Abstract
Purpose
Limited data exist on the molecular biology, treatment, and outcomes of breast cancer in men, and much of our understanding in this area remains largely an extrapolation from data in women with breast cancer.
Materials and Methods
We studied men and women with hormone receptor–positive breast cancer and the 21-gene Breast Recurrence Score (RS) results. Differences in clinical characteristics and gene expression were determined, and distribution of RS results was correlated with 5-year breast cancer–specific survival (BCSS) and overall survival.
Results
There were 3,806 men and 571,115 women. Men were older than women (mean age, 64.2 v 59.1 years; P < .001). RS < 18 predominated in both genders, but RS ≥ 31 was more frequent in men (12.4% v 7.4%; P < .001), as were very low scores (RS < 11; 33.8% v 22.1%; P < .001). Mean gene expression was higher in men for the estrogen receptor (ER), proliferation, and invasion groups. ER was lowest and progesterone receptor was highest in women younger than 50 years of age, with a progressive increase in ER with age. Men younger than 50 years of age had slightly lower ER and progesterone receptor compared with older men. Survival data were available from SEER for 322 men and 55,842 women. Five-year BCSS was 99.0% (95% CI, 99.3% to 99.9%) and 95.9% (95% CI, 87.6% to 98.7%) for men with RS < 18 and RS 18-30, respectively, and for women, it was 99.5% (95% CI, 99.4% to 99.6%) and 98.6% (95% CI, 98.4% to 98.8%), respectively. RS ≥ 31 was associated with an 81.0% 5-year BCSS in men (95% CI, 53.3% to 93.2%) and 94.9% 5-year BCSS (95% CI, 93.9% to 95.7%) in women. Five-year BCSS and overall survival were lower in men than in women.
Conclusion
This study reveals some distinctive biologic features of breast cancer in men and an important prognostic role for RS testing in both men and women.
INTRODUCTION
Breast cancer is uncommon in men, with an estimated 2,600 patients diagnosed in the United States in 2016,1 accounting for 1% of all new patients with breast cancer. For unclear reasons, the incidence of breast cancer in men seems to be increasing.2,3 Approximately 95% of breast cancers diagnosed in men express the estrogen receptor (ER) and progesterone receptor (PR), which is a higher percentage than in women4 and suggests a key role for ER in the biology of breast cancer in men. So far, much of our understanding of breast cancer in men is extrapolated from knowledge of breast cancer in women, although recent data suggest that there are some potential distinctions.5 Additional study of the molecular characteristics of breast cancer in men is therefore necessary to better understand this disease.
The 21-gene Breast Recurrence Score (RS) test provides individualized estimates of distant recurrence risk and helps predict benefit from adjuvant chemotherapy in women with ER-positive breast cancer.6-8 We studied the 21-gene RS assay in men with ER-positive breast cancer and compared the results with those for women, and evaluated breast cancer–specific survival (BCSS) and overall survival (OS) from the SEER Program.
MATERIALS AND METHODS
Study Population
Men and women with breast cancer specimens submitted for Genomic Health 21-gene RS testing in North America between June 2004 and January 2017 were identified. The de-identified data set of patients with reported RS results and ER-positive and/or PR-positive and human epidermal growth factor receptor 2 (HER2)–negative disease, as defined by quantitative single-gene scores determined by the 21-gene assay, included 3,806 men and 571,115 women with negative nodal involvement (N0, including isolated tumor cells), micrometastases only (N1mi), and involvement of one to three lymph nodes (1-3LN; Appendix Fig A1, online only). Gene expression was quantified for each of the 21 genes included in the assay by reverse transcription polymerase chain reaction on a scale from 0 to 15 relative to reference genes, where a one-unit increment was associated with a two-fold change in expression. The assay was conducted on RNA extracted from formalin-fixed paraffin-embedded tumors. The RS ranges from 0 to 100. Tumor histology was recorded by Genomic Health pathologists reviewing the submitted patient samples according to WHO criteria. The Genomic Health Laboratory database is updated and maintained according to regulations and internal operating procedures.
For the analysis of outcomes, the National Cancer Institute’s SEER population of patients with breast cancer diagnosed between 2004 and 2012 was linked to the 21-gene RS results from the Genomic Health Laboratory (2004 to 2013) by a third party, Information Management Services (IMS; Calverton, MD; Appendix Fig A1). IMS met Federal Information Security Act standards for data security and individual SEER registries information technology security requirements. RS is a required data element for cancer surveillance. Manual collection by cancer registrars is suboptimal (incomplete and time consuming). The linkage ensured data on population level and vastly increased the effectiveness of data collection. IMS released the de-identified, linked data set to the study team for analyses after SEER approval.9 Overall, the linkage success rate was high.
The SEER study population included 322 men and 55,842 women from all the individual registries with ER- and/or PR-positive invasive breast cancer as defined by both the SEER-reported ER and PR (positive or borderline) immunohistochemistry results and 21-gene assay quantitative ER and PR score. Patients were excluded if they had any of the following characteristics: (1) had distant metastases at diagnosis, (2) were affected by Hurricane Katrina, (3) were older than 99 years of age at diagnosis, (4) had zero years of survival time, (5) had autopsy data only, or (6) were without a state death certificate. Patients with HER2-positive disease as determined by the single-gene HER2 score of the 21-gene assay were excluded. HER2 status was unavailable in SEER before 2010. The smaller number of patients in the SEER study population is attributed to three factors: (1) SEER constitutes only 30% of the US population; (2) RS was ordered on fewer than half of the patients, especially in the earlier years; and (3) the requirement for more than 1 year of survival follow-up.
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics as well as gene expression and RS distribution. The χ2 test was used to compare categorical characteristics, and Student t test was used to compare continuous variables between genders.
Five-year BCSS was calculated using cause of death data in the SEER registry, which is derived from linkage with state death certificates and the National Center for Health Statistics’ National Death Index.10 These analyses used a variable derived by the SEER program that uses a mapping to dichotomize causes of death as breast cancer specific or other-cause specific. In the estimates of BCSS, outcome is censored at the time of other-cause deaths or last known follow-up. OS was estimated by including all deaths as events regardless of cause.
Survival analysis was performed on eligible patients and was stratified by the 21-gene RS cut points: RS < 18, RS 18-30, or RS ≥ 31, and by lymph node status. Actuarial estimates of survival and 95% CIs were calculated through 5 years. A two-degrees-of-freedom log-rank test was used to determine differences across the three categorical RS groups, and CIs were computed using the log-log transformation.
All analyses were performed using SAS, Version 9.4 (SAS Institute, Cary, NC). All statistical tests were two sided, with a .05 level of significance. Analyses were performed on de-identified Genomic Health data with institutional review board approval (Aspire Institutional Review Board, Santee, CA).
RESULTS
Distribution of Age, Histologic Subtypes, and Nodal Status
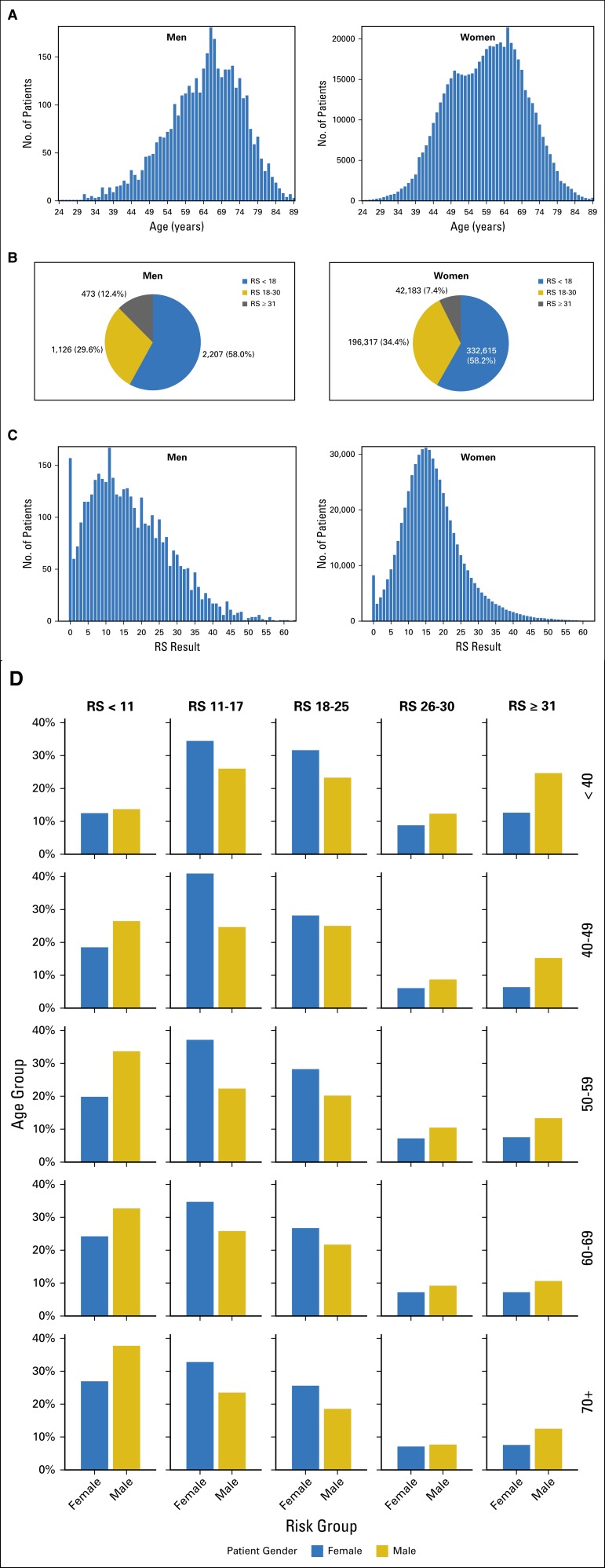
A total of 3,806 men and 571,115 women with ER-positive breast cancer and RS results were included in this analysis (Table 1). Men were older than women at the time of diagnosis (mean age, 64.2 v 59.1 years; P < .001; Table 1). Women had a second earlier peak at approximately age 50, with no similar second earlier peak in men (Fig 1A).
Table 1.
Age, Nodal Status, and Histologic Types in Men and Women With RS Testing
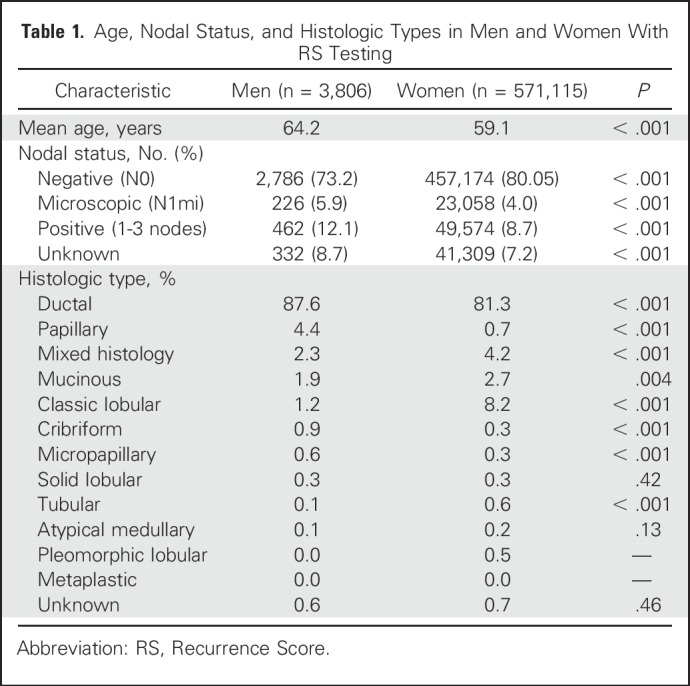
Fig 1.
Age and Recurrence Score (RS) distribution in men (n = 3,806) and women (n = 571,115). (A) The number of patients plotted on the y-axis against age on the x-axis for men (left side of graph) and women (right side of graph). (B) A pie chart for the breakdown of RS distribution in men (left side) versus women (right side), with RS < 18 in blue, RS 18-30 in gold, and RS ≥ 31 in gray. (C) The number of patients on the y-axis plotted against RS results on the x-axis for men (left side of graph) and women (right side of graph). (D) RS by age group on the y-axis against gender on the x-axis. Age groups plotted are < 40, 40 to 49, 50 to 59, 60 to 69, and ≥ 70 years, with proportion of RS ranges (RS < 11, RS 11-17, RS 18-25, RS 26-30, and RS ≥ 31) shown for each age group. Men are represented in gold and women in blue.
Infiltrating ductal carcinoma was the most common histology in both genders, but predominated in men (87.6% v 81.3%; P < .001), whereas lobular cancer was rarely encountered in men compared with women (1.2% v 8.2%; P < .001; Table 1). Papillary histology was more common in men (4.4% v 0.7%; P < .001), whereas other special subtypes were rare (Table 1). Most patients had N0 disease (73.2% of men and 80.1% of women), but lymph node involvement (1-3LN) was more common in men (12.1% v 8.7%; P < .001; Table 1).
RS Distribution
The average RS in the 3,806 men was comparable to that in the 571,115 women (16.8 v 17.0; P = .08), and most men (58.0%) and women (58.2%) had RS < 18 (Fig 1B). Significantly more men than women had high RS ≥ 31 (12.4% v 7.4%; P < .001; Fig 1B). This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age (Figs 1C and 1D). Conversely, very low RS results (RS < 11), and specifically, RS 0, were more common in men than in women, except in those younger than 40 years of age (Fig 1D). These differences in RS distribution between men and women were observed in the N0, N1mi, and 1-3LN subsets (data not shown).
Individual Gene Expression
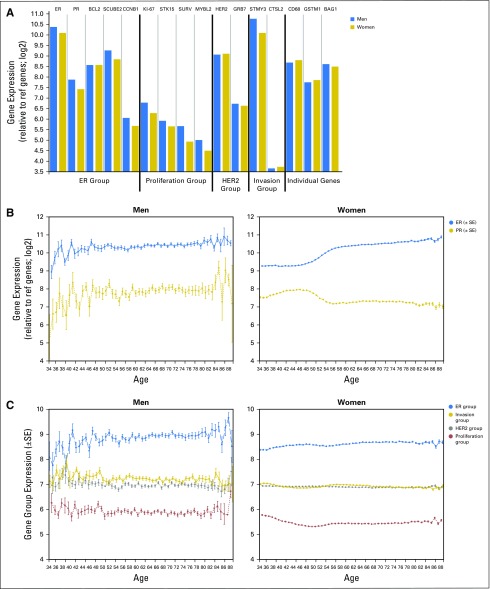
Mean quantitative gene expression was approximately 0.5 units higher (40% greater in absolute amounts) in the 3,806 men than the 571,115 women for the ER gene group, for the proliferation gene group, and for stromelysin in the invasion gene group (Fig 2A). The pattern of gene expression as a function of age, however, was different depending on the genes considered. Quantitative ER expression was relatively low in women younger than 50 years of age but increased with age (Fig 2B). PR had the reverse pattern, with higher quantitative PR expression in those younger than age 50. ER and PR were slightly lower in men younger than 50 years of age than in older men, but interpretation was limited by the smaller numbers and greater variance in the youngest (younger than 40 years) and oldest (older than 80 years) age groups (Fig 2B). Expression levels for the proliferation, overall ER, HER2, and invasion gene groups did not differ as a function of age for either men or women (Fig 2C).
Fig 2.
Individual gene expression in men (n = 3,806) and women (n = 571,115). (A) Individual gene expression levels plotted for men (blue) and women (gold). The y-axis represents fold change in expression value, and the x-axis represents the different genes and gene groups for men and women as follows from left to right: the estrogen-receptor (ER) group (ER, progesterone receptor [PR], BCL2, SCUBE2, and CCNB1); the proliferation group (Ki-67, STK15, SURV, and MYLB2); the human epidermal growth factor receptor 2 (HER2) group (HER2 and GRB7); the invasion group (stromelysin [STMY3] and CTLS2); and the additional individual genes (CD68, GSTM1, and BAG1). Each group is separated by a vertical black line. (B) Individual ER and PR expression for men (left side of graph) and women (right side of graph). The y-axis represents fold change in expression value (mean ± SE), and x-axis represents age. ER is in blue and PR is in gold. (C) The mean quantitative expression of the different gene groups (axes): proliferation group (red),HER2 group (gray), ER group (blue), and invasion group (gold). The y-axis represents fold change in expression (mean ± SE), and the x-axis represents age.
BCSS and OS
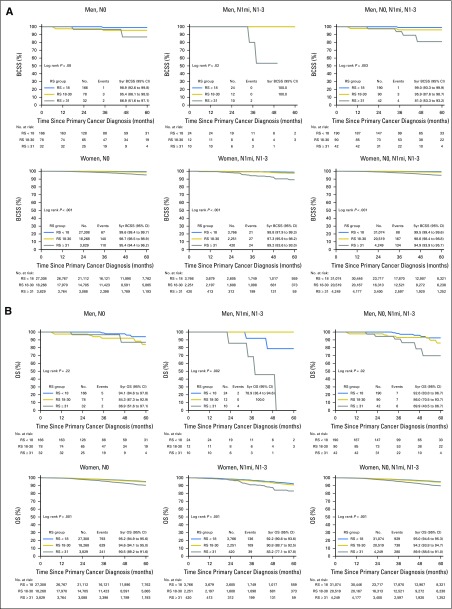
Survival data from SEER was available for 322 men and 55,842 women, and there was a wide range of RS results in both genders. Men had a higher proportion of larger tumors and grade 3 tumors than women (Tables 2 and 3). Figure 3A shows the 5-year BCSS for both men and women by nodal status and RS groups. Although the number of events in men with breast cancer was relatively small, estimates for BCSS differed significantly across risk groups, regardless of nodal status (P = .003 in the overall group). Five-year BCSS was 99.0% (95% CI, 99.3% to 99.9%) and 95.9% (95% CI, 87.6% to 98.7%) in men with RS < 18 and RS 18-30, respectively, and 81.0% in men with RS ≥ 31 (95% CI, 53.3% to 93.2%). In women, the RS < 18 and RS 18-30 groups had largely similar 5-year BCSS of 99.5% (95% CI, 99.4% to 99.6%) and 98.6% (95% CI, 98.4% to 98.8%), respectively (P < .001), whereas the 5-year BCSS in the RS ≥ 31 group was 94.9% (95% CI, 93.9% to 95.7%), better than that observed in men (Fig 3A). The lower BCSS in men and women with RS ≥ 31 was observed despite the more prevalent use of chemotherapy in this highest risk group (Tables 2 and 3).
Table 2.
Tumor Size, Grade, and Reported Chemotherapy Use by Nodal Status and RS Group in Men Included in the Survival Analysis
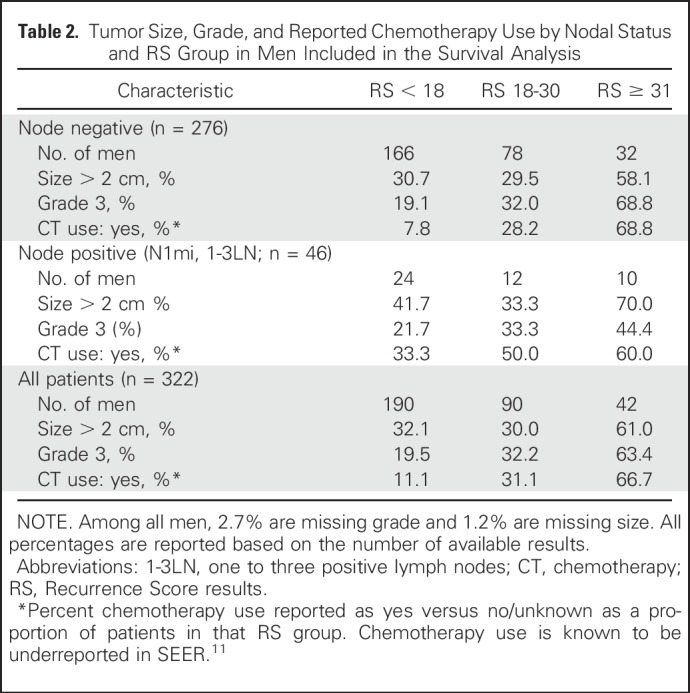
Table 3.
Tumor Size, Grade, and Reported Chemotherapy Use by Nodal Status and RS Group in Women in the Survival Analysis
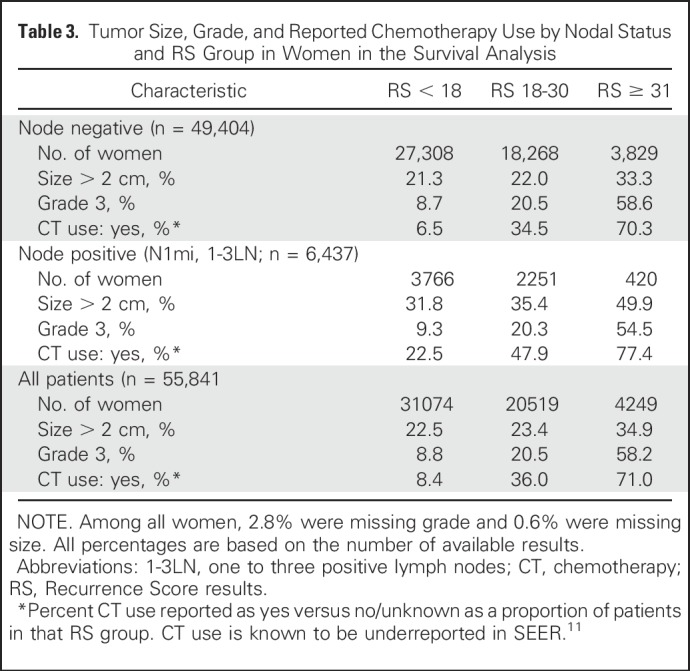
Fig 3.
Breast cancer-specific survival (BCSS) and overall survival (OS) by Recurrence Score (RS) group and nodal status in men (n = 322) and women (n = 55,842). (A) BCSS (y-axis) plotted against time in months from cancer diagnosis (x-axis) according to RS and nodal status in men (top panel) versus women (lower panel). Left-side graphs are for N0, middle graphs are for N1mi and N1-3, and right-side graphs are for all nodal groups together (N0, N1mi, and N1-3). (B) Overall survival (y-axis) plotted against RS and nodal status in men (top panel) versus women (lower panel). Left-side graphs are for N0, middle graphs are for N1mi and N1-3, and right-side graphs are for all nodal groups together (N0, N1mi, and N1-3). RS < 18 (blue), RS 18-30 (gold), and RS ≥ 31 (gray). Log rank P values for each survival graph are shown.
OS estimates were significantly different between RS groups, regardless of nodal status for both men and women (P = .02 and < .001, respectively; Fig 3B). The 5-year OS was 92.6% and 86.0% for men with RS < 18 and RS 18-30, respectively, compared with 69.9% for men in the RS ≥ 31 group. For women, the 5-year OS was 95% and 94.2% in the RS < 18 and RS 18-30 groups, respectively, compared with 89.9% in the RS ≥ 31 group (Fig 3B).
DISCUSSION
In this large genomic study, we characterized the molecular features of breast cancer in men versus women using the 21-gene RS assay and correlated our findings with clinical characteristics and with disease survival in the subset of patients with available data from the SEER database. Men with breast cancer were older than women, which may reflect a second peak at diagnosis at approximately 50 years of age in women and no similar earlier peak in men. The difference for this age distribution is unclear, but may reflect the distinct physiologic milieu of the estrogen environment in women related to menopausal status and no equivalent state in men. The use of screening mammography in women starting at 40 years of age may also be a contributing factor.12 Interestingly, there is evidence of increased breast cancer incidence in both women and men in recent decades, although the increase in men has been reported primarily in men older than 50 years.1,3 This suggests that in younger men, a more constant etiologic factor, such as genetics, may predominate, whereas in women and older men, other etiologies might be at play to explain the increasing incidence, such as yet-unknown environmental factors common to both sexes.
A larger proportion of men than women had RS ≥ 31, particularly men younger than 40 years of age. Although a larger proportion of men than women ≥ 60 years of age had RS < 11, including RS 0, a similar proportion of men and women younger than 40 years of age had very low RS results (Fig 1D). These observed differences in RS distribution suggest that men may have more biologically distinct ER-positive disease subtypes that can be defined by RS results: a very low RS disease subtype in older men and a high RS disease subtype in younger men. BRCA2 mutations, which occur in 4% to 16% of men with breast cancer,13 may help explain the observed RS distribution with age in our study and suggest that BRCA2-associated breast cancer may be a distinct entity in younger men.14 This is supported by the increased frequency of a biologically more aggressive phenotype for BRCA2-associated breast cancer in general, characterized by high tumor grade and stage, as well as the association with younger age at diagnosis, compared with patients without BRCA2 mutation.15-17 Recent studies have also shown that women with breast cancer and BRCA mutations have a much higher proportion of RS results ≥ 31,18-20 and on the basis of our data, future studies should next examine the association between BRCA mutations and RS results in men with breast cancer. Other genetic mutations seem to be associated with breast cancer in men such as CHEK2 and PALB2, but not BRCA1, which seems to be infrequently encountered in men compared with women.16,21 With increasing age, breast cancer in men starts resembling breast cancer in older women and may reflect increasing independence from genetic risk factors.22
Confirming prior observations, the most common breast cancer histology in men is ductal carcinoma, which primarily reflects the smaller proportion of lobular carcinoma in men.12,23 For yet unclear reasons, papillary histology seems to be more common in men than in women, in keeping with prior observations.23 Other histologic subtypes are exceedingly rare in men.
Mean gene expression levels were higher in men than in women for both the ER and proliferation gene groups, with higher expression of stromelysin from the invasion group in men. The mean expression of HER2 and GRB7 were similar between men and women, which reflects the exclusion of HER2-positive tumors. Interestingly, the pattern of ER and PR expression as a function of age differed between the two genders. ER was lowest in younger women, whereas PR was highest in this group. After 50 years of age, however, PR expression decreased and stayed relatively constant, whereas ER expression continued to increase with age. Men younger than 50 years of age had slightly lower ER and PR expression compared with older men. Higher PR expression in younger women may reflect the premenopausal hormonal milieu and is similar to prior observations in younger women with ER-positive/PR-positive breast cancer, compared with women with ER-positive/PR-negative disease.24 The exclusion of HER2-positive breast cancer from this cohort might have affected the trends in ER because of the known reciprocal relationship between hormone receptor expression and HER2.25
The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women. Patients with RS < 18 and RS 18-30 have an excellent prognosis regardless of nodal status, suggesting indolent disease biology and better outcomes than the RS ≥ 31 group. The number of events in men was small, but the prognostic impact of RS ≥ 31 was evident for both men and women. Because chemotherapy use was more often reported as yes in the RS ≥ 31 group than in the RS < 18 and RS 18-30 groups, the prognostic utility of RS results is evident despite adjuvant chemotherapy use (Tables 2 and 3).
In men with breast cancer, RS < 18 and RS 18-30 were associated with an OS of 92.6% and 86.0%, respectively, with a significant decrease to 69.9% in the RS ≥ 31 group. Furthermore, BCSS was 81.0% (95% CI 53.3% to 93.2%) for men with RS equal to or >31, whereas for women with RS of equal to or >31 it was 94.9% (95% CI 93.9% to 95.7%). The numbers in men are small, but it seems that women do better than men in the RS ≥ 31 group. The explanation for this finding is not clear, but could possibly be related to excess use of aromatase inhibitors (AIs) in men instead of tamoxifen on the basis of a perception of superiority extrapolating from data in women.26 Tamoxifen is associated with improved survival in men with breast cancer,26 whereas AI use was inferior to tamoxifen in the adjuvant setting.30 The inferiority of AIs versus tamoxifen in men may be related to gonadal physiology resembling premenopausal women, which would dictate the need for gonadal suppression should an AI be used in men.27 It is important to note that tamoxifen should be used as the standard adjuvant endocrine therapy for men with breast cancer, and the use of AIs should be discouraged until more data become available. Another possible explanation for the inferior BCSS in men compared with women in the RS ≥ 31 group may be related to rates of adherence to tamoxifen in men, although data are limited.28
Not surprisingly, men have lower 5-year OS than women, which reflects their older age at diagnosis, and more competing causes of mortality. This higher all-cause mortality observed in men with breast cancer is similar to prior observations.29
It is important to note that there are limitations to our study. First, although these data focus on men and women with hormone receptor–positive/HER2-negative breast cancer, we want to emphasize that this does not reflect the whole population with this disease because RS testing was only ordered, on average, in fewer than half of the patients, with less frequent testing in the earlier years. Patterns of testing in men versus women may also be variable because there are no clear guidelines for testing in men. Other limitations are that SEER constitutes 30% of the US population, and recurrence events are not recorded, only mortality. Furthermore, adjuvant systemic therapy data in SEER are limited, particularly on endocrine therapy, but also details of chemotherapy regimens used. Finally, there are, to date, a small number of events in men with breast cancer with relatively short-term follow-up in the lifetime of ER-positive breast cancer. Longer-term follow-up of these data are needed.
In conclusion, this is a large genomic study that provides new insights into the molecular biology, treatment, and outcomes of breast cancer in men and women. In addition to the biologic distinctions observed for breast cancer in men versus women, our study shows that both men and women with lower RS results have low mortality from ER-positive breast cancer, and many can be spared the risks associated with overtreatment, particularly from chemotherapy. Although there are limitations to our study, it may be appropriate to limit the use of chemotherapy in appropriately selected patients with RS 0-30, including those with 1-3LN. Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS ≥ 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy.
Appendix
Fig A1.
Genomic Health Laboratory data were merged with SEER registry data, resulting in patients diagnosed between 2004 and 2013, with and without Recurrence Score (RS) testing. Final analytic cohorts are represented in shaded boxes: Genomic Health Laboratory cohort (n = 574,921) and SEER cohort with RS results (n = 56,164). 0-3LN, zero to three positive lymph nodes; HER2, human epidermal growth factor receptor 2; HR, hormone receptor.
AUTHOR CONTRIBUTIONS
Conception and design: Suleiman Alfred Massarweh, George W. Sledge, Steven Shak
Collection and assembly of data: Suleiman Alfred Massarweh, Debbie McCullough, Steven Shak
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Molecular Characterization and Mortality From Breast Cancer in Men
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Suleiman Alfred Massarweh
Stock or Other Ownership: Radius Health
Consulting or Advisory Role: Novartis
Research Funding: Novartis (Inst), Bayer/Onyx (Inst), Novartis (Inst), Genentech/Roche (Inst), Gilead Sciences (Inst)
George W. Sledge
Leadership: Syndax
Stock or Other Ownership: Syndax
Honoraria: Symphogen
Consulting or Advisory Role: Symphogen, Coherus Biosciences, Radius Health, Peregrine Pharmaceuticals, Taiho Pharmaceutical
Research Funding: Genentech/Roche (Inst)
Travel, Accommodations, Expenses: Radius Health, Taiho Pharmaceutical
Dave P. Miller
Employment: Genomic Health, Verily Life Sciences
Stock or Other Ownership: Genomic Health, Verily Life Sciences
Debbie McCullough
Employment: Genomic Health
Stock or Other Ownership: Genomic Health
Valentina I. Petkov
No relationship to disclose
Steven Shak
Employment: Genomic Health
Leadership: Genomic Health
Stock or Other Ownership: Genomic Health
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2016. CA Cancer J Clin 66:7-30, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Anderson WF, Jatoi I, Tse J, et al. : Male breast cancer: A population-based comparison with female breast cancer. J Clin Oncol 28:232-239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreiter E, Richardson A, Potter J, et al. : Breast cancer: Trends in international incidence in men and women. Br J Cancer 110:1891-1897, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavez-Macgregor M, Clarke CA, Lichtensztajn D, et al. : Male breast cancer according to tumor subtype and race: A population-based study. Cancer 119:1611-1617, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piscuoglio S, Ng CK, Murray MP, et al. : The genomic landscape of male breast cancers. Clin Cancer Res 22:4045-4056, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paik S, Tang G, Shak S, et al. : Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726-3734, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Paik S, Shak S, Tang G, et al. : A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817-2826, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Albain KS, Barlow WE, Shak S, et al. : Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol 11:55-65, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petkov VI, Miller DP, Howlader N, et al. : Breast-cancer-specific mortality in patients treated based on the 21-gene assay: A SEER population-based study. NPJ Breast Cancer 2:16017, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howlader N, Ries LA, Mariotto AB, et al. : Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst 102:1584-1598, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noone AM, Lund JL, Mariotto A, et al. : Comparison of SEER treatment data with Medicare claims. Med Care 54:e55-e64, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massarweh SA, Sledge GW: Breast cancer in men. Textbook of Uncommon Cancer. Hoboken, NJ, John Wiley & Sons, 2017, pp. 364-376. [Google Scholar]
- 13.Johansen Taber KA, Morisy LR, Osbahr AJ, III, et al. : Male breast cancer: Risk factors, diagnosis, and management (Review). Oncol Rep 24:1115-1120, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Ding YC, Steele L, Kuan CJ, et al. : Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Res Treat 126:771-778, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvestri V, Barrowdale D, Mulligan AM, et al. : Male breast cancer in BRCA1 and BRCA2 mutation carriers: Pathology data from the Consortium of Investigators of Modifiers of BRCA1/2. Breast Cancer Res 18:15, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ottini L, Silvestri V, Rizzolo P, et al. : Clinical and pathologic characteristics of BRCA-positive and BRCA-negative male breast cancer patients: Results from a collaborative multicenter study in Italy. Breast Cancer Res Treat 134:411-418, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Tai YC, Domchek S, Parmigiani G, et al. : Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst 99:1811-1814, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah PD, Patil S, Dickler MN, et al. : Twenty-one-gene recurrence score assay in BRCA-associated versus sporadic breast cancers: Differences based on germline mutation status. Cancer 122:1178-1184, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Halpern N, Sonnenblick A, Uziely B, et al. : Oncotype Dx recurrence score among BRCA1/2 germline mutation carriers with hormone receptors positive breast cancer. Int J Cancer 140:2145-2149, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Lewin R, Sulkes A, Shochat T, et al. : Oncotype-DX recurrence score distribution in breast cancer patients with BRCA1/2 mutations. Breast Cancer Res Treat 157:511-516, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Pritzlaff M, Summerour P, McFarland R, et al. : Male breast cancer in a multi-gene panel testing cohort: Insights and unexpected results. Breast Cancer Res Treat 161:575-586, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diab SG, Elledge RM, Clark GM: Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 92:550-556, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Giordano SH, Cohen DS, Buzdar AU, et al. : Breast carcinoma in men: A population-based study. Cancer 101:51-57, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Alqaisi A, Chen L, Romond E, et al. : Impact of estrogen receptor (ER) and human epidermal growth factor receptor-2 (HER2) co-expression on breast cancer disease characteristics: Implications for tumor biology and research. Breast Cancer Res Treat 148:437-444, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Konecny G, Pauletti G, Pegram M, et al. : Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst 95:142-153, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Harlan LC, Zujewski JA, Goodman MT, et al. : Breast cancer in men in the United States: A population-based study of diagnosis, treatment, and survival. Cancer 116:3558-3568, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagani O, Regan MM, Walley BA, et al. : Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 371:107-118, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anelli TF, Anelli A, Tran KN, et al. : Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer 74:74-77, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Gnerlich JL, Deshpande AD, Jeffe DB, et al. : Poorer survival outcomes for male breast cancer compared with female breast cancer may be attributable to in-stage migration. Ann Surg Oncol 18:1837-1844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eggeman H, Ignator A, Smith BJ, et al. : Adjuvant therapy with tamoxifen compared to aromatase inhibitors for 257 male breast cancer patients. Breast Cancer Research and Treatment 137:465-70, 2013 [DOI] [PubMed] [Google Scholar]