Abstract
Subsequent malignant neoplasms (SMNs) in childhood cancer survivors cause substantial morbidity and mortality. This review summarizes recent literature on SMN epidemiology, risk factors, surveillance, and interventions. Survivors of childhood cancer experience long-term increased SMN risk compared with the general population, with a greater than twofold increased solid tumor risk extending beyond age 40 years. There is a dose-dependent increased risk for solid tumors after radiotherapy, with the highest risks for tumors occurring in or near the treatment field (eg, greater than fivefold increased risk for breast, brain, thyroid, skin, bone, and soft tissue malignancies). Alkylating and anthracycline chemotherapies increase the risk for development of several solid malignancies in addition to acute leukemia/myelodysplasia, and these risks may be modified by other patient characteristics, such as age at exposure and, potentially, inherited genetic susceptibility. Strategies for identifying survivors at risk and initiating long-term surveillance have improved and interventions are underway to improve knowledge about late-treatment effects among survivors and caregivers. Better understanding of treatment-related risk factors and genetic susceptibility holds promise for refining surveillance strategies and, ultimately, upfront cancer therapies.
INTRODUCTION
Survival after childhood cancer now exceeds 80% throughout the United States and much of Europe.1,2 With this improvement in survival over the last five decades, there has been increased recognition of late health complications, including subsequent malignant neoplasms (SMNs), among survivors. SMNs, defined as new primary malignancies after an initial cancer diagnosis, are the most frequent cause of nonrelapse late mortality, accounting for nearly half of nonrelapse deaths among 5-year survivors.3 This review highlights up-to-date evidence on SMN risk, risk factors, and surveillance efforts.
CUMULATIVE INCIDENCE AND RISK FOR SUBSEQUENT NEOPLASMS
Multi-institution and population-based cohort studies, based in Europe and North America, designed to follow long-term survivors of childhood cancer, have been instrumental in describing SMN epidemiology. These cohort consortia have led efforts to characterize late effects experienced by survivors and have provided important data that have helped guide current cancer therapies and guidelines for surveillance of survivors of childhood cancer.
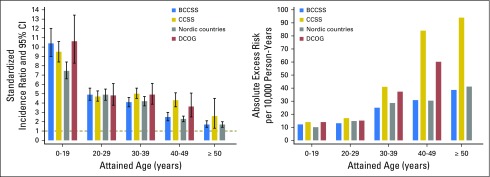
The largest SMN series have been reported by the North American Childhood Cancer Survivor Study (CCSS), the British Childhood Cancer Survivor Study (BCCSS), a collaborative effort from the Nordic countries cancer registries, and the Dutch Childhood Cancer Oncology Group-Long-Term Effects After Childhood Cancer (DCOG-LATER) cohort. These groups have shown that an increased SMN risk persists with advancing attained age. All four studies reported that beyond age 40 years, the standardized incidence ratio (SIR) was at least twofold and that the absolute excess risk (AER) increased with attained age (Fig 1).7,10-12 Despite consistent SIRs and AERs across cohorts before age 40 years, the CCSS and DCOG-LATER studies reported higher SIRs and AERs than the BCCSS or Nordic countries for older attained ages. A potential explanation is that the BCCSS and the Nordic country cohorts include patients who received their diagnosis between 1940 and 1969, an era of low overall survival rates for pediatric cancer. Most survivors from this era received treatments with minimal carcinogenic potential, such as surgery alone, low-energy radiotherapy, or single-agent chemotherapy. Because survivors treated before 1970 make up the majority of patients with higher attained ages, it is not surprising that the treatment-related excess risks are lower.4
Fig 1.
Standardized incidence ratios and absolute excess risk of subsequent malignant neoplasm by attained age in international cohorts of survivors of childhood cancer.
The first analysis of SMN risk from the CCSS reported a 20-year cumulative incidence of 3.2%, with a sixfold increased risk compared with the general population (SIR, 6.4; 95% CI, 5.7 to 7.1).5 In a follow-up report, SMN cumulative incidence was reported at 30 years and had increased to 7.9%, whereas the risk for malignancy remained stable from the previous report (SIR, 6.0; 95% CI, 5.5 to 6.4), with the greatest SIRs observed for cancers of the bone, CNS, thyroid, head and neck, and breast.6 Survivors experienced a fourfold increased risk of developing a malignancy after age 40 years compared with the general population (SIR, 4.4; 95% CI, 3.8 to 5.0), with the greatest risk observed for cancers of the breast, kidney, thyroid, and soft tissue sarcoma (STS).7 The most recent comprehensive report of SMNs from the CCSS reported 15-year cumulative SMN incidence by decade of diagnosis and showed that those whose cancer was diagnosed in the 1990s had a significantly lower incidence of subsequent malignancy compared with those diagnosed in the 1970s (1.3% v 2.1%; P < .001).8
Within Great Britain, before the development of the BCCSS, SMN incidence and risk were reported from a retrospective cohort of 16,541 3-year survivors of childhood cancer who were identified through the National Register of Childhood Tumors.9 Among survivors of nonretinoblastoma primary cancers, the 20-year cumulative SMN incidence was 2.8% and survivors experienced a nearly sixfold increased risk for malignancy compared with the general population (SIR, 5.8; 95% CI, 5.0 to 6.7). The greatest risk was observed for cancers of the bone, CNS, endocrine system, and STS.9 Subsequently, the population-based BCCSS reported on long-term risks of SMNs in 17,981 5-year survivors whose cancer was diagnosed when they were between age 0 and 14 years between 1940 and 1991.10 The study identified a fourfold increased risk for SMNs compared with the general population (SIR, 3.9; 95% CI, 3.6 to 4.2).10 The BCCSS showed that survivors remain at increased risk for SMNs beyond age 40 years, with a 2.5-fold increased SIR for ages 40 to 49 years (95% CI, 2.1 to 3.0) and 1.7-fold increased SIR beyond 50 years (95% CI, 1.4 to 2.1). The greatest AER after age 40 years was for SMNs of the GI (AER, 5.9 per 10,000 person-years) and genitourinary (AER, 6.0 per 10,000 person-years) systems, with these two sites accounting for 36% of the total AER after age 40 years.10
The combined Nordic cohort, which consists of registry data from Denmark, Finland, Iceland, Norway, and Sweden, spans the diagnosis years between 1943 and 2005. This study11 identified a threefold increased risk for a SMN compared with the general population (SIR, 3.3; 95% CI, 3.1 to 3.5) and showed the highest risk for developing SMNs of the bone, connective tissue, CNS, and endocrine glands. The risk for a SMN occurring between ages 40 and 70 years was 1.5- to 2.3-fold that of the general population. Individuals treated in the most recent era of study (1975 to 2005) experienced higher age-specific incidence rates compared with those treated earlier.11
The DCOG-LATER study reported a fivefold increase in SMN SIR compared with the general population (SIR, 5.2; 95% CI, 4.6 to 5.8) among 6,165 5-year survivors diagnosed between 1963 and 2001, and a 25-year cumulative SMN incidence of 3.9%.12 The SIR was still significantly increased after ≥ 30 years (SIR, 3.8; 95% CI, 2.8 to 4.9) and the AER substantially increased with increasing follow-up time. There was no significant decrease in cumulative incidence of SMNs for survivors treated in the 1990s compared with those treated earlier, in contrast to what was reported by the CCSS.8,12
Collaborative work among multiple European countries is forthcoming under the umbrella of the Pan-European Network for Care of Survivors after Childhood and Adolescent Cancer (PanCare). Pooled cohort and case-control studies on the risk of SMNs among 69,460 5-year survivors across 12 European countries are underway.13 This large-scale study provides a means for consistent data collection and reporting across cohorts and overcomes the limitations in size and available data observed in previous studies.14
RISK FACTORS
Radiotherapy and SMN Development
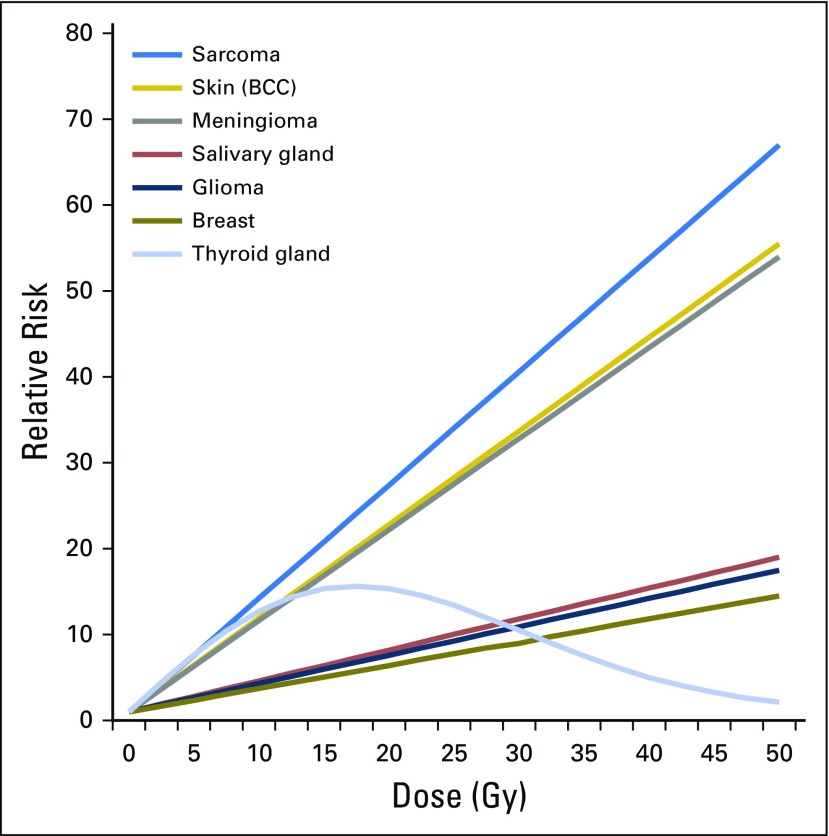
Radiation dose–related SMN risk has been studied for many cancers, as summarized in Fig 2.15 Dose-response studies rely on the radiotherapy target dose (ie, the dose delivered to the tumor and its surroundings), which is valid for proximal tissues. For more distant tissues, exposures are estimated using dose-reconstruction methods, accounting for patient and treatment characteristics (eg, treatment dose, beam energy, field size and configuration).16
Fig 2.
Fitted radiation dose response by type of second cancer, on the basis of previously published reports from the Childhood Cancer Survivor Study. Reprinted with permission.15
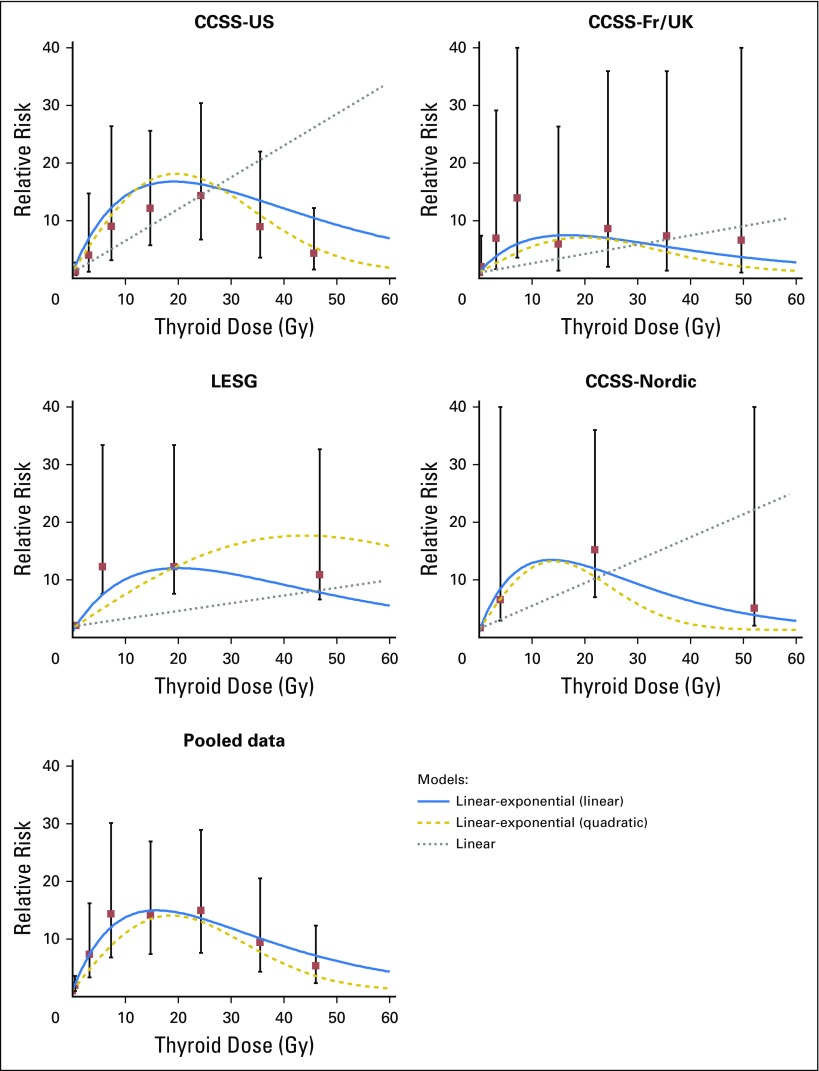
CNS tumors occur in excess after cranial radiotherapy, mainly among survivors of pediatric brain tumors and acute leukemia.10 The CCSS and BCCSS reported dose-response trends for glioma (excess odds ratio [EOR] per Gy, 0.33 and 0.079, respectively) and nonmalignant and malignant meningioma (EOR per Gy, 1.06 and 5.1, respectively).17,18 A 39-fold excess risk of salivary gland tumors was reported by the CCSS, with an estimated excess relative risk per Gy of 0.36, with most observed cases occurring after leukemia or lymphoma.19 An international pooled analysis of thyroid cancer in survivors of childhood cancer showed a dose-response plateau between 10 and 30 Gy and decreasing risk at higher doses (Fig 3),20 hypothesized to be due to cell killing, with stronger dose-responses for those who were youngest at the time of exposure.20 Additional analysis of low-dose radiation exposure showed significant dose-response trends at < 0.2 and < 0.1 Gy (P < .01) persisting > 45 years after exposure.21
Fig 3.
Relative risks and 95% CIs for categories of radiation dose and fitted dose-response models for four original studies and for all data combined (pooled analysis) for subsequent thyroid cancer. Category-specific relative risks for the LESG and CCSS-Nordic Studies were adjusted using the fitted linear-exponential (linear) model to reflect a referent of zero dose. CCSS, Childhood Cancer Survivor Study; Fr/UK, France and United Kingdom; LESG, Late Effects Study Group. Reprinted with permission.20
Data on lung cancer in cohorts of survivors of childhood cancer are limited. The Nordic cohort reported eight cases, for a 3.9-fold increased risk (95% CI, 1.7 to 7.6) in survivors with an attained age of 40 to 79 years.11 In addition, large studies of survivors of Hodgkin lymphoma (HL) who were treated as children and young adults showed that lung cancer risk was elevated after chest radiation and that radiation incurred a multiplicative effect in smokers.22,23
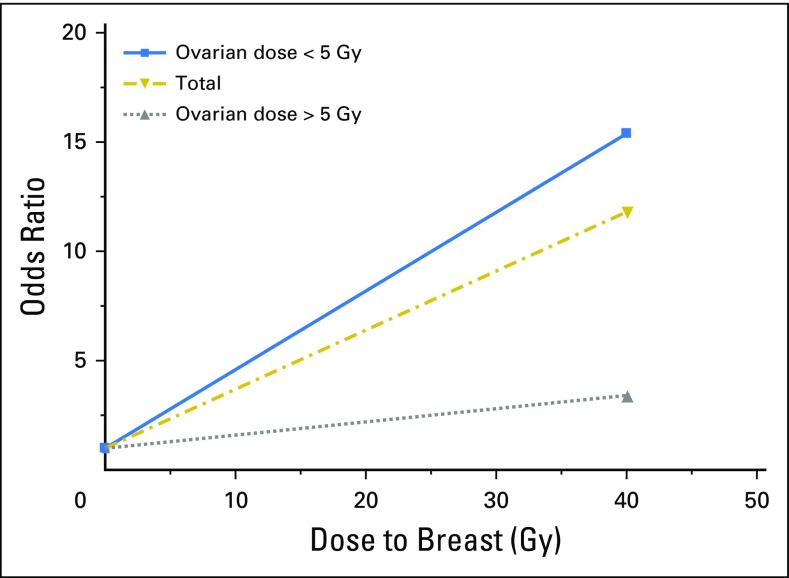
Female breast cancer risk is increased, particularly after chest/absorbed doses > 10 Gy,24 with an established linear dose-response relationship (Fig 4).25 There is growing evidence for heightened radiation sensitivity surrounding menarche,26 with risk persisting 40 to 45 years after radiotherapy,7,22 and reduction of radiation-related risk among women with premature menopause (Fig 4).25 Cumulative incidence of breast cancer by age 50 years in women treated with chest irradiation for HL has been estimated at 35%. The cumulative incidence by age 45 years for other survivors of childhood cancer treated with chest irradiation was 15%, presumably lower because of lower radiation treatment doses.24 Excess risk for breast cancer was also shown after total-body irradiation at a young age.12
Fig 4.
Breast cancer risk by radiation dose to the breast and ovary. Reprinted with permission.25
GI tract SMNs occur in excess many years after childhood cancer (SIRs range from 2.0 to 30.0).10,27-29 The CCSS reported that survivors treated with abdominal radiation experienced an 11-fold increased risk for GI SMNs (SIR, 11.2; 95% CI, 7.6 to 16.4).27 The St Jude Lifetime Cohort (SJLIFE) showed a radiation dose-response by 10-Gy increments of prescribed dose for colon cancer,28 whereas a relative risk per dose (Gy) to digestive organs of 1.13 was reported in Europe.29 The BCCSS investigators reported a nearly fivefold increased risk for GI cancers (SIR, 4.6; 95% CI, 3.8 to 5.6). Cumulative incidence of colorectal cancer by age 50 years was 1.4% (95% CI, 0.7% to 2.6%) for survivors treated with abdominopelvic irradiation, similar to rates observed in individuals with two or more first-degree relatives affected by colorectal cancer.10
A summary of six studies of subsequent sarcomas among survivors of childhood cancer30 showed a linear dose-response > 10 Gy, with a possible decrease at doses > 40 Gy for bone sarcoma, and with higher relative risks for bone sarcoma compared with STS. Nonmelanoma skin cancer, most often basal cell carcinoma, represents the most common subtype of solid cancer after radiotherapy, of which > 90% occur in the radiation field (EOR per Gy, 1.09).31
Most studies examining radiotherapy-associated SMNs analyze radiotherapy from the era of two-dimensional imaging. However, dose distribution across healthy tissues is changed with modern radiotherapy techniques, such as intensity-modulated radiotherapy and proton therapy. Proton beam radiotherapy involves no dose deposition in tissues behind the tumor, which could reduce SMN risk, but because of small sample size and other methodologic challenges, the single study on SMN risk after proton therapy is inconclusive.32 It will be critical to study how these changes in technique have effected SMN risk among survivors treated more recently.
Chemotherapy and SMN Development
The best-established association between chemotherapy and SMNs is for therapy-related acute myeloid leukemia (t-AML) and therapy-related myelodysplastic syndrome (t-MDS). Dose-dependent risks for t-AML and t-MDS are high (> 10-fold increased) after almost all alkylating agents, as well as topoisomerase II inhibitors33-36; however, the leukemogenicity of different agents varies substantially and the AER is low because of the low background risk. t-AML after alkylating-agent exposure typically arises after a latency of 5 to 8 years, is frequently preceded by MDS, and often has a complex karyotype with chromosome 5 and 7 abnormalities.33 In contrast, t-AML after topoisomerase II inhibitor exposure typically arises < 3 years after therapy, is rarely preceded by MDS, and is most frequently characterized by 11q23 rearrangements.37
Chemotherapy also increases risk for nonhematologic SMNs, which typically occur > 10 years after exposure.33 Alkylating-agent exposure increases risk for GI, thyroid, lung, breast, and bladder cancers, as well as sarcoma.22,23,28,38-43 Specifically, cyclophosphamide increases sarcoma risk in a dose-dependent manner.12,38,42,44 Furthermore, cyclophosphamide equivalent doses of > 18,000 mg/m2 increase breast cancer risk by threefold (SIR, 3.0; 95% CI, 1.2 to 7.7),41 and procarbazine and platinum have been associated with 3.2-fold (95% CI, 1.1 to 9.4) and 7.6-fold (95% CI, 2.3 to 25.5) increased risks, respectively, for GI SMNs.40 Procarbazine-related risks for the GI tract may be related to direct exposure with the mucosa,28,39,44 whereas the mechanisms of carcinogenesis for agents administered intravenously and for other malignancies are unknown.
Risk for breast cancer and other solid malignancies, including sarcoma, are increased after anthracycline exposure.12,41,44 In the CCSS cohort, risk for breast cancer in survivors treated with > 250 mg/m2 anthracycline and without a history of chest radiotherapy is increased by nearly fourfold compared with risk in the general population (SIR, 3.8; 95% CI, 1.7 to 8.3).41 The DCOG-LATER cohort reported similar findings, with a dose-dependent relationship between breast cancer risk and doxorubicin (Ptrend < .001).12 In the CCSS and DCOG-LATER reports, breast cancer risk was highest after Li-Fraumeni syndrome–associated cancers, suggesting a possible interaction between chemotherapy and genetic predisposition.12,41
Chemotherapy can also indirectly affect SMN risk. In studies of adolescent and young adult survivors of HL,22,45,46 higher cumulative procarbazine exposure was associated with a greater reduction of breast cancer risk, with 30% and 67% risk reductions for regimens of < 8.4 g/m2 and > 8.4 g/m2 procarbazine, respectively.45,46 This risk reduction seems to be due to the higher frequency of premature menopause in patients treated more intensively with chemotherapy and their resultant reduced exposure to ovarian hormones.46-48 Similarly, high cumulative alkylator exposure significantly reduced breast cancer risk in the CCSS cohort,26 in contrast to earlier CCSS results that did not show a reduced breast cancer risk after alkylator therapy.49 Breast cancer risk also increases in women with > 10 years of ovarian function after chest radiotherapy compared with those with less.26,46,48
Genetics and SMN Development
Genomic advances in the last decade have expanded our understanding of cancer predisposition. Broadly, genetic contributions to cancer range from rare, highly penetrant variants that are often associated with familial cancer susceptibility syndromes to more common genetic variants associated with weakly or modestly elevated risk for cancer in the general population.
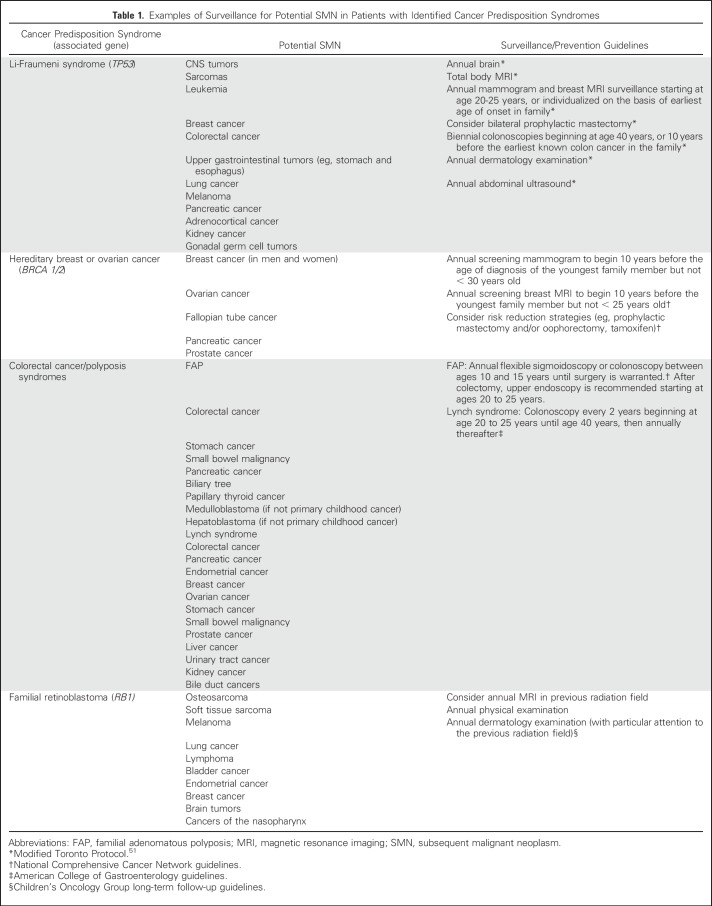
Multiple primary cancers within an individual can occur in several cancer susceptibility syndromes50; diagnoses often include rare histologies or occurrence at younger than expected ages. Most variants confer risk through an autosomal dominant inheritance pattern, although a few exhibit autosomal recessive, X-linked, or Y-linked patterns. Examples of inherited cancer predisposition syndromes are listed in Table 1. Understanding of the penetrance of and risks associated with these mutations, particularly in the absence of established family history, is evolving rapidly with the expansion of gene panel testing in recent years.
Table 1.
Examples of Surveillance for Potential SMN in Patients with Identified Cancer Predisposition Syndromes
Research is increasingly focused on whether germline genetic variation modifies risk for treatment-related SMNs. Sensitivity to damage from ionizing radiation exposure has been reported among individuals with several cancer predisposition syndromes, such as ataxia telangiectasia, and in experimental studies demonstrating cellular radiosensitivity.52,53 In the general population, most studies have focused on genetic variation in DNA damage detection and repair mechanisms as potential modifiers of treatment-related SMN risks, as reviewed recently.54 However, these studies are limited by small sample sizes, insufficient treatment exposure data, or lack of replication of the reported findings. More recently, studies have agnostically interrogated common genetic variation across the genome to identify variants associated with SMN risk, including studies of t-MDS and t-AML,55 SMNs after HL,56 and breast cancer after childhood cancer.57 Expansion of these studies through large-scale genomics efforts in survivors of cancer, such as the CCSS and the SJLIFE Cohort, should provide important insights into the role of genetic susceptibility in multiple primary cancers.
SURVEILLANCE AND INTERVENTION
In 2003, the Institute of Medicine called for lifelong risk-based health care for survivors of childhood cancer.58 Given the high risk for morbidity and mortality resulting from SMNs, the Children’s Oncology Group (COG) and others developed consensus-based surveillance guidelines for SMNs,59,60 with the goal of detecting SMNs at earlier, more treatable stages. Guideline groups worldwide have formed the International Guideline Harmonization Group (IGHG) to provide harmonized evidence-based guidelines.61
Examination of other populations at increased cancer risk have shown that, for some solid cancers, early initiation of surveillance may improve outcomes. Breast cancer surveillance guidelines60,62-64 have been prioritized, given the increased risk among survivors exposed to chest irradiation. Mammogram screening in high-risk survivors is associated with earlier breast cancer detection,65 and combination breast magnetic resonance imaging and mammogram screening in survivors exposed to chest radiotherapy before age 30 years increases the specificity and detection of invasive breast cancer and ductal carcinoma in situ, a finding that is now reflected in screening guidelines.60,62-64,66 The COG, DCOG-LATER, and the IGHG guidelines recommend that screening begin at age 25 years or 8 years after treatment, whichever occurs later. Recently, the COG has decreased the radiation exposure threshold to 10 Gy for initiating screening, consistent with the 2010 Dutch recommendations.60,62 The COG, unlike other guidelines, recommends annual colonoscopy in survivors exposed to abdominal or pelvic radiation therapy, beginning at age 35 or 10 years after radiation exposure, whichever occurs last.60 IGHG recommendations for colorectal cancer surveillance are expected in 2018. For skin cancer screening, the COG recommends yearly dermatologic examinations of the radiation field.62
Routine screening for thyroid cancer and CNS neoplasms remains controversial. Studies examining annual thyroid ultrasound surveillance suggest that a yearly physical examination is sufficient and may minimize the harm associated with overdiagnosis and overtreatment.67 For survivors exposed to neck radiation, the COG recommends ultrasound and fine needle aspiration for palpable nodules. Similarly, routine radiographic screening for meningiomas is currently not recommended.62
Screening programs are recommended for survivors with known germline cancer predisposition syndromes, such as Li-Fraumeni syndrome, Lynch syndrome, and familial retinoblastoma. According to one study, nearly 10% of survivors of childhood cancer may harbor an actionable germline genetic mutation68; thus, it is imperative that risk-based care include yearly review of family history and referral for genetic counseling for survivors with a history suggestive of a cancer predisposition syndrome.
Despite surveillance recommendations, many primary care providers are unaware of,69 and many survivors are often nonadherent with,70,71 recommended screenings. Interventions have been developed to improve awareness and adherence to screening guidelines for breast and skin cancer.72,73 Additional study is necessary to inform SMN surveillance recommendations and to improve adherence as well as survivor and provider knowledge of these recommendations.
Few primary prevention strategies are available for SMN reduction. A phase II, multicenter, randomized, placebo-controlled trial is currently evaluating the use of low-dose tamoxifen for 2 years in female patients who received ≥ 12 Gy of chest irradiation before age 40 years. Prophylactic mastectomy is also offered to women exposed to chest radiotherapy at a young age.
In conclusion, we have learned a great deal about SMN risk, risk factors, genetic predisposition, and surveillance. New therapies in clinical practice necessitate ongoing research on SMN risk; prioritization of surveillance efforts and survivor and provider education are also necessary. Improved survival and recognition of late effects, including SMNs, reinforce the need for ongoing upfront therapy modifications to moderate late health risks and to improve long-term survivor health.
AUTHOR CONTRIBUTIONS
Conception and design: Lucie M. Turcotte, Joseph P. Neglia, Tara O. Henderson
Collection and assembly of data: Lucie M. Turcotte, Raoul C. Reulen, Yutaka Yasui, Tara O. Henderson
Data analysis and interpretation: Joseph P. Neglia, Raoul C. Reulen, Cecile M. Ronckers, Flora E. van Leeuwen, Lindsay M. Morton, David C. Hodgson, Yutaka Yasui, Kevin C. Oeffinger, Tara O. Henderson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Risk, Risk Factors, and Surveillance of Subsequent Malignant Neoplasms in Survivors of Childhood Cancer: A Review
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Lucie M. Turcotte
No relationship to disclose
Joseph P. Neglia
No relationship to disclose
Raoul C. Reulen
No relationship to disclose
Cecile M. Ronckers
No relationship to disclose
Flora E. van Leeuwen
No relationship to disclose
Lindsay M. Morton
No relationship to disclose
David C. Hodgson
No relationship to disclose
Yutaka Yasui
No relationship to disclose
Kevin C. Oeffinger
No relationship to disclose
Tara O. Henderson
Research Funding: Seattle Genetics
REFERENCES
- 1.Howlader N NA, Krapcho M, Miller D, et al. (eds): SEER cancer statistics review, 1975-2014. https://seer.cancer.gov/csr/1975_2014/
- 2.Gatta G, Zigon G, Capocaccia R, et al. : Survival of European children and young adults with cancer diagnosed 1995-2002. Eur J Cancer 45:992-1005, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Armstrong GT, Chen Y, Yasui Y, et al. : Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 374:833-842, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardous-Ubbink MC, Heinen RC, Bakker PJ, et al. : Risk of second malignancies in long-term survivors of childhood cancer. Eur J Cancer 43:351-362, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Neglia JP, Friedman DL, Yasui Y, et al. : Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst 93:618-629, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Friedman DL, Whitton J, Leisenring W, et al. : Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst 102:1083-1095, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turcotte LM, Whitton JA, Friedman DL, et al. : Risk of subsequent neoplasms during the fifth and sixth decades of life in the Childhood Cancer Survivor Study Cohort. J Clin Oncol 33:3568-3575, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turcotte LM, Liu Q, Yasui Y, et al. : Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970-2015. JAMA 317:814-824, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkinson HC, Hawkins MM, Stiller CA, et al. : Long-term population-based risks of second malignant neoplasms after childhood cancer in Britain. Br J Cancer 91:1905-1910, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reulen RC, Frobisher C, Winter DL, et al. : Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA 305:2311-2319, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Olsen JH, Möller T, Anderson H, et al. : Lifelong cancer incidence in 47,697 patients treated for childhood cancer in the Nordic countries. J Natl Cancer Inst 101:806-813, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Teepen JC, van Leeuwen FE, Tissing WJ, et al. : Long-term risk of subsequent malignant neoplasms after treatment of childhood cancer in the DCOG LATER study cohort: Role of chemotherapy. J Clin Oncol 35:2288-2298, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Fidler MM, Reulen RC, Winter DL, et al. : Risk of subsequent bone cancers among 69 460 five-year survivors of childhood and adolescent cancer in Europe. J Natl Cancer Inst 10.1093/jnci/djx165 [epub ahead of print on February 1, 2018] [DOI] [PubMed] [Google Scholar]
- 14.Hjorth L, Haupt R, Skinner R, et al. : Survivorship after childhood cancer: PanCare: A European Network to promote optimal long-term care. Eur J Cancer 51:1203-1211, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inskip PD, Sigurdson AJ, Veiga L, et al. : Radiation-related new primary solid cancers in the Childhood Cancer Survivor Study: Comparative radiation dose response and modification of treatment effects. Int J Radiat Oncol Biol Phys 94:800-807, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stovall M, Weathers R, Kasper C, et al. : Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res 166:141-157, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Neglia JP, Robison LL, Stovall M, et al. : New primary neoplasms of the central nervous system in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 98:1528-1537, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Taylor AJ, Little MP, Winter DL, et al. : Population-based risks of CNS tumors in survivors of childhood cancer: The British Childhood Cancer Survivor Study. J Clin Oncol 28:5287-5293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boukheris H, Stovall M, Gilbert ES, et al. : Risk of salivary gland cancer after childhood cancer: A report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys 85:776-783, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veiga LH, Lubin JH, Anderson H, et al. : A pooled analysis of thyroid cancer incidence following radiotherapy for childhood cancer. Radiat Res 178:365-376, 2012 [Erratum: Radiat Res 180:e41, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubin JH, Adams MJ, Shore R, et al. : Thyroid cancer following childhood low-dose radiation exposure: A pooled analysis of nine cohorts. J Clin Endocrinol Metab 102:2575-2583, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaapveld M, Aleman BM, van Eggermond AM, et al. : Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med 373:2499-2511, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Travis LB, Gospodarowicz M, Curtis RE, et al. : Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst 94:182-192, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Moskowitz CS, Chou JF, Wolden SL, et al. : Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol 32:2217-2223, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inskip PD, Robison LL, Stovall M, et al. : Radiation dose and breast cancer risk in the Childhood Cancer Survivor Study. J Clin Oncol 27:3901-3907, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskowitz CS, Chou JF, Sklar CA, et al. : Radiation-associated breast cancer and gonadal hormone exposure: A report from the Childhood Cancer Survivor Study. Br J Cancer 117:290-299, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson TO, Oeffinger KC, Whitton J, et al. : Secondary gastrointestinal cancer in childhood cancer survivors: A cohort study. Ann Intern Med 156:757-766, W-260, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nottage K, McFarlane J, Krasin MJ, et al. : Secondary colorectal carcinoma after childhood cancer. J Clin Oncol 30:2552-2558, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Tukenova M, Diallo I, Anderson H, et al. : Second malignant neoplasms in digestive organs after childhood cancer: A cohort-nested case-control study. Int J Radiat Oncol Biol Phys 82:e383-e390, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Berrington de Gonzalez A, Kutsenko A, Rajaraman P: Sarcoma risk after radiation exposure. Clin Sarcoma Res 2:18, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watt TC, Inskip PD, Stratton K, et al. : Radiation-related risk of basal cell carcinoma: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 104:1240-1250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bekelman JE, Schultheiss T, Berrington De Gonzalez A: Subsequent malignancies after photon versus proton radiation therapy. Int J Radiat Oncol Biol Phys 87:10-12, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Travis LB, Demark Wahnefried W, Allan JM, et al. : Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol 10:289-301, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Hawkins MM, Wilson LM, Stovall MA, et al. : Epipodophyllotoxins, alkylating agents, and radiation and risk of secondary leukaemia after childhood cancer. BMJ 304:951-958, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Deley MC, Leblanc T, Shamsaldin A, et al. : Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: A case-control study by the Société Française d’Oncologie Pédiatrique. J Clin Oncol 21:1074-1081, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Pui CH, Ribeiro RC, Hancock ML, et al. : Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N Engl J Med 325:1682-1687, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Pendleton M, Lindsey RH, Jr., Felix CA, et al. : Topoisomerase II and leukemia. Ann N Y Acad Sci 1310:98-110, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkins MM, Wilson LM, Burton HS, et al. : Radiotherapy, alkylating agents, and risk of bone cancer after childhood cancer. J Natl Cancer Inst 88:270-278, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Morton LM, Dores GM, Curtis RE, et al. : Stomach cancer risk after treatment for Hodgkin lymphoma. J Clin Oncol 31:3369-3377, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson TO, Oeffinger KC, Whitton J, et al. : Secondary gastrointestinal cancer in childhood cancer survivors: A cohort study. Ann Intern Med 156:757-766, W-260, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson TO, Moskowitz CS, Chou JF, et al. : Breast cancer risk in childhood cancer survivors without a history of chest radiotherapy: A report from the Childhood Cancer Survivor Study. J Clin Oncol 34:910-918, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tucker MA, D’Angio GJ, Boice JD, Jr., et al. : Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med 317:588-593, 1987 [DOI] [PubMed] [Google Scholar]
- 43.Veiga LHS, Bhatti P, Ronckers CM, et al. : Chemotherapy and thyroid cancer risk: A report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev 21:92-101, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henderson TO, Rajaraman P, Stovall M, et al. : Risk factors associated with secondary sarcomas in childhood cancer survivors: A report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys 84:224-230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swerdlow AJ, Cooke R, Bates A, et al. : Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin’s lymphoma in England and Wales: A National Cohort Study. J Clin Oncol 30:2745-2752, 2012 [DOI] [PubMed] [Google Scholar]
- 46.De Bruin ML, Sparidans J, van’t Veer MB, et al. : Breast cancer risk in female survivors of Hodgkin’s lymphoma: Lower risk after smaller radiation volumes. J Clin Oncol 27:4239-4246, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Cooke R, Jones ME, Cunningham D, et al. : Breast cancer risk following Hodgkin lymphoma radiotherapy in relation to menstrual and reproductive factors. Br J Cancer 108:2399-2406, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krul IM, Opstal-van Winden AWJ, Aleman BMP, et al. : Breast cancer risk after radiation therapy for Hodgkin lymphoma: Influence of gonadal hormone exposure. Int J Radiat Oncol Biol Phys 99:843-853, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Kenney LB, Yasui Y, Inskip PD, et al. : Breast cancer after childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Intern Med 141:590-597, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Lindor NM, McMaster ML, Lindor CJ, et al. Concise handbook of familial cancer susceptibility syndromes, 2nd ed. J Natl Cancer Inst Monogr 38:1-93 2008 [DOI] [PubMed] [Google Scholar]
- 51.Kratz CP, Achatz MI, Brugières L, et al. : Cancer screening recommendations for individuals with Li-Fraumeni syndrome. Clin Cancer Res 23:e38-e45, 2017 [DOI] [PubMed] [Google Scholar]
- 52.Barnett GC, West CM, Dunning AM, et al. : Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat Rev Cancer 9:134-142, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.West CM, Barnett GC: Genetics and genomics of radiotherapy toxicity: Towards prediction. Genome Med 3:52, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhatia S: Genetic variation as a modifier of association between therapeutic exposure and subsequent malignant neoplasms in cancer survivors. Cancer 121:648-663, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knight JA, Skol AD, Shinde A, et al. : Genome-wide association study to identify novel loci associated with therapy-related myeloid leukemia susceptibility. Blood 113:5575-5582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Best T, Li D, Skol AD, et al. : Variants at 6q21 implicate PRDM1 in the etiology of therapy-induced second malignancies after Hodgkin’s lymphoma. Nat Med 17:941-943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morton LM, Sampson JN, Armstrong GT, et al. : Genome-wide association study to identify susceptibility loci that modify radiation-related risk for breast cancer after childhood cancer. J Natl Cancer Inst 10.1093/jnci/djx058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hewitt M, Weiner SL, Simone JV. (eds): Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC, The National Academies Press, 2003 [PubMed] [Google Scholar]
- 59. Skinner R, Wallace WHB, Levitt GA (eds): Therapy-based long-term follow-up: practice statement. https://www.uhb.nhs.uk/Downloads/pdf/CancerPbTherapyBasedLongTermFollowUp.pdf.
- 60. Dutch Childhood Oncology Group/SKION: Guidelines for follow-up in survivors of childhood cancer 5 years after diagnosis. https://www.skion.nl/workspace/uploads/vertaling-richtlijn-LATER-versie-final-okt-2014_2.pdf.
- 61.Kremer LC, Mulder RL, Oeffinger KC, et al. : A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer 60:543-549, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. http://www.survivorshipguidelines.org/pdf/ltfuguidelines.pdf CureSearch Children’s Oncology Group: Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 4.0. [PubMed]
- 63.Saslow D, Boetes C, Burke W, et al. : American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 57:75-89, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Mulder RL, Kremer LC, Hudson MM, et al. : Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 14:e621-e629, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diller L, Medeiros Nancarrow C, Shaffer K, et al. : Breast cancer screening in women previously treated for Hodgkin’s disease: A prospective cohort study. J Clin Oncol 20:2085-2091, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Ng AK, Garber JE, Diller LR, et al. : Prospective study of the efficacy of breast magnetic resonance imaging and mammographic screening in survivors of Hodgkin lymphoma. J Clin Oncol 31:2282-2288, 2013 [DOI] [PubMed] [Google Scholar]
- 67.Tonorezos ES, Barnea D, Moskowitz CS, et al. : Screening for thyroid cancer in survivors of childhood and young adult cancer treated with neck radiation. J Cancer Surviv 11:302-308, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang J, Walsh MF, Wu G, et al. : Germline mutations in predisposition genes in pediatric cancer. N Engl J Med 373:2336-2346, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nathan PC, Daugherty CK, Wroblewski KE, et al. : Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv 7:275-282, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Nathan PC, Ness KK, Mahoney MC, et al. : Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: A report from the childhood cancer survivor study. Ann Intern Med 153:442-451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oeffinger KC, Ford JS, Moskowitz CS, et al. : Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA 301:404-414, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oeffinger KC, Ford JS, Moskowitz CS, et al. : The EMPOWER study: Promoting breast cancer screening—A randomized controlled trial (RCT) in the Childhood Cancer Survivor Study (CCSS). J Clin Oncol 34, 2016. (suppl; abstr 10506) [Google Scholar]
- 73.Daniel CL, Armstrong GT, Keske RR, et al. : Advancing Survivors’ Knowledge (ASK) about skin cancer study: Study protocol for a randomized controlled trial. Trials 16:109, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]