Abstract
Purpose
DNX-2401 (Delta-24-RGD; tasadenoturev) is a tumor-selective, replication-competent oncolytic adenovirus. Preclinical studies demonstrated antiglioma efficacy, but the effects and mechanisms of action have not been evaluated in patients.
Methods
A phase I, dose-escalation, biologic-end-point clinical trial of DNX-2401 was conducted in 37 patients with recurrent malignant glioma. Patients received a single intratumoral injection of DNX-2401 into biopsy-confirmed recurrent tumor to evaluate safety and response across eight dose levels (group A). To investigate the mechanism of action, a second group of patients (group B) underwent intratumoral injection through a permanently implanted catheter, followed 14 days later by en bloc resection to acquire post-treatment specimens.
Results
In group A (n = 25), 20% of patients survived > 3 years from treatment, and three patients had a ≥ 95% reduction in the enhancing tumor (12%), with all three of these dramatic responses resulting in > 3 years of progression-free survival from the time of treatment. Analyses of post-treatment surgical specimens (group B, n = 12) showed that DNX-2401 replicates and spreads within the tumor, documenting direct virus-induced oncolysis in patients. In addition to radiographic signs of inflammation, histopathologic examination of immune markers in post-treatment specimens showed tumor infiltration by CD8+ and T-bet+ cells, and transmembrane immunoglobulin mucin-3 downregulation after treatment. Analyses of patient-derived cell lines for damage-associated molecular patterns revealed induction of immunogenic cell death in tumor cells after DNX-2401 administration.
Conclusion
Treatment with DNX-2401 resulted in dramatic responses with long-term survival in recurrent high-grade gliomas that are probably due to direct oncolytic effects of the virus followed by elicitation of an immune-mediated antiglioma response.
INTRODUCTION
Newly diagnosed glioblastoma (GBM) is treated with surgery, radiochemotherapy, and adjuvant chemotherapy, with a median survival time of approximately 15 months.1-3 Despite maximal initial therapy, tumors invariably recur, after which the median survival time is typically only 6 months, even with reirradiation, repeat surgery, or chemotherapy.1 Oncolytic adenoviruses are attractive therapeutic agents because they can kill tumor stem cells4 and induce cell death by several mechanisms, including direct lysis, expression of toxic proteins, induction of cytokines, and T-cell–mediated immunity.5,6
DNX-2401 (Delta-24-RGD; tasadenoturev) is an oncolytic adenovirus designed to be tumor selective, infectivity enhanced, and replication competent.7 Tumor selectivity is achieved by a 24-base pair deletion in the E1A gene, which renders the virus unable to replicate in normal cells that maintain a functional Rb pathway but fully replication competent in tumor cells.7,8 To enhance potency, an RGD-motif was engineered into the fiber H-loop, enabling the virus to use αvβ3 or αvβ5 integrins to enter cells.7 These integrins are typically enriched on tumor cells, including glioma stem cells.4,9,10 In preclinical models, DNX-2401 kills glioma cells by direct oncolysis and by inducing immune responses against tumor antigens, leading to sustained antitumor immunity and tumor regression.6,7,11
To determine safety, evaluate efficacy, and define the biologic effect of escalating doses of DNX-2401, we performed a phase I clinical trial with biologic end points in patients with recurrent malignant glioma. Remarkably, 20% of patients (five of 25) survived more than 3 years after a single DNX-2401 administration, and three of these five patients had > 95% reduction in tumor volumes that evolved over several months after treatment and that extended survival for > 3 years from the time of treatment. Biologic evaluations of post-treatment tumor specimens demonstrate that tumor regression induced by DNX-2401 seems to be due to the direct oncolytic effects of virus infection as well as activation of an immune-mediated antiglioma response.
METHODS
Study Design
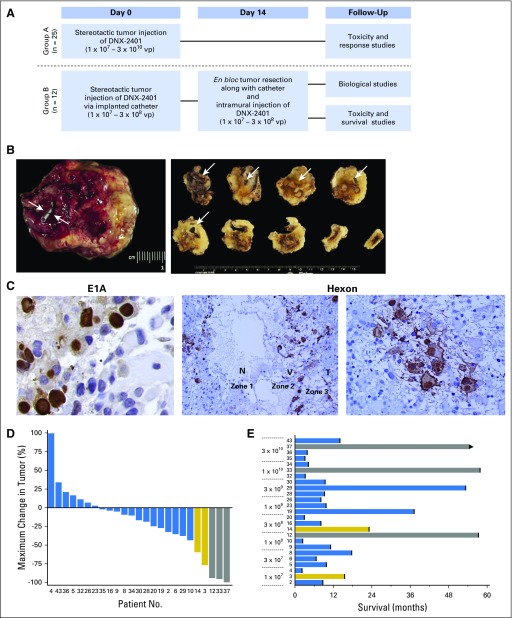
A dose-escalation trial in patients with recurrent malignant glioma was conducted. Patients were enrolled in one of two groups (Fig 1A). Group A (treatment-only group) underwent stereotactic biopsy to document recurrence, followed by a single intratumoral injection of DNX-2401 at the assigned dose through the biopsy needle into the contrast-enhancing tumor mass. Group B (treat-resect-treat group) underwent stereotactic biopsy and intratumoral injection of DNX-2401 through an implanted catheter (to mark the injection site), followed 14 days later by craniotomy with en bloc tumor resection, including the catheter, and injection of a second dose of DNX-2401 into multiple sites in the wall of the resection cavity. This approach provided a post-treatment specimen in which the injection site was marked by the catheter. Dose escalation in group A used a 3 + 3 design, starting at 1 × 107 viral particles (vp; Data Supplement). Enrollment in group B lagged behind group A, opening only after safety of the dose level was established for intratumoral injection. The study was approved by the MD Anderson Institutional Review Board.
Fig 1.
In situ replication and clinical activity of DNX-2401 in recurrent malignant glioma. (A) Schema outlining treatment strategies for group A (single injection of DNX-2401 into recurrent tumor without other therapeutic interventions to evaluate safety and efficacy), and group B (two-stage surgical design to provide post-treatment specimens for analysis). (B) Photomicrograph of en bloc resected post-treatment surgical specimen (left) obtained at open craniotomy from a patient in group B who underwent intratumoral injection of DNX-2401 14 days before this specimen was obtained. The arrows indicate the location of the proximal end of the injection catheter whose distal end extends into the center of the tumor and marks the site of DNX-2401 administration. After fixation, the en bloc specimen was blocked perpendicular to the catheter, and these blocks (right) were then sectioned and analyzed for viral replication; the arrows indicate the location of the catheter in each block, which allowed for precise identification of the site of DNX-2401 injection. (C) Photomicrographs of sections from en bloc resection specimens taken 14 days after virus injection showing immunohistochemical staining for viral E1A protein (left, ×100), which is a marker of viral infection, and for viral hexon protein (middle, ×20; right, ×40), which is a marker of replication. E1A immunostaining (left) is primarily intranuclear, as would be expected for actively infecting virus. The middle photomicrograph shows three distinct concentric zones indicative of active viral spread: a central zone of virus-induced necrosis without pseudopalisading, where the virus has infected and lysed the tumor cells leaving necrosis (N; zone 1), an intermediate zone of active viral replication as demonstrated by high hexon protein expression, which surrounds the central area of necrosis consistent with the centrifugal spread of the virus (V; zone 2), and a peripheral zone of yet-to-be-infected tumor cells (T; zone 3). The right panel shows a high-power view (×40) of tumor cells immunostaining positively for hexon protein; note the viral inclusions in the cytoplasm and the viral blebbing from the cell membrane consistent with active replication. (D) Waterfall plot showing maximal change in tumor size for all patients enrolled in group A after a single DNX-2401 treatment (n = 25). Bars represent the maximal tumor change from baseline on the basis of contrast-enhanced magnetic resonance imaging (complete responses, gray; partial responses, gold). Numbers at the bottom correspond to each patient’s identification number. (E) Bar plots showing the survival for each patient in group A by DNX-2401 dose. The patient numbers and bar colors correspond to Figure 1D. The length of the bar represents survival. The arrow indicates ongoing survival. vp, viral particles.
Patients and Study Treatment
Eligible patients were enrolled between 2009 and 2012, were older than 18 years of age with a Karnofsky performance scale score ≥ 70, and had histologically confirmed recurrent malignant glioma. Failure of standard or additional therapy was demonstrated by tumor progression as visualized on magnetic resonance imaging (MRI), and all patients underwent stereotactic biopsy before DNX-2401 administration (Data Supplement). DNX-2401 was manufactured at the Biopharmaceutical Development Program at SAIC-Frederick (Frederick, Maryland; Data Supplement).
Study Evaluations
Medical history, MRI, blood counts, and chemistry values were documented at screening and follow-ups (Data Supplement). Adverse events (AEs) were graded using Common Terminology Criteria for Adverse Events (version 3), and the relationship to DNX-2401 was assessed. Dose-limiting toxicity was defined as any DNX-2401–related, nonhematologic AE ≥ grade 3. Tumor response was based on Macdonald criteria, with the modification that (1) increases in tumor size (contrast-enhancing volume) were allowed within the first 3 to 4 months to account for potential immune-mediated inflammatory responses as long as the patient was clinically stable, and (2) responses were judged as a complete response (CR) if there was ≥ 95% reduction in size of the enhancing tumor, a partial response if there was > 50% reduction, progressive disease if there was > 25% increase, and stable disease in all other situations.12
Biologic Assessments
Pretreatment biopsy specimens obtained before injection were stained with hematoxylin and eosin and evaluated by a certified neuropathologist to document recurrent tumor. Post-treatment surgical specimens were immunostained for adenovirus proteins (hexon [AB1056; Millipore, Burlington, MA] and E1A [sc-430; Santa Cruz Biotech, Dallas, TX]) and for immune cell infiltrates (CD3 [LN10; Vector Laboratories, Burlingame, CA], CD4 and CD8 [both VP-C320; Vector Laboratories], and T-bet [H-210; Santa Cruz Biotech]). Independent studies were also performed to quantify immune cell density (CD3, CD4, CD8) and checkpoint proteins from pretreatment and post-treatment specimens (programmed death ligand-1 [PD-L1], programmed death-1 [PD-1], transmembrane immunoglobulin mucin-3 [TIM-3], indoleamine 2,3-dioxygenase [IDO-1]). Quantification was performed as previously described (Data Supplement).13
Damage-associated molecular patterns were evaluated by enzyme-linked immunosorbent assay in glioma sphere-forming cell (GSC) lines, GSC327 and GSC308, which were generated from fresh surgical tumor specimens4 from patients in group B (Data Supplement).
Serum, sputum, and urine were analyzed for the presence of adenovirus genomes using the polymerase chain reaction technique, as previously described14 (Data Supplement). Serum was tested for antiadenovirus (type 5; anti-Ad5) by an indirect immunofluorescence assay before and at multiple times after treatment.
Statistics
Statistical justification is shown in the Data Supplement.
RESULTS
Demographics and Safety
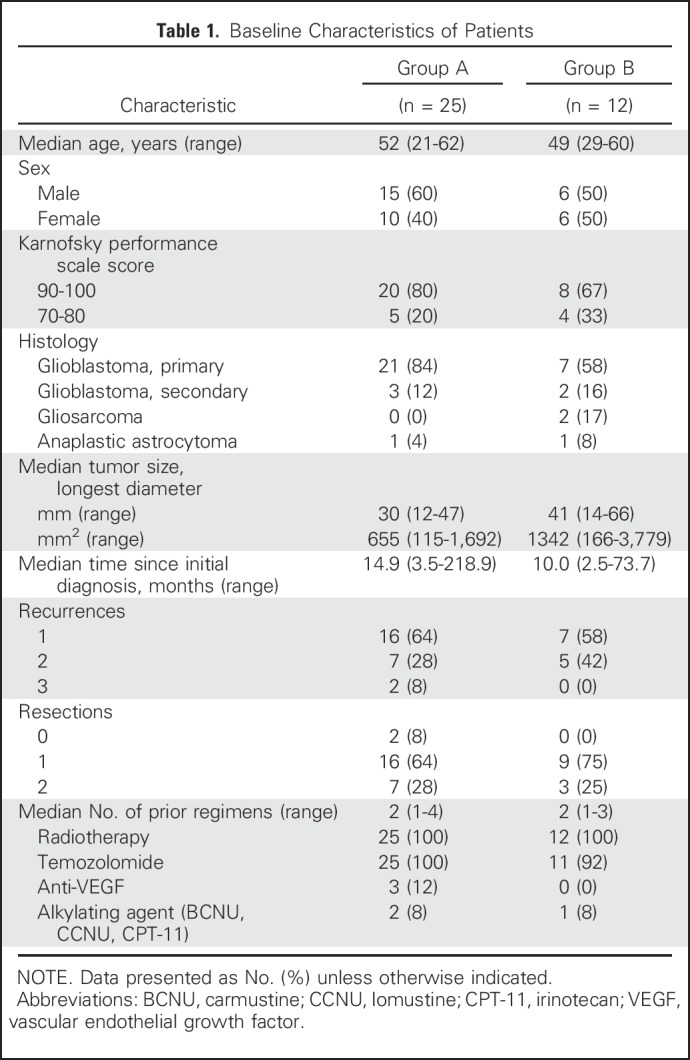
Thirty-seven patients were enrolled in groups A (n = 25; Table 1) or B (n = 12) and received DNX-2401 according to the assigned regimen (Fig 1A). Histology across both groups included GBM (89%), gliosarcoma (5%), or anaplastic astrocytoma (5%; Table 1). Median tumor diameter was 30 mm for group A and 41 mm for group B. Patients were enrolled at first (62%), second (32%), or third recurrence (5%) and had received a median of two prior treatment regimens. Three patients in group A (12%) were refractory to prior anti–vascular endothelial growth factor therapy.
Table 1.
Baseline Characteristics of Patients
In group A, eight dose-escalation cohorts were administered a single intratumoral dose of DNX-2401 into the contrast-enhancing portion of the tumor (1 × 107 − 3 × 1010 vp in 1 mL; Data Supplement). In group B, 11 patients underwent initial injection of DNX-2401 across four cohorts (1 × 107 − 3 × 108 vp) via an implanted catheter, and 14 days later underwent en bloc tumor resection and second injections into the wall of the resection cavity. One patient underwent surgical resection and injection of DNX-2401 into the residual tumor. Enrollment in group B was stopped at cohort 4 after assessment of post-treatment specimens indicated that the predefined biologic end point of viral replication had been achieved (see Viral Replication).
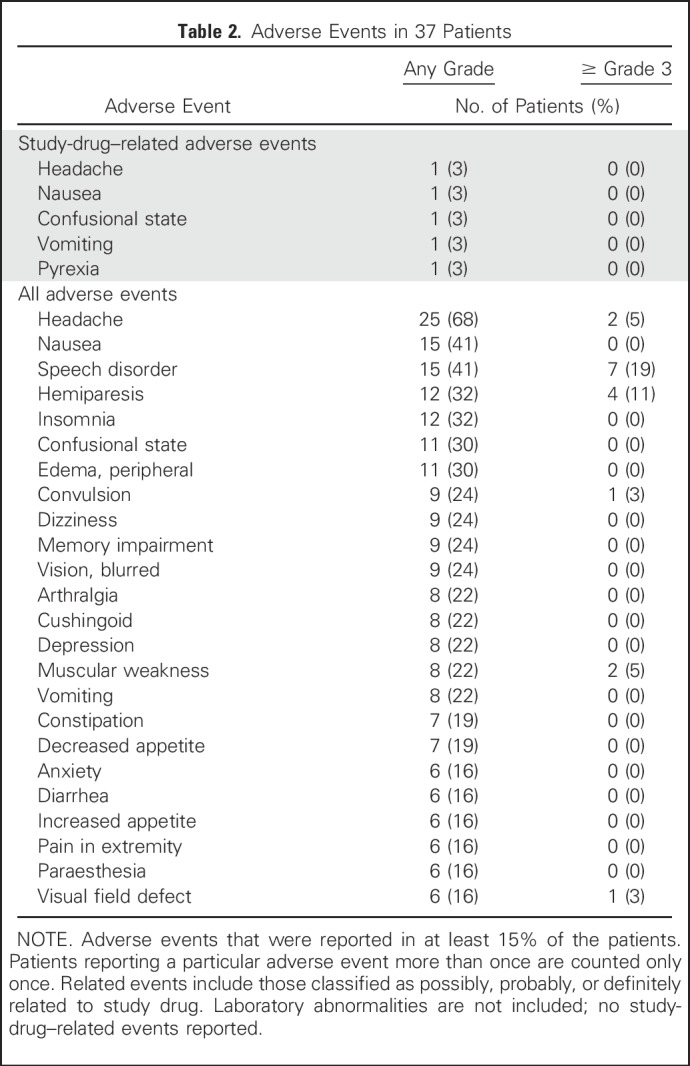
No dose-limiting toxicities were observed in the study; therefore, a maximum tolerated dose was not identified (Data Supplement), and 3 × 1010 vp in 1 mL (cohort 8), the highest concentration of virus that could be manufactured, was deemed the maximal achieved dose. Only two patients experienced AEs related to DNX-2401, including grade 1 to 2 headache, nausea, confusion, vomiting, and pyrexia (Table 2). In one patient with a fever, adenoviral DNA was detected by the polymerase chain reaction method in serum and bone marrow; repeat testing was negative 4 months later (Data Supplement). Overall, adenoviral DNA was detected in < 3% of post-treatment serum, sputum, or urine samples and was not associated with AEs.
Table 2.
Adverse Events in 37 Patients
Viral Replication
The ability of DNX-2401 to replicate within human glioma was evaluated by assessing surgical specimens from patients in group B (n = 11). Tumor samples were cut perpendicular to the catheter, which marked the injection site (Fig 1B). Immunostaining revealed viral E1A or hexon protein in six of 11 tumors (55%), thus demonstrating virus replication in these tumors 14 days after treatment (Fig 1C; Data Supplement). Expression of E1A or hexon coexisted with prominent inclusion bodies, characteristic of adenovirus replication (Fig 1C, left and right). The pattern of staining resembled the three zones of viral spread observed in animal models (zone 1: virus-induced necrosis; zone 2: active virus replication; zone 3: peripheral uninfected cells).4,7 These data demonstrate the ability of DNX-2401 to lyse glioma cells and provide evidence for active oncolytic adenovirus replication in human gliomas.
Efficacy
Tumor reductions were observed in 72% of patients (18 of 25) enrolled in group A (Fig 1D), with a median overall survival time of 9.5 months regardless of dose. Most remarkably, five patients (20%) in group A survived for more than 3 years, three of whom had ≥ 95% reduction in a cross-sectional area of the enhancing tumor (designated as CRs; see Methods), and two patients had sustained stable disease after an initial tumor regression, all occurring at or above the 1 × 108 vp dose level (cohort 3; Fig 1E). Importantly, all three of these dramatic (≥ 95%) responses resulted in progression-free periods of ≥ 3 years from the time of treatment, attesting to the durability of the treatment effects of DNX-2401 (Fig 1E; Data Supplement). Because of tumor resection on day 14, patients in group B were assessed only for survival; two patients (17%) survived for 2 years (Data Supplement), and the overall median survival time was 13.0 months across all cohorts.
Complete Responder No. 1 (Patient No. 12).
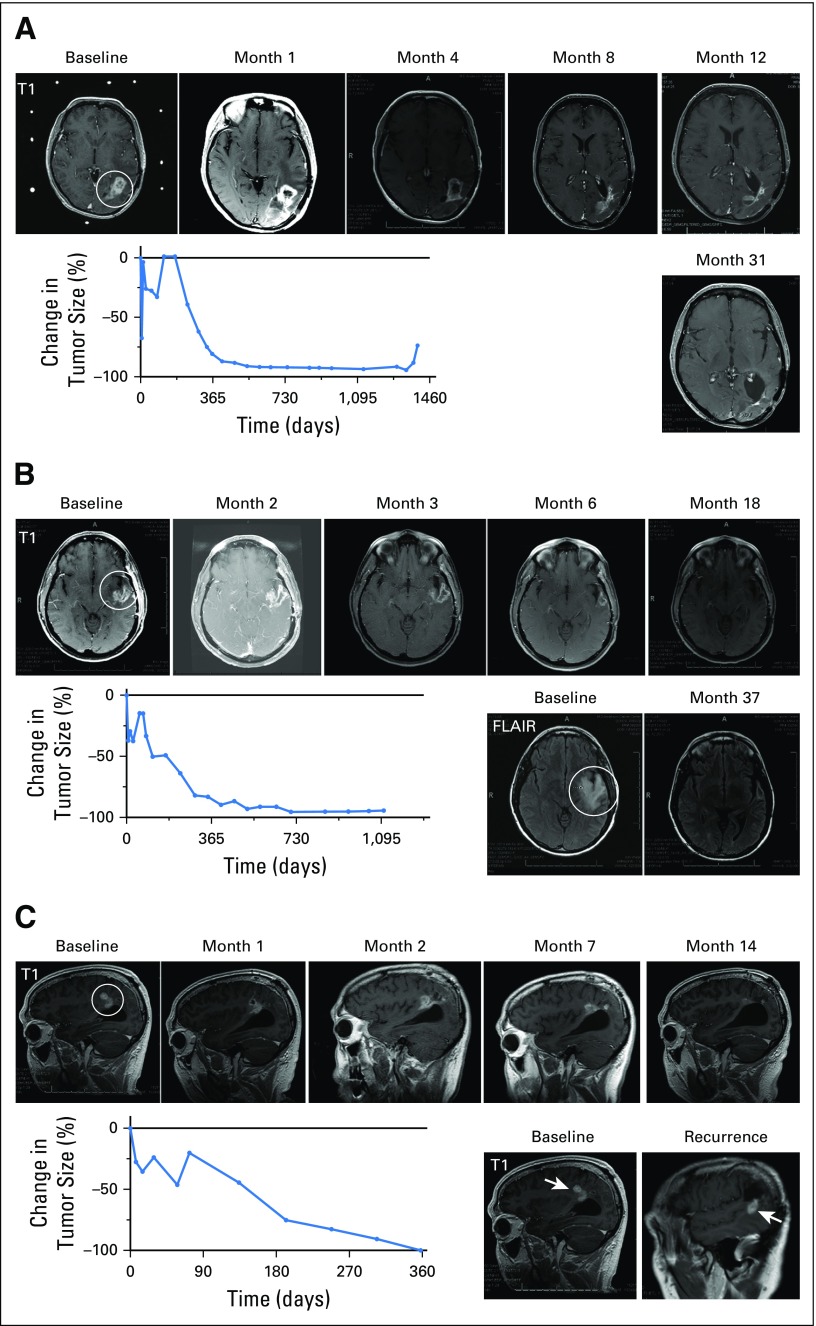
A 53-year-old woman (Fig 2A) with a left parietal GBM underwent subtotal resection followed by radiation therapy and concurrent temozolomide plus dasatinib. One and one-half months after completing radiation therapy, MRI revealed progressive contrast enhancement at the site of the residual tumor that was present after the initial resection. Advanced brain imaging (including dynamic contrast enhancement MRI, dynamic susceptibility MRI, and magnetic resonance spectroscopy) was consistent with recurrent disease. The patient underwent stereotactic biopsy of the enhancing mass, documenting recurrent GBM (IDH1 wild type) and was treated with intratumoral injection of DNX-2401 (1 × 108 vp; cohort 3). MRI scans after virotherapy showed an initial decrease in contrast-enhancing tumor, followed by increased enhancement that peaked after 4 months and then progressively decreased, to achieve dramatic regression (95% reduction) 17.6 months after treatment. From this point, serial MRI scans remained stable for 24.9 months, for a total progression-free period of 42.5 months (3.5 years) from initial treatment (Fig 2A). At this time, an area of new enhancement appeared and progressed, requiring surgical resection that showed gliosarcoma. The patient died 14 months after the second recurrence (4.8 years after DNX-2401 treatment).
Fig 2.
Summary of the three complete tumor responses of recurrent glioblastomas after a single dose of DNX-2401. (A) Magnetic resonance imaging (MRI) scans and graph of tumor size for Patient No. 12 demonstrating a complete response of a recurrent left parietal glioblastoma treated with 1 × 108 viral particles (vp) DNX-2401. Contrast enhancement was observed until 4 months post-treatment, followed by complete regression, which lasted until 41.5 months after DNX-2401 treatment. MRI scans at baseline and months 1, 4, 8, 12, and 31 after treatment are shown. Circle indicates tumor mass. The graph shows the percent change in tumor size (contrast enhancement) over time; note the initial increase in enhancement followed by a progressive decrease. (B) MRI scans and graph of tumor size for Patient No. 33 demonstrating a recurrent glioblastoma of the left superior temporal gyrus treated with 1 × 1010 vp DNX-2401. MRI scans at baseline and months 2, 3, 6, 18, and 37 after treatment are shown. Circle indicates tumor mass. Complete regression was concurrent with a decrease in peritumoral hyperintensity on fluid-attenuated inversion recovery (FLAIR) images (bottom left). As in Figure 2A, the graph of percent change in tumor size (contrast enhancement) over time shows an initial increase in enhancement followed by a progressive decrease. (C) MRI scans and graph of tumor size for Patient No. 37 with a recurrent glioblastoma of his right parietal lobe injected with 3 × 1010 vp DNX-2401. Complete regression was observed 1 year after treatment, as shown on MRIs from baseline and months 1, 2, 7, and 14. The graph of percent change in tumor size (contrast enhancement) over time shows a staggering initial increase in enhancement followed by a progressive decrease. A contrast-enhanced nodule (bottom right) arose away from the initial recurrence 29 months after DNX-2401 treatment (arrows note location of new lesion); surgical resection of this lesion revealed necrosis and inflammation and no evidence of tumor.
Complete responder No. 2 (Patient No. 33).
A 38-year-old woman, diagnosed with a GBM of her left superior temporal gyrus, underwent resection, followed by concurrent radiation therapy, temozolomide, and adjuvant isotretinoin (Fig 2B). Three months after the completion of radiation therapy, progressive enhancement was identified, and advanced brain tumor imaging was consistent with recurrent tumor. The patient underwent stereotactic biopsy documenting recurrent GBM with active microvascular proliferation (IDH1 [R132H] and p53 [R248W] mutant) followed by intratumoral injection of DNX-2401 (1 × 1010 vp; cohort 7). MRI scans 2 months after virotherapy showed an increase in the contrast enhancement before steady decreases and complete resolution (96% reduction) after 17.1 months, with the decrease in enhancement on T1-weighted images occurring concurrently with a decrease in peritumoral hyperintensity on fluid-attenuated inversion recovery images (Fig 2B). From this point, serial MRI scans remained stable for 19.3 months, for a total progression-free period of 36.4 months (3.25 years) from the time of treatment, after which biopsy of a new enhancing lesion showed recurrent GBM. The patient died 23 months after recurrence (4.8 years after DNX-2401 treatment).
Complete responder No. 3 (Patient No. 37).
A 47-year-old man, diagnosed with a GBM of his right parietal lobe, underwent total resection, followed by concurrent radiation therapy and temozolomide treatment (Fig 2C). He then received adjuvant temozolomide plus memantine. A new enhancing mass occurred 4 months after radiation therapy. The patient underwent stereotactic biopsy, documenting recurrent GBM with atypical cells and microvascular proliferation (IDH1 wild type), followed by intratumoral injection of DNX-2401 (3 × 1010 vp; cohort 8). The patient resumed temozolomide treatment per his oncologist’s preference. MRI initially showed an increase in tumor size that was followed by tumor regression until a complete response (100% reduction) was achieved after 12 months (Fig 2C). Seventeen months after achieving a complete response, a new enhancing lesion was detected distant from the treated recurrence (Fig 2C), and surgical resection of this lesion revealed only necrosis and no tumor cells. The patient remained stable for another 12 months until he experienced recurrence (ie, 29 months from the time of complete response and 41 months [3.4 years] from the treatment with DNX-2401). Resection of this recurrent mass revealed gliosarcoma. Despite repeated radiation therapy and chemotherapy, the recurrent tumor progressed, and 9 months after the second surgery, the patient was retreated with DNX-2401. He is currently alive 4.5 years after initial treatment with DNX-2401.
Immune Effects
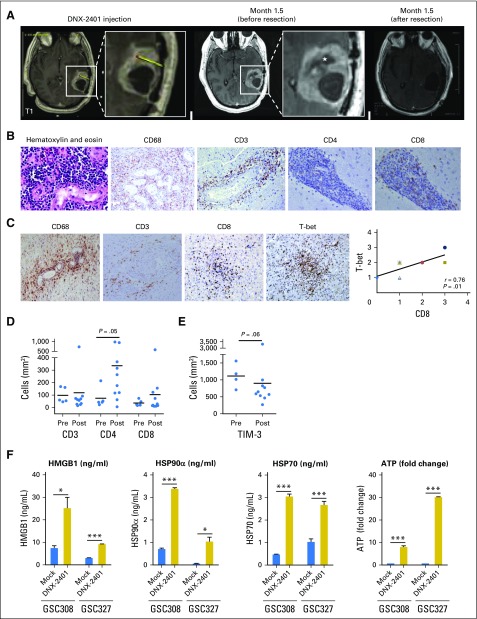
In preclinical studies, DNX-2401 induces a TH1-skewed CD8+ cytotoxic T-cell response.11 Consistent with an immune-mediated response, contrast enhancement on MRI increased in all three complete responders from group A within 4 months after injection of DNX-2401, followed by a reduction in the size of the enhancing lesion over the next 12 to 18 months (Fig 2). One patient with recurrent GBM of the left temporal lobe (Patient No. 20) underwent surgical resection 1.5 months after injection of DNX-2401 (3 × 108 vp) because of progressive symptoms. At this time, MRI showed necrosis at the injection site but increased contrast enhancement throughout the tumor (Fig 3A). Analyses of the resected tumor revealed that 80% of the mass was necrotic, and the remaining specimen was largely acute with chronic inflammatory cell infiltration (macrophages and CD8+ T cells; Fig 3B), without evidence of adenovirus-infected cells. Equally important, 2.5 years after treatment with DNX-2401, complete responder No. 3 (Patient No. 37) underwent resection for a new enhancing nodule distant from the initial lesion as described previously (Fig 2C, bottom right). Pathologic examination revealed necrosis and inflammation, but no evidence of tumor, potentially indicative of an adaptive memory antitumor response.
Fig 3.
Immune-mediated response to DNX-2401. (A and B) Magnetic resonance imaging (MRI) scans and pathologic specimens of left temporal glioblastoma from Patient No. 20. (A) Baseline MRI (far left) and the exact site of DNX-2401 injection into recurrent glioblastoma of the left temporal lobe (second from left shows enlarged image with yellow line and red dot showing trajectory of injection needle and site of injection of DNX-2401, respectively). MRI 1.5 months after treatment (third and fourth from left) shows necrosis near injection site (star) and worsening of enhancement. This entire tumor was resected, and postoperative MRI shows complete removal of the mass (far right). (B) The pathologic examination of the tumor resected in Figure 3A, including hematoxylin and eosin–stained section (far left) demonstrating large numbers of small hematoxylin-stained inflammatory cells around blood vessels. Immunostaining shows infiltration of macrophages (CD68; second from left), T cells (CD-3; middle), few CD4+ T cells (second from right), and large numbers of CD8+ T cells (far right), all consistent with a cytotoxic immune response. The patient died of a pulmonary embolism 1.5 months after the surgery; therefore, his outcome related to the tumor could not be assessed. (C) Representative cases of post-treated specimens from patients from group B taken 14 days after treatment with DNX-2401 and immunostained for macrophages (CD68), T cells (CD3), cytotoxic T cells (CD8), and T-bet+ T cells; note the prominent perivascular cuffing of all these cell types. Each dot represents a different patient. Different colors and shapes were used in the plotted points because many overlapped. The CD8 and T-bet specimens are serial sections from the same patient. T-bet expression correlated with increased CD8+ T cells as expected of a Th1 immune response (far right). Graphs showing the density of (D) CD3+, CD4+, and CD8+ cells and (E) TIM-3+ cells on the basis of quantitative analyses of pretreatment (pre; n = 5) and post-treatment (post; n = 10) tumor specimens. Post-treatment specimens were from patients in group B treated with DNX-2401 14 days previously, and five pretreatment specimens were the matched pretreatment biopsies of three patients in group B and two unmatched specimens (one from group B and one from group A). The mean values are noted by horizontal bars; although CD3+ changes were not evident, both CD4+ and CD8+ cells increased after treatment, with increases in CD4+ cells reaching statistical significance. A decrease in the exhaustion marker TIM-3 was seen after treatment with DNX-2401. (F) Graphs showing analyses of damage-associated molecular patterns (DAMPs) in glioma stem cells cultured from the surgical specimens of two patients enrolled in group B. Glioma sphere-forming cells (GSC) 327 and GSC308 were cultured and treated with either mock infection (blue bars) or with DNX-2401 (dose of 20 active viral particles per cell; gold bars). After 72 hours, the supernatant was collected and analyzed by enzyme-linked immunosorbent assay for the following damage-associated molecular patterns: high-mobility group proteins B1 (HMGB1), heat-shock protein (HSP)90α, HSP70, and ATP. Data represent mean ± standard deviation. *P ≤ .05; ***P ≤ .001.
Group B tumor specimens resected 14 days after DNX-2401 treatment (n = 9) were analyzed by immunohistochemistry for the presence of CD3, CD4, CD8, and T-bet (Fig 3C; Data Supplement). CD3+ T cells were detected in all specimens either throughout the tumor or around the vessels (perivascular cuffing). The majority were CD8+, with lower representations of CD4+ cells. Lymphocytes were positive for T-bet, suggestive of a TH1 response (Fig 3C), as in preclinical experiments.11 To quantify these results, we conducted an independent automated image analysis of immunohistochemically stained tumors13 and determined cell density. For post-treatment specimens (n = 10), the mean density of CD3+, CD4+, and CD8+ cells was 119 ± 217, 336 ± 350, and 104 ± 177 cells/mm2, respectively. Compared with five pretreatment specimens (three of five matched to post-treatment specimens), an increase in CD4 and CD8 cell density after treatment with DNX-2401 was observed, although statistically significant only for CD4 by this method (Fig 3D; P = .05). In addition, we performed a quantitative immunohistochemical analysis of the checkpoint proteins PD-1, PD-L1, IDO-1H, and TIM-3. A decrease in the expression of TIM-3 was evident when tumors (n = 10) after treatment with DNX-2401 were compared with tumors before treatment (n = 4; Fig 3E; P = .06), without changes in the expression of PD-1, PD-L1, or IDO-1H (Data Supplement). Together, these data suggest that DNX-2401 is capable of increasing immune cell infiltrates in gliomas and can potentially alter checkpoint protein expression.
To evaluate immune-related signals, GSCs were isolated from the surgical specimens of two patients enrolled in group B,15 treated with DNX-2401 in vitro, and assayed for immunogenic cell death. A significant increase in damage-associated molecular patterns16 (high-mobility group proteins B1, heat-shock protein [HSP]90α, HSP70, ATP) was detected in GSCs after treatment, indicating that DNX-2401 induces immunogenic cell death (Fig 3F).
Anti-Ad5 Titers
Anti-Ad5 antibodies were detected in low titers (1:8) before treatment in five of 25 patients (20%) in group A and none of 12 patients (0%) in group B (Data Supplement). Increases in anti-Ad5 titers were observed in 12 patients in group A and four patients in group B after treatment with DNX-2401. Two of the three patients with CR had an increase in anti-Ad5 titer.
DISCUSSION
In this dose-escalation study, we show that DNX-2401, a novel tumor-selective adenovirus, is safe and capable of robust viral replication and killing of recurrent high-grade glioma cells. Secondary antiglioma immune-mediated responses were observed. Importantly, these biologic mechanisms translated into clinical benefit, including three dramatic responses (≥ 95% reduction in tumor size) with long progression-free intervals and long-term survival after treatment with DNX-2401. To our knowledge, this is the first clinical study to show direct oncolysis and replication of a therapeutic adenovirus in human brain tumors and to provide correlative evidence for a viral-induced antiglioma immune response.
By marking the injection with a catheter, we were able to localize the site of virus injection and assess viral replication. DNX-2401 infection, replication, and killing followed the three-concentric-zone pattern that we described in preclinical animal models (Fig 1C).4,7 However, not all patients in group B showed evidence of infection, possibly because of inefficient delivery, inadequate infection/replication, or rapid immune-mediated elimination. To standardize delivery, current clinical studies are focused on assessing purpose-built cannulas to deliver DNX-2401.17-20 Although viral replication was evident in post-treatment specimens collected 2 weeks after treatment, specimens taken beyond 1 month did not show evidence of virus, suggesting that DNX-2401 does not persist long term or falls below the detection limit over time, consistent with observations in immunocompetent animal models.11 However, clinical responses (≥ 95% reduction in tumor size) were typically first evident at more than 3 months after treatment, pointing to a robust secondary antitumor immune effect.
Immune responses were observed after a single injection of DNX-2401 into human glioma. Widespread necrosis with infiltration of T cells, consistent with a TH1 immune response, was observed in surgical specimens obtained 14 days after DNX-2401 treatment, as in preclinical studies,11,21 and in surgical specimens obtained at the time of increased tumor enhancement seen on MRI. Examination of a recurrent enhancing nodule located away from the originally injected tumor 2.5 years after a complete response to DNX-2401 (Fig 2C) revealed only inflammation without evidence of tumor, suggestive of an adaptive immune memory effect. In all three patients with a reduction of ≥ 95% in tumor size (ie, CRs), tumor regression occurred over a 1- to 1.5-year period, and the pattern of tumor enhancement on MRI was reminiscent of pseudo-progression (ie, therapy-mediated tumor enhancement), which is considered a radiographic hallmark of inflammation and is observed with immune checkpoint inhibitors.22-24
One of the mechanisms of tumor immune suppression is T-cell exhaustion.25 Combinations of inhibitory receptors such as PD-1 and TIM-3 coregulate exhausted T cells26 and cooperate to induce deficiency of CD8+ T cells.27,28 Here, although PD-1 was not significantly modified after DNX-2401 treatment, tumor infection induced a decrease in TIM-3 expression (Fig 3E), indicating that DNX-2401 may partially overcome some features of T-cell exhaustion and suggesting that combining DNX-2401 with checkpoint-based therapies may further augment clinical benefit. The combination of DNX-2401 and anti–PD-1 antibodies is currently being evaluated (ClinicalTrials.gov identifier: NCT02798406).
In all, these results provide the first clinical correlates that DNX-2401 can induce a direct oncolytic effect followed by an antitumor immune response. Insofar as DNX-2401 is highly efficient because of expanded infectivity conferred by the RGD motif, and insofar as it is capable of enhancing antigen presentation,11 we speculate that these effects lead to immune cell infiltration and antitumor activity. DNX-2401 warrants further study as a single agent and in combination with other immune-modulatory therapeutics.
ACKNOWLEDGMENT
We thank the patients who participated in this trial; Xiarong Ji, Lammone Crutcher, and Dima Suki, PhD, for assistance with conducting the trial; and Xuejun Fan, Barbara Mina, and Weihua Tian for technical expertise.
Footnotes
Supported by the National Cancer Institute (P50 CA127001), The Marcus Foundation, The Broach Foundation for Brain Cancer Research, the Elias Family Fund, Dr. Marnie Ross Foundation, the Will Power Foundation, and the J.P. Harris Brain Tumor Research Fund.
Clinical trial information: NCT00805376.
See accompanying article on page 1440
AUTHOR CONTRIBUTIONS
Conception and design: Frederick F. Lang, Charles Conrad, Candelaria Gomez-Manzano, W.K. Alfred Yung, Gregory N. Fuller, Clemens M.F. Dirven, Frank Tufaro, Juan Fueyo
Financial support: Frederick F. Lang
Administrative support: Frederick F. Lang, Joanna J. Peterkin, Frank Tufaro
Provision of study materials or patients: Frederick F. Lang, Charles Conrad, W.K. Alfred Yung, Raymond Sawaya, Jeffrey S. Weinberg, Sujit S. Prabhu, Ganesh Rao, Gregory N. Fuller, Kenneth D. Aldape
Collection and assembly of data: Frederick F. Lang, Charles Conrad, Candelaria Gomez-Manzano, Jeffrey S. Weinberg, Sujit S. Prabhu, Ganesh Rao, Kenneth D. Aldape, Joy Gumin, Brett Ewald, Joanna J. Peterkin, Juan Fueyo
Data analysis and interpretation: Frederick F. Lang, Candelaria Gomez-Manzano, Raymond Sawaya, Joy Gumin, Luis M. Vence, Ignacio Wistuba, Jaime Rodriguez-Canales, Pamela A. Villalobos, Sonia Tejada, Ricardo D. Valle, Marta M. Alonso, Brett Ewald, Juan Fueyo
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Frederick F. Lang
Patents, Royalties, Other Intellectual Property: Patent holder on DNX-2401
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Charles Conrad
Stock or Other Ownership: DNAtrix (I)
Patents, Royalties, Other Intellectual Property: Patent holder on DNAtrix (I)
Candelaria Gomez-Manzano
Stock or Other Ownership: DNAtrix, DNAtrix (I)
Consulting or Advisory Role: DNAtrix, DNAtrix (I)
Research Funding: DNAtrix, DNAtrix (I)
Patents, Royalties, Other Intellectual Property: Delta-24-RGD intellectual property, Delta-24-RGD intellectual property (I)
Travel, Accommodations, Expenses: DNAtrix (I)
W.K. Alfred Yung
Stock or Other Ownership: DNAtrix
Honoraria: DNAtrix
Consulting or Advisory Role: DNAtrix
Patents, Royalties, Other Intellectual Property: DNAtrix
Raymond Sawaya
No relationship to disclose
Jeffrey S. Weinberg
No relationship to disclose
Sujit S. Prabhu
No relationship to disclose
Ganesh Rao
Honoraria: Monteris Medical
Gregory N. Fuller
No relationship to disclose
Kenneth D. Aldape
Consulting or Advisory Role: Celldex
Speakers' Bureau: Merck
Travel, Accommodations, Expenses: Merck
Joy Gumin
No relationship to disclose
Luis M. Vence
No relationship to disclose
Ignacio Wistuba
Consulting or Advisory Role: Genentech/Roche, Eli Lilly, Bristol-Myers Squibb, Ariad, HTG Molecular Diagnostics, Asuragen, Pfizer, AstraZeneca/MedImmune
Speakers' Bureau: Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol-Myers Squibb
Research Funding: Genentech, Merck, OncoPlex Diagnostics, Myriad Genetics, HTG Molecular Diagnostics, Silicon Biosytems, Adaptimmune, EMD Serono, Pfizer, MedImmune
Jaime Rodriguez-Canales
Employment: MedImmune
Pamela A. Villalobos
No relationship to disclose
Clemens M.F. Dirven
Consulting or Advisory Role: DNAtrix (Inst)
Sonia Tejada
No relationship to disclose
Ricardo D. Valle
No relationship to disclose
Marta M. Alonso
Patents, Royalties, Other Intellectual Property: MD Anderson and DNAtrix
Brett Ewald
Employment: DNAtrix
Stock or Other Ownership: DNAtrix
Joanna J. Peterkin
Employment: DNAtrix
Leadership: DNAtrix
Stock or Other Ownership: DNAtrix
Consulting or Advisory Role: DNAtrix
Patents, Royalties, Other Intellectual Property: DNAtrix
Travel, Accommodations, Expenses: DNAtrix
Other Relationship: DNAtrix (I)
Frank Tufaro
Employment: DNAtrix
Leadership: DNAtrix
Stock or Other Ownership: DNAtrix
Consulting or Advisory Role: DNAtrix
Patents, Royalties, Other Intellectual Property: DNAtrix
Travel, Accommodations, Expenses: DNAtrix
Juan Fueyo
Stock or Other Ownership: DNAtrix, DNAtrix (I)
Consulting or Advisory Role: DNAtrix, DNAtrix (I)
Research Funding: DNAtrix, DNAtrix (I)
Patents, Royalties, Other Intellectual Property: Delta-24-RGD intellectual property, Delta-24-RGD intellectual property (I)
Travel, Accommodations, Expenses: DNAtrix
REFERENCES
- 1.Wen PY, Kesari S: Malignant gliomas in adults. N Engl J Med 359:492-507, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. : Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459-466, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Liao P, et al. : CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro-oncol 16:iv1-iv63, 2014. (suppl 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang H, Gomez-Manzano C, Aoki H, et al. : Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: Role of autophagic cell death. J Natl Cancer Inst 99:1410-1414, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Kirn D: Replication-selective oncolytic adenoviruses: Virotherapy aimed at genetic targets in cancer. Oncogene 19:6660-6669, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Jiang H, Gomez-Manzano C, Rivera-Molina Y, et al. : Oncolytic adenovirus research evolution: From cell-cycle checkpoints to immune checkpoints. Curr Opin Virol 13:33-39, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fueyo J, Alemany R, Gomez-Manzano C, et al. : Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst 95:652-660, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Fueyo J, Gomez-Manzano C, Alemany R, et al. : A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 19:2-12, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Bello L, Francolini M, Marthyn P, et al. : Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery 49:380-389, 2001; discussion 390 [DOI] [PubMed] [Google Scholar]
- 10.Desgrosellier JS, Cheresh DA: Integrins in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer 10:9-22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, Clise-Dwyer K, Ruisaard KE, et al. : Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS One 9:e97407, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macdonald DR, Cascino TL, Schold SC, Jr, et al. : Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277-1280, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Parra ER, Behrens C, Rodriguez-Canales J, et al. : Image analysis-based assessment of PD-L1 and tumor-associated immune cells density supports distinct intratumoral microenvironment groups in non-small cell lung carcinoma patients. Clin Cancer Res 22:6278-6289, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heim A, Ebnet C, Harste G, et al. : Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol 70:228-239, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Singh SK, Hawkins C, Clarke ID, et al. : Identification of human brain tumour initiating cells. Nature 432:396-401, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Krysko DV, Garg AD, Kaczmarek A, et al. : Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 12:860-875, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Sampson JH, Archer G, Pedain C, et al. : Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg 113:301-309, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Mehta AI, Choi BD, Ajay D, et al. : Convection enhanced delivery of macromolecules for brain tumors. Curr Drug Discov Technol 9:305-310, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olbricht WL, Neeves KB, Foley CP: Microfluidic probes in the treatment of brain-related diseases. Drug News Perspect 23:491-497, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Brady ML, Raghavan R, Singh D, et al. : In vivo performance of a microfabricated catheter for intraparenchymal delivery. J Neurosci Methods 229:76-83, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Kleijn A, Kloezeman J, Treffers-Westerlaken E, et al. : The in vivo therapeutic efficacy of the oncolytic adenovirus Delta24-RGD is mediated by tumor-specific immunity. PLoS One 9:e97495, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoos A, Eggermont AM, Janetzki S, et al. : Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 102:1388-1397, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolchok JD, Hoos A, O’Day S, et al. : Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res 15:7412-7420, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Wolchok JD, Kluger H, Callahan MK, et al. : Nivolumab plus ipilimumab In advanced melanoma. N Engl J Med 369:122-133, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wherry EJ, Kurachi M: Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15:486-499, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fourcade J, Sun Z, Benallaoua M, et al. : Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 207:2175-2186, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin HT, Anderson AC, Tan WG, et al. : Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA 107:14733-14738, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson AC: Tim-3: An emerging target in the cancer immunotherapy landscape. Cancer Immunol Res 2:393-398, 2014 [DOI] [PubMed] [Google Scholar]