely on numeric variables (eg, cell count or level of transaminases). However, there may be a disconnect between patient and provider perceptions of reported symptoms such as fatigue, malaise, and pain, which are subject to individual patient and provider interpretation and reporting as well as recall and reporting biases.11,12 In one study, patients with cancer participating in a lifestyle intervention during chemotherapy self-reported 366% higher levels of physical activity compared with objective measures.13 Physical activity monitors can therefore identify at-risk patients with suboptimal performance, which is the first step in targeting interventions aimed at increasing physical activity. Clinicians and clinical trial investigators do not currently have tools to accurately assess or report the functional status or impact, whether positive or detrimental, of cancer therapy on activity. Most trials, including large phase III randomized studies, depend on functional assessment scales as surrogates for levels of physical activity. Commonly used scales, such as the Karnofsky performance status (PS) and Eastern Cooperative Oncology Group (ECOG) PS scales, have evolved into generally accepted tools for clinical care in assessing physical activity level, prognosis, and tolerability of therapy and even for determining clinical trial eligibility.14,15 These scales are generally reproducible but have low associations with QOL and symptom measures.16 Although there is acceptable agreement in comparing good (ECOG PS, 0 to 2) versus poor (ECOG PS, 3 to 4) functional statuses, interobserver agreement is lost when making observations with higher resolution (eg, ECOG PS, 0 v 1 v 2).17 In part because of the lack of accurate assessment of tolerability, some therapies deemed to be effective in clinical trials have not seen broad adoption in clinical practice as a result of poorer-than-expected treatment tolerance in the real world.18,19 Physical activity monitors may be able to provide a more accurate measure of functional status, thus guiding therapy and eligibility in clinical trials. Patient-reported outcomes have been adopted in oncology clinical trial designs in the form of treatment- and disease-specific tools.20-23 These tools may not accurately reflect the fluctuation of symptoms between visits and can be compromised by missing data and user fatigue, which can pose a challenge in assigning clinical meaningfulness of the obtained data. Physical activity monitoring devices, however, can be worn for extended periods of time, allow relatively automated passive data capture, and therefore provide more clinically relevant longitudinal data.
FIG 1.
Current applications of commercially available physical activity monitors in oncology.
Physical activity has been associated with improved outcomes in patients with cancer across pooled studies.24 Higher levels of physical activity have been reported to decrease both cancer mortality and recurrence rates.25 Physical activity monitors can also increase levels of physical activity among patients with cancer (Fig 1). Exercise can be safely undertaken by patients with cancer and can improve both cancer- (eg, mortality and risk of recurrence) and patient-reported outcomes (eg, QOL and fatigue). Providing physical activity monitors to patients with early-stage breast cancer along with weekly wellness coaching led to increase in physical activity and improved mental health and QOL scores.26 Physical activity monitors may act as motivational tools and improve levels of physical activity via self-monitoring and feedback.27 Supplementing physical activity monitors with reminder systems, such as text messages and telephone-based counseling, can further improve outcomes.28,29 In one study, 377 patients with breast cancer were randomly assigned to receive physical activity print materials, a pedometer, or both. The combined intervention (ie, printed materials and pedometer) achieved maximum improvement in QOL measures and fatigue at 12 weeks.30 Pilot studies have successfully assessed the feasibility of using mobile health components (ie, self-monitoring of selected dietary behaviors via daily text messages and wireless devices to automatically track weight and steps) for weight management in overweight survivors of breast cancer.31 Modern monitors are expanding their scope and can measure non–activity-related variables, such as sleep, posture, and vital signs, which have been recognized to both represent and affect health outcomes. Trials evaluating whether physical activity monitor–based interventions can improve cognitive outcomes in sedentary survivors of breast cancer with self-reported problems with cognition are under way.32 Physical activity monitors may be a valuable outcome measure and validation anchor in clinical research, as demonstrated in a trial of 32 hematopoietic stem-cell transplant recipients, where more severe symptoms, impaired physical health, and restrictions in the performance of usual daily activities were associated with statistically significant decrements in objectively measured daily steps.33
Physical activity monitors have demonstrated their utility in rehabilitation among patients with congestive heart failure (CHF).34,35 This application of physical activity monitors extends into the oncology setting as well. In a systematic review and meta-analysis of 21 studies, prehabilitation and increased physical activity before surgery were associated with reduced length of stay and improved postoperative status in patients undergoing surgery,36 and this has been demonstrated in patients undergoing surgery for colorectal or prostate cancer as well.37,38 Thus, a potential application of physical activity monitors is to provide telemonitoring and prehabilitation services to patients preparing to undergo major cancer surgery, especially in rural areas.39,40 The benefit of physical activity monitor–based rehabilitation programs may extend to the postsurgical setting as well, even in patients receiving adjuvant chemotherapy.41 Pedometer-based walking programs have also been demonstrated to be a feasible way to improve objective measures, such as the 6-minute walk test, and subjective measures, such as happiness (measured by the Oxford Happiness Questionnaire) in survivors of breast or head and neck cancer.42 Physical activity monitors may also play a role in cancer survivorship; in a study of 46 adult survivors of childhood cancer, the stimulation of daily physical activity using exercise counseling and a pedometer over 10 weeks led to a significant decrease in fatigue, and this benefit lasted more than 3 years.43
PRIOR USE OF PHYSICAL ACTIVITY MONITORS IN OTHER CHRONIC MEDICAL CONDITIONS
In 2011, the Centers for Disease Prevention and Control released its first-ever manual studying the use of physical activity monitors, which established the framework for use of these devices in multiple medical conditions.44 Efforts to recognize the most relevant physical activity monitor variables associated with health outcomes have been ongoing in chronic obstructive pulmonary disease (COPD), CHF, and chronic kidney disease.45-47 The Proactive Project has identified and attempted to validate physical activity monitors in several chronic health conditions, including COPD, CHF, type 2 diabetes, primary pulmonary hypertension, chronic lower back pain, fibromyalgia syndrome, and obesity.48 Accelerometers were able to accurately predict performance and even provide additional data compared with the 6-minute walk test in a study of patients with CHF.49 Newer implanted cardioverter defibrillator devices have accelerometers implanted in them, and trials using these internal physical activity monitors are under way in patients with advanced CHF.50 A meta-analysis of randomized trials reported significant improvements in several health markers, including mean steps per day, with use of physical activity monitors and physical activity monitor–based counseling in patients with type 2 diabetes mellitus.50 Patients undergoing physical activity monitor intervention also had significantly lower hemoglobin A1c levels, body mass index, and systolic blood pressure. In a study of patients with COPD not undergoing rehabilitation, an outpatient pedometer-based exercise counseling program enhanced daily physical activity, physical fitness, health-related QOL measures, and intrinsic motivation.51 The benefit of physical activity monitor–based counseling has also been shown to be complimentary to established pulmonary rehabilitation programs in COPD.52 Physical activity monitors may thus be used to augment existing programs for increasing activity in oncology patients as well.
Feedback from physical activity monitors has led to improved levels of activity in elderly patients, which is especially pertinent in oncology patients, who tend to be older than the general population.53 On the opposite end of the age spectrum, Lu et al27 assigned survivors of childhood cancer to wear a physical activity monitor daily for 6 months. Retention was 79% at the end of the study, and on the basis of motivational feedback, levels of total weekly moderate to vigorous physical activity increased from 265.6 to 301.4 minutes. Use of physical activity monitors was associated with increasing step count and reduced fatigue (correlation coefficient between steps per day and fatigue, −0.66) in children with acute lymphoblastic leukemia undergoing corticosteroid pulse.54
PRIOR VALIDATION OF PHYSICAL ACTIVITY MONITORS IN ONCOLOGY
Previous-generation accelerometers provided a valid and reliable measure of activity in oncology, but their use was largely limited to the research setting. One study used accelerometers in patients undergoing surgical resection of GI cancers and demonstrated that objective physical activity scores correlated significantly with disease stage, functional status, and QOL.55 In this study, accelerometer data revealed patients with early-stage disease had higher levels of activity compared with patients with advanced disease. There was a high rate of correlation between ECOG PS and time active and steps taken, and these variables also differed between ECOG PS of 1 versus 2. Physical activity as measured by the devices also correlated with mean Karnofsky PS and the physical and role domains of the European Organisation for Research and Treatment of Cancer QOL Questionnaire–C30. Physical activity measures by wearable devices may serve as an important outcome for medical care and research end points. In a study of women with breast cancer receiving chemotherapy, temporal changes with longitudinal follow-up in accelerometer data correlated with changes in fatigue, mood, and depression as measured by validated tools including the Fatigue Symptom Checklist, Multidimensional Assessment of Fatigue, Profile of Mood States, Center for Epidemiologic Studies Depression Scale, and Hamilton Depression Inventory.56 In another prospective study, 100 treatment-naïve patients with cancer wore an accelerometer before their oncology office visit.57 A significant inverse association between objectively measured activity and ECOG PS was reported. Accelerometer-assessed activity was able to differentiate patients with clinician-assigned ECOG PS of 0 from those with ECOG PS of 2 to 3 but not from those with ECOG PS of 1. This lack of ability to differentiate ECOG PS of 0 from 1 despite using monitored data could reflect limitations of PS scales like the ECOG scale that are inherently prone to over- and underestimation by clinicians. In this study, older patients were assigned poorer ECOG PS compared with younger patients despite similar levels of activity, which could be explained by provider bias. Additionally, monitored data can also lose their reliability as a result of patient compliance or systematic bias introduced into the databases via the reduction algorithm used in the study. Increased aesthetics and comfort, paralleled with increasing affordability, can allow for longer wear time and may allow newer-generation physical activity monitors to quantify longer observation periods, which may in turn help differentiate ECOG PS of 0 from 1. Accelerometers may provide more clinically relevant data compared with clinician assessment, because patient-reported physical activity using the International Physical Activity Questionnaire correlated poorly with physician-assigned ECOG PS, with patients far more sedentary than estimated using physician-assigned ECOG PS.58
Because physical activity is associated with improved cancer outcomes, better understanding and assessment of activity using physical activity monitors can allow studies to adjust for activity and improve the evaluation of treatment strategies. Prospective studies in multiple cancer types have suggested that physical activity after cancer diagnosis consistently improves QOL measures.59 The impacts of exercise intensity, duration, and adherence in the real-world setting and the effect of longer follow-up are not well established. Physical activity monitoring devices can provide investigators the opportunity to assess adherence and enable long-term follow-up, which may provide accurate objective assessment to compare with cancer outcomes.
CHALLENGES AND FUTURE DIRECTIONS
Translating large amounts of data collected from an individual physical activity monitoring device into clinically relevant variables requires compiling data into a standardized database to allow application of data-reduction algorithms. Existing Web-based programs allow tracking of a large number of individuals using a specific commercial product. However, for clinical and research purposes, a device- or brand-agnostic solution would be needed to achieve standardized data compiling and reduction. Areas for standardization include identifying a valid patient daily wear time (ie, minimum daily wear time requirement for which data would be considered representative or evaluable) and automated screening for spurious data. Studies have not consistently reported their decision rules, which have the potential for affecting outcome variables; this emphasizes the importance of standardizing the data-handling process.60 Rigorous examination of use patterns is needed before these decisions are standardized, because they can have significant impact on the validity of data, and additional considerations to individual study end points will need to be anticipated. Studies focusing on surveillance and intervention may focus on data at the population level and need less restrictive criteria to enable higher rates of inclusion and participant retention during the study period. In contrast, association studies focus on individual-level data and may need to concentrate on intraindividual variation specifically as it relates to natural variability in behavior with time and environmental circumstances (eg, home v work).2
The RE-AIM (ie, reach, efficacy/effectiveness, adoption, implementation, and maintenance) framework can be used to provide a structure to develop, implement, and sustain physical activity monitoring device approaches in real-life research and clinical settings.61 It is critical to not only understand who would be best studied using physical activity monitor–derived end points, but also study why patients refuse or are unable to comply with requirements. This will affect future applications in the clinic and in research. The uptake of physical activity monitors may not be seen consistently across all demographics. Most patients with cancer are elderly and may not be able to use wearable devices, which need to be charged intermittently and require access to a Web- or mobile-based software. This may be less of an issue as the adoption of smart phones across demographics makes access to mobile platforms more ubiquitous. Elderly, frail patients who have cautious movement patterns may not be measured accurately, and absolute measurement variables will depend on the sensitivity of the devices being used.62 Also, in patients who lack the ability to care for themselves or perform activities of daily living, physical activity monitors are not likely to capture steps reliably, but other wearable devices can be beneficial in estimating body position and transfer.63 Because wearable devices are now being incorporated into articles of daily living, including clothing, headphones, eyeglasses, shoes, and caps, attrition may become less of an issue. However, validation of these devices is needed, because uptake, compliance, and reliability of technology will be expected to vary.
Implementation in the clinic and in research requires standardized processes. As an example, the study or clinic team may assume responsibility for setting up physical activity monitors for patients and allow centralized monitoring. Additional burden to research centers or health systems regarding gathering and processing data, performing quality control, and covering the cost of equipment should be anticipated. These will vary based on the primary objective being pursued. Clinics and researchers can offer services to sync patients’ devices during their office visits using wireless USB dongles or office wireless Internet networks. Centers may have inconsistent uptake of physical activity monitors based on their levels of resources, interest, physical capacity, and technical expertise. To help maximize efficiency, implementation of physical activity monitors should be incorporated into daily staff workflow whenever possible. Individual physical activity monitor startup kits with clear, easy-to-follow instructions are one example that can help standardize such processes, but they should be flexible enough to be customized to the specific environment (clinic v research, hospital based v office based, urban v rural). The cost of implementing physical activity monitors goes beyond simply purchasing a device; therefore, continuous assessment and reporting of the impact and added value of physical activity monitors to the clinic or research study should be incorporated from the inception of the program.
Commercially available physical activity monitors are being rapidly adopted by oncology patients, clinicians, and researchers and have the potential to greatly improve our ability to quantify physical activity in patients with cancer. Prior work on quantified-self data was based on older-generation, research-grade accelerometers, which laid the foundation for consumer-based physical activity monitoring devices to be validated as a feasible and reliable tool in patients with cancer. Before broader implementation in patients with cancer can be advocated, further work is needed to understand what parameters these devices are best suited to measure. Clinical and research programs should prepare to systematically implement these devices into their workflows in an adaptable and iterative manner.
ACKNOWLEDGMENT
M.S.B. and A.G. contributed equally to this article.
AUTHOR CONTRIBUTIONS
Conception and design: Muhammad S. Beg, Arjun Gupta, Chad D. Rethorst
Collection and assembly of data: Arjun Gupta, Tyler Stewart
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Promise of Wearable Physical Activity Monitors in Oncology Practice
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jop/site/misc/ifc.xhtml.
Muhammad S. Beg
Consulting or Advisory Role: Genentech, Ipsen, Bayer HealthCare Pharmaceuticals, Celgene, Boston Biomedical
Speakers’ Bureau: Ipsen
Research Funding: miRNA Therapeutics (Inst), Precision BioLogic (Inst), Genentech (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), CASI Pharmaceuticals (Inst)
Arjun Gupta
No relationship to disclose
Tyler Stewart
No relationship to disclose
Chad D. Rethorst
No relationship to disclose
REFERENCES
- 1.Broderick JM, Ryan J, O’Donnell DM, et al. A guide to assessing physical activity using accelerometry in cancer patients. Support Care Cancer. 2014;22:1121–1130. doi: 10.1007/s00520-013-2102-2. [DOI] [PubMed] [Google Scholar]
- 2.Matthews CE, Hagströmer M, Pober DM, et al. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44:S68–S76. doi: 10.1249/MSS.0b013e3182399e5b. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul SS, Tiedemann A, Hassett LM, et al. Validity of the Fitbit activity tracker for measuring steps in community-dwelling older adults. BMJ Open Sport Exerc Med. 2015;1:e000013. doi: 10.1136/bmjsem-2015-000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tully MA, McBride C, Heron L, et al. The validation of Fibit Zip™ physical activity monitor as a measure of free-living physical activity. BMC Res Notes. 2014;7:952. doi: 10.1186/1756-0500-7-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz KM, Krupka DJ, Chang MJ, et al. Fitbit®: An accurate and reliable device for wireless physical activity tracking. Int J Cardiol. 2015;185:138–140. doi: 10.1016/j.ijcard.2015.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberger ME, Buman MP, Haskell WL, et al. 24 hours of sleep, sedentary behavior, and physical activity with nine wearable devices. Med Sci Sports Exerc. 2016;48:457–465. doi: 10.1249/MSS.0000000000000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tudor-Locke C, Ainsworth BE, Thompson RW, et al. Comparison of pedometer and accelerometer measures of free-living physical activity. Med Sci Sports Exerc. 2002;34:2045–2051. doi: 10.1097/00005768-200212000-00027. [DOI] [PubMed] [Google Scholar]
- 8.El-Amrawy F, Nounou MI. Are currently available wearable devices for activity tracking and heart rate monitoring accurate, precise, and medically beneficial? Healthc Inform Res. 2015;21:315–320. doi: 10.4258/hir.2015.21.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep Breath. 2012;16:913–917. doi: 10.1007/s11325-011-0585-y. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Sonn GA, Sadetsky N, Presti JC, et al. Differing perceptions of quality of life in patients with prostate cancer and their doctors. J Urol. 189:S59–S65. doi: 10.1016/j.juro.2012.11.032. discussion S65, 2013 (suppl) [DOI] [PubMed] [Google Scholar]
- 12.Slevin ML, Plant H, Lynch D, et al. Who should measure quality of life, the doctor or the patient? Br J Cancer. 1988;57:109–112. doi: 10.1038/bjc.1988.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vassbakk-Brovold K, Kersten C, Fegran L, et al. Cancer patients participating in a lifestyle intervention during chemotherapy greatly over-report their physical activity level: A validation study. BMC Sports Sci Med Rehabil. 2016;8:10. doi: 10.1186/s13102-016-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karnofsky DA, Abelmann WH, Craver LF, et al. The use of the nitrogen mustards in the palliative treatment of carcinoma with particular reference to bronchogenic carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 15.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 16.Suh SY, Leblanc TW, Shelby RA, et al. Longitudinal patient-reported performance status assessment in the cancer clinic is feasible and prognostic. J Oncol Pract. 2011;7:374–381. doi: 10.1200/JOP.2011.000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sørensen JB, Klee M, Palshof T, et al. Performance status assessment in cancer patients: An inter-observer variability study. Br J Cancer. 1993;67:773–775. doi: 10.1038/bjc.1993.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 19.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 20.Scott CB, Stetz J, Bruner DW, et al. Radiation Therapy Oncology Group quality of life assessment: Design, analysis, and data management issues. Qual Life Res. 1994;3:199–206. doi: 10.1007/BF00435385. [DOI] [PubMed] [Google Scholar]
- 21.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy–Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 22.Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5:139–150. doi: 10.1007/BF00435979. [DOI] [PubMed] [Google Scholar]
- 23.Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12:199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 24.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 25.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175:959–967. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan C, Klepsa A. Encouraging regular physical activity for hormone-receptor positive breast cancer patients to improve mental and physical health during treatment and survivorship. J Clin Oncol. 2015;33 (suppl 28S; abstr 91) [Google Scholar]
- 27.Le A, Mitchell HR, Zheng DJ, et al. A home-based physical activity intervention using activity trackers in survivors of childhood cancer: A pilot study. Pediatr Blood Cancer. doi: 10.1002/pbc.26235. [epub ahead of print on September 12, 2016] [DOI] [PubMed] [Google Scholar]
- 28.Wang JB, Cadmus-Bertram LA, Natarajan L, et al. Wearable sensor/device (Fitbit One) and SMS text-messaging prompts to increase physical activity in overweight and obese adults: A randomized controlled trial. Telemed J E Health. 2015;21:782–792. doi: 10.1089/tmj.2014.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartman SJ, Nelson SH, Cadmus-Bertram LA, et al. Technology- and phone-based weight loss intervention: Pilot RCT in women at elevated breast cancer risk. Am J Prev Med. 2016;51:714–721. doi: 10.1016/j.amepre.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallance JK, Courneya KS, Plotnikoff RC, et al. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 31.Quintiliani LM, Mann DM, Puputti M, et al. Pilot and feasibility test of a mobile health-supported behavioral counseling intervention for weight management among breast cancer survivors. JMIR Cancer. 2016;2:e4. doi: 10.2196/cancer.5305. [DOI] [PubMed] [Google Scholar]
- 32.Hartman SJ, Natarajan L, Palmer BW, et al. Impact of increasing physical activity on cognitive functioning in breast cancer survivors: Rationale and study design of Memory & Motion. Contemp Clin Trials. 2015;45:371–376. doi: 10.1016/j.cct.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett AV, Reeve BB, Basch EM, et al. Evaluation of pedometry as a patient-centered outcome in patients undergoing hematopoietic cell transplant (HCT): A comparison of pedometry and patient reports of symptoms, health, and quality of life. Qual Life Res. 2016;25:535–546. doi: 10.1007/s11136-015-1179-0. [DOI] [PubMed] [Google Scholar]
- 34.Hassett L, van den Berg M, Lindley RI, et al. Effect of affordable technology on physical activity levels and mobility outcomes in rehabilitation: A protocol for the Activity and MObility UsiNg Technology (AMOUNT) rehabilitation trial. BMJ Open. 2016;6:e012074. doi: 10.1136/bmjopen-2016-012074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorup C, Hansen J, Grønkjær M, et al. Cardiac patients’ walking activity determined by a step counter in cardiac telerehabilitation: Data from the intervention arm of a randomized controlled trial. J Med Internet Res. 2016;18:e69. doi: 10.2196/jmir.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santa Mina D, Clarke H, Ritvo P, et al. Effect of total-body prehabilitation on postoperative outcomes: A systematic review and meta-analysis. Physiotherapy. 2014;100:196–207. doi: 10.1016/j.physio.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Angenete E, Angerås U, Börjesson M, et al. Physical activity before radical prostatectomy reduces sick leave after surgery: Results from a prospective, non-randomized controlled clinical trial (LAPPRO) BMC Urol. 2016;16:50. doi: 10.1186/s12894-016-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: Argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150:505–514. doi: 10.1016/j.surg.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 39.Cuadros L, Ismail H, Ho K. Evaluation of reliability of MYZONE MZ-3 heart rate monitor: A study for the future of telephysiotherapy for preoperative prehabilitation in cancer patients. Telemed J E Health. doi: 10.1089/tmj.2016.0138. [epub ahead of print on August 18, 2016] [DOI] [PubMed] [Google Scholar]
- 40.Frensham LJ, Zarnowiecki DM, Parfitt G, et al. The experiences of participants in an innovative online resource designed to increase regular walking among rural cancer survivors: A qualitative pilot feasibility study. Support Care Cancer. 2014;22:1923–1929. doi: 10.1007/s00520-014-2177-4. [DOI] [PubMed] [Google Scholar]
- 41.Munro J, Adams R, Campbell A, et al. CRIB: The use of cardiac rehabilitation services to aid the recovery of patients with bowel cancer—A pilot randomised controlled trial (RCT) with embedded feasibility study. BMJ Open. 2014;4:e004684. doi: 10.1136/bmjopen-2013-004684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Javaheri PA, Nekolaichuk C, Haennel R, et al. Feasibility of a pedometer-based walking program for survivors of breast and head and neck cancer undergoing radiation therapy. Physiother Can. 2015;67:205–213. doi: 10.3138/ptc.2014-24O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaauwbroek R, Bouma MJ, Tuinier W, et al. The effect of exercise counselling with feedback from a pedometer on fatigue in adult survivors of childhood cancer: A pilot study. Support Care Cancer. 2009;17:1041–1048. doi: 10.1007/s00520-008-0533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Appelboom G, Yang AH, Christophe BR, et al. The promise of wearable activity sensors to define patient recovery. J Clin Neurosci. 2014;21:1089–1093. doi: 10.1016/j.jocn.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Asakuma S, Ohyanagi M, Iwasaki T. Simple methods of assessing physical activity in patients with chronic heart failure. Congest Heart Fail. 2000;6:250–255. doi: 10.1111/j.1527-5299.2000.80163.x. [DOI] [PubMed] [Google Scholar]
- 46.Demeyer H, Burtin C, Van Remoortel H, et al. Standardizing the analysis of physical activity in patients with COPD following a pulmonary rehabilitation program. Chest. 2014;146:318–327. doi: 10.1378/chest.13-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson-Cohen C, Littman AJ, Duncan GE, et al. Assessment of physical activity in chronic kidney disease. J Ren Nutr. 2013;23:123–131. doi: 10.1053/j.jrn.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Remoortel H, Giavedoni S, Raste Y, et al. Validity of activity monitors in health and chronic disease: A systematic review. Int J Behav Nutr Phys Act. 2012;9:84. doi: 10.1186/1479-5868-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jehn M, Schmidt-Trucksäess A, Schuster T, et al. Accelerometer-based quantification of 6-minute walk test performance in patients with chronic heart failure: Applicability in telemedicine. J Card Fail. 2009;15:334–340. doi: 10.1016/j.cardfail.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Vaes AW, Cheung A, Atakhorrami M, et al. Effect of “activity monitor-based” counseling on physical activity and health-related outcomes in patients with chronic diseases: A systematic review and meta-analysis. Ann Med. 2013;45:397–412. doi: 10.3109/07853890.2013.810891. [DOI] [PubMed] [Google Scholar]
- 51.Hospes G, Bossenbroek L, Ten Hacken NH, et al. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: Results of an exercise counseling program. Patient Educ Couns. 2009;75:274–278. doi: 10.1016/j.pec.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 52.de Blok BM, de Greef MH, ten Hacken NH, et al. The effects of a lifestyle physical activity counseling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD: A pilot study. Patient Educ Couns. 2006;61:48–55. doi: 10.1016/j.pec.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Victor CR, Rogers A, Woodcock A, et al. What factors support older people to increase their physical activity levels? An exploratory analysis of the experiences of PACE-Lift trial participants. Arch Gerontol Geriatr. 2016;67:1–6. doi: 10.1016/j.archger.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hooke MC, Gilchrist L, Tanner L, et al. Use of a fitness tracker to promote physical activity in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2016;63:684–689. doi: 10.1002/pbc.25860. [DOI] [PubMed] [Google Scholar]
- 55.Ferriolli E, Skipworth RJ, Hendry P, et al. Physical activity monitoring: A responsive and meaningful patient-centered outcome for surgery, chemotherapy, or radiotherapy? J Pain Symptom Manage. 2012;43:1025–1035. doi: 10.1016/j.jpainsymman.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 56.Roscoe JA, Morrow GR, Hickok JT, et al. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer. 2002;10:329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 57.Broderick JM, Hussey J, Kennedy MJ, et al. Patients over 65 years are assigned lower ECOG PS scores than younger patients, although objectively measured physical activity is no different. J Geriatr Oncol. 2014;5:49–56. doi: 10.1016/j.jgo.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 58.Walsh J, Hussey J, O'Donnell D. A pilot study comparing objective physical activity to the physical component of the Eastern Cooperative Oncology Group (ECOG) performance status scale. J Clin Oncol. 2009;27 (suppl; abstr e20501) [Google Scholar]
- 59.Streckmann F, Kneis S, Leifert JA, et al. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol. 2014;25:493–499. doi: 10.1093/annonc/mdt568. [DOI] [PubMed] [Google Scholar]
- 60.Mâsse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: A comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005;37:S544–S554. doi: 10.1249/01.mss.0000185674.09066.8a. (suppl) [DOI] [PubMed] [Google Scholar]
- 61.Phillips SM, Alfano CM, Perna FM, et al. Accelerating translation of physical activity and cancer survivorship research into practice: Recommendations for a more integrated and collaborative approach. Cancer Epidemiol Biomarkers Prev. 2014;23:687–699. doi: 10.1158/1055-9965.EPI-13-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Storti KL, Pettee KK, Brach JS, et al. Gait speed and step-count monitor accuracy in community-dwelling older adults. Med Sci Sports Exerc. 2008;40:59–64. doi: 10.1249/mss.0b013e318158b504. [DOI] [PubMed] [Google Scholar]
- 63.Skipworth RJ, Stene GB, Dahele M, et al. Patient-focused endpoints in advanced cancer: Criterion-based validation of accelerometer-based activity monitoring. Clin Nutr. 2011;30:812–821. doi: 10.1016/j.clnu.2011.05.010. [DOI] [PubMed] [Google Scholar]