Abstract
Background:
Local disruption of the cord that causes contracture of the finger in Dupuytren disease can be achieved either through mechanical division by percutaneous needle fasciotomy (PNF) or through enzymatic digestion by injectable collagenase Clostridium histolyticum (CCH). This study was designed to compare clinical and patient-reported outcomes between patients who had been treated with each method.
Methods:
A prospective, randomized, single-blinded, controlled trial was designed and included 156 patients with a contracture of the metacarpophalangeal (MCP) joint of ≥20°. The patients were allocated to treatment with either PNF or CCH. The primary outcome was a reduction of the MCP contracture to <5°. Secondary outcomes included the reduction of any concomitant contracture of the proximal interphalangeal (PIP) joint, the presence of Dupuytren cords, and changes in patient-reported outcomes as measured with the URAM (Unité Rhumatologique des Affections de Main) and QuickDASH (an abbreviated version of the Disabilities of the Arm, Shoulder and Hand [DASH]) questionnaires and visual analog scales for patient satisfaction. All treatments were performed by a single surgeon and all blinded follow-up measurements were made by a single physiotherapist. The participants were assessed at 1 week, 6 months, and 1 and 2 years after the interventions.
Results:
A total of 152 patients (97%) were examined at 2 years, at which time 58 patients (76%) treated with CCH and 60 (79%) treated with PNF retained a straight MCP joint. No cords were detectable in >50% of the patients at 2 years. There were no significant differences in the reduction of PIP contracture, range of motion, or patient-reported outcomes between the 2 treatments.
Conclusions:
This trial demonstrated no advantage of CCH treatment compared with PNF in terms of clinical outcome at any time during the 2-year follow-up. The significant decrease in the number of pathological cords (p < 0.0001, Wilcoxon signed-rank test) after disruption regardless of the method used may indicate that resorption of pathological collagen occurs when the tension in the Dupuytren cord is diminished.
Level of Evidence:
Therapeutic Level I. See Instructions for Authors for a complete description of levels of evidence.
Dupuytren disease is a common progressive disease in which pathological changes in the aponeurosis of the hand lead to the formation of a rigid cord that eventually compromises extension in the affected finger, a Dupuytren contracture1. Until recently, open surgery involving excision of the pathological tissue has been the mainstay treatment option2, but alternative minimally invasive methods have gained in popularity in recent years3.
Percutaneous needle fasciotomy (PNF) is a percutaneous technique in which the Dupuytren cord is divided mechanically through repeated perforations by a needle, a method that has been refined since the 19th century4-6. Injectable collagenase Clostridium histolyticum (CCH) for the enzymatic division of the Dupuytren cord was introduced in 20097 and was approved by the U.S. Food and Drug Administration (Xiaflex) and by the European Medicines Agency (Xiapex) in 2011. Both methods disrupt the Dupuytren cord, resulting in subsequent extension of the affected finger, and patients require considerably less rehabilitation after these methods than after open surgery3,8. However, the cost of CCH treatment is substantially higher than that of PNF, and 2 visits by the patient are required instead of 19-12. The 1-year follow-up of this trial and another recent randomized controlled trial failed to show any significant difference in clinical or patient-reported outcome10,13, whereas a third trial of the treatment for proximal interphalangeal (PIP) joint contracture showed CCH to have inferior results after 2 years14. Recurrence of the Dupuytren contracture is common8,15. Because we found no clinical trials with >1 of year of follow-up that compared CCH with any other treatment for the metacarpophalangeal (MCP) joint12, we investigated whether there were any differences at 2 years that could justify the substantially higher cost of CCH.
Materials and Methods
This prospective, randomized, single-blinded, controlled trial was approved by the Regional Ethical Committee (EPN 2012:513-12). It was conducted according to the CONSORT (Consolidated Standards of Reporting Trials) guidelines16 and was registered in a database for prospective trials (www.researchweb.org; project number 213221). From October 2012 to October 2014, all patients referred to the Department of Hand Surgery at Sahlgrenska University Hospital, Gothenburg, Sweden, were assessed for participation in the study. All patients were finally recruited and treated by the same senior hand surgeon (J.S.), and were assessed by the same physiotherapist at blinded follow-up in the same department at 6, 12, and 24 months after the intervention.
Inclusion and Exclusion Criteria
The inclusion criteria were (1) a palpable Dupuytren cord with (2) an extension deficit of ≥20° in (3) a single finger in (4) an adult patient who (5) agreed to participate and signed the written informed consent form. The exclusion criteria were (1) any earlier treatment of, including surgery on, the finger to be treated; (2) any other pathological condition, or limited range of motion, of the finger to be treated; (3) any contraindication to CCH treatment (for example, anticoagulant treatment or intake of acetylsalicylic acid exceeding 150 mg per day); (4) any clinical signs of, or medical records indicating, alcohol or drug abuse; and (5) any chronic neuromuscular disease compromising hand function.
Sample Size
An a priori sample-size estimate indicated that 67 patients were required in each group given a significance level (α) of 0.05 and a power (β) of 0.85 for a minimal clinically important difference of 5° in passive joint extension between the 2 groups. Anticipating a loss to follow-up, we added 11 patients to each group for a total of 78 patients.
Randomization and Allocation
The patients were included in a consecutive manner throughout the study. The protocol was explained and both treatments were described in detail by the hand surgeon prior to allocation and treatment, and the patients signed an informed consent form. A new treatment cycle was initiated when 10 patients had been placed on the waiting list; thus, groups of 10 patients were treated per cycle. Before treatment, 5 patients were randomized to CCH and 5 were randomized to PNF according to a computer-generated block randomization process performed using a statistical software program (MEDSTAT, version 2.1, 1988; Astra Group). The randomization created a list that assigned the letter A or B to the numbers 1 through 10, and a secretary prepared 10 numbered envelopes containing one or the other of these letters according to this list for each set of 10 patients. Before treatment began for each group, the surgeon decided which of the 2 letters would correspond to which treatment using a simple lottery. The envelopes were then opened consecutively, and treatment was chosen accordingly. Both the injection of the CCH and the PNF were performed in a small operating room adjacent to the outpatient ward at the Department of Hand Surgery.
Study Outcomes
The primary outcome was a straight finger, defined as reduction of the contracture to <5°. All measurements of joint movement throughout the trial were made with 1 specific goniometer (Zimmer). Secondary outcomes included recurrence, changes in passive MCP or PIP joint extension, improvement in motion compared with baseline, active range of motion, and presence of a Dupuytren cord at the MCP or PIP joint level. Secondary outcome measures also included scores on the 9-item URAM (Unité Rhumatologique des Affections de la Main) scale, a validated patient-reported outcome measure specific to Dupuytren-disease-associated disability17,18; the QuickDASH (an abbreviated version of the Disabilities of the Arm, Shoulder and Hand [DASH]), a general questionnaire for hand and arm function19; and a VAS (visual analog scale) for patient satisfaction completed by the patients. For both the URAM and the QuickDASH, a higher score indicates a greater level of disability and severity. The score range is 0 to 45 for the URAM and 0 to 100 for the QuickDASH.
CCH Treatment
After the patient’s forearm was prepared and was draped with an arm cover according to the standard procedure for minor surgery, 0.58 mg of collagenase Clostridium histolyticum (Xiapex) reconstituted in 0.39 mL of sterile diluent was injected into the pretendinous cord at the MCP level in 3 portions according to the instructions from the manufacturer. A bulky dressing was then applied, and the patient was given instructions not to use the hand. The next day, 2.5 mL of mepivacaine (Carbocaine, 20 mg/mL) was injected with a 25-gauge needle around the first injection site to provide local anesthesia. A forced extension maneuver was performed to disrupt the cord and, if this was not accomplished after 3 trials, the patient was scheduled for a second treatment in 1 month.
PNF
The patient’s forearm was prepared and was draped with an arm cover according to the standard procedure for minor surgery. A 2.5-mL syringe with 1 mL of methylprednisolone (Depo Medrol, 40 mg/mL) and 1.5 mL of mepivacaine (Carbocaine, 20 mg/mL) was used with a 25-gauge needle. A small volume was injected volarly and dorsally in relation to the pretendinous cord at the MCP level and, with the finger gently extended passively, the needle was passed through the cord repeatedly in various directions from the skin puncture site until the cord ruptured.
Procedure at 1-Week Follow-up
The patients in both groups were assessed by the hand surgeon at a follow-up visit 1 week after treatment, at which time joint movement, grip strength, flexor tendon and nerve function, and any side effects of the given treatment were recorded. Patients who had a difference of 10° between active and passive extension of the MCP joint were referred to an occupational therapist for a night splint with full extension of the finger to be used for 3 months. No specific training instructions, other than to stretch the finger passively, were given. Patients with an inadequate outcome were offered the opportunity to undergo another treatment at 1 month after the 1-week follow-up visit, thus entering another treatment cycle. All of the patients who had a disrupted cord were randomly assigned a blinded-follow-up identity by choosing a sealed envelope containing a number referring to the treatment group and an identification letter (e.g., 3D).
Outcome Assessment
The patients were examined 6, 12, and 24 months after treatment by a single physiotherapist who was unaware of the treatment that each patient had received. A special administrative protocol was established in which an assistant nurse who had access to the identities of the patients made appointments. The physiotherapist was given only the blinded identities and thus was unable to check any medical records relating to the patients, who were reminded not to yield any information on which treatment they had received. In order to detect recurrence, which was defined as a loss of extension of ≥20° compared with the postoperative results at 1 week, the physiotherapist had access to these measurements for MCP and PIP joint movement. Clinical examination included the measurement of joint movement with the same goniometer used at the time of inclusion and visual and palpatory examination for remaining Dupuytren cords. The patients also completed the URAM and QuickDASH questionnaires as well as the VAS for patient satisfaction at every follow-up visit.
Statistical Methodology
Cross tables were used to compare the treatment groups. Nonparametric data were analyzed with the Mann-Whitney U test to compare the distribution of the 2 unmatched groups. Categorical data were analyzed with the Pearson chi-square test. Repeated individual measurements were analyzed with the Wilcoxon signed-rank test. A significance level of 5% (α = 0.05) was used for all statistical tests of the outcomes, so that a p value of <0.05 was considered significant. SPSS software (version 24; IBM) and Excel for Mac 2011 (Microsoft) were used for the statistical analysis.
Results
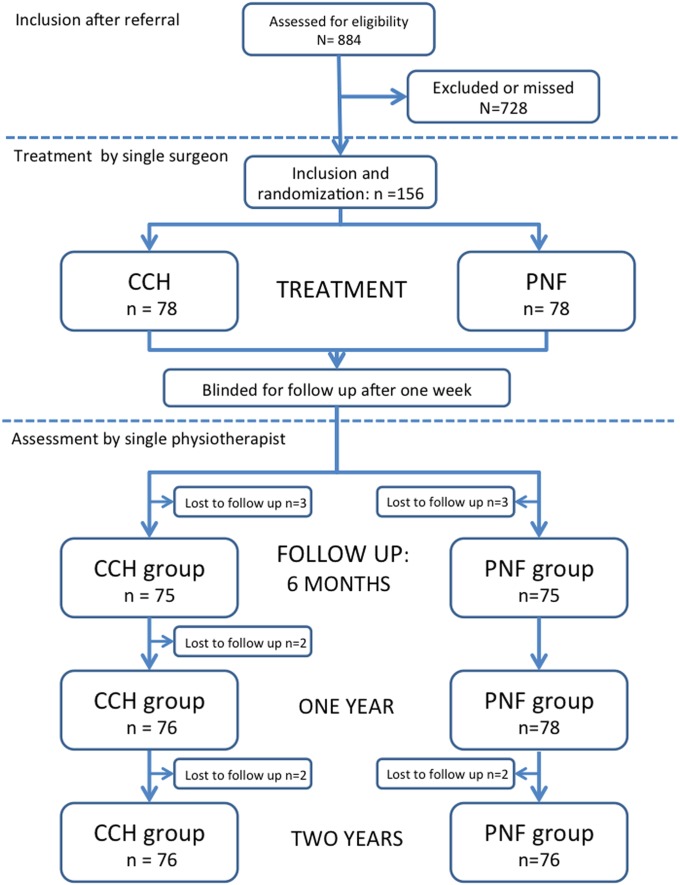
Between October 2012 and October 2014, 884 patients with Dupuytren contracture were referred to our unit, and 169 patients were initially enrolled in the study. However, a secondary assessment by the hand surgeon (J.S.) prior to treatment allocation revealed that 13 of these patients did not meet the inclusion criteria, and they were excluded. Of the 156 patients who were included, 78 were allocated to each group (Fig. 1). The groups were considered homogeneous in terms of baseline characteristics (Table I). Twenty-seven patients in the CCH group and 25 in the PNF group had a concomitant PIP contracture of >5°. The Dupuytren cord was ruptured in all patients in the PNF group and in all but 2 patients in the CCH group. One of these patients had another CCH injection with subsequent rupture after 1 month, but the other refused another injection. The percentage of patients assessed at each follow-up was >96% (Fig. 1). The primary results (at 1 week) and the results at 1 year for part of the study population have been reported previously10.
Fig. 1.
Flowchart of the study. The boxes show the number of patients assessed at each follow-up. The losses to follow-up are not cumulative—e.g., 1 patient could be lost to follow-up at 6 months but return for assessment at 1 and 2 years.
TABLE I.
Patient Characteristics at Baseline
| Patient Characteristics at Baseline | CCH (N = 78) | PNF (N = 78) |
| Age (yr) | ||
| Mean ± stand. dev. | 65 ± 8.1 | 68 ± 9.1 |
| Median (range) | 66 (42-80) | 69 (29-86) |
| Sex (no. [%]) | ||
| Male | 65 (83%) | 68 (87%) |
| Female | 13 (17%) | 10 (13%) |
| Hand with contracture (no.) | ||
| Right | 48 | 43 |
| Left | 30 | 35 |
| Finger involved (no.) | ||
| Little | 40 | 40 |
| Ring | 32 | 33 |
| Long | 6 | 5 |
| Flexion contracture at MCP joint (°) | ||
| Median | 44 | 45 |
| Mean | 46 | 46 |
| Range | 20-90 | 20-87 |
| Active range of motion of MCP joint (°) | ||
| Median | 41 | 41 |
| Mean | 40 | 41 |
| Range | 6-73 | 3-68 |
| Flexion contracture at all PIP joints (°) | ||
| Median | 0 | 0 |
| Mean | 11 | 7 |
| Range | −20-74 | −20-48 |
| Active range of motion of all PIP joints (°) | ||
| Median | 85 | 84 |
| Mean | 80 | 82 |
| Range | 30-116 | 42-116 |
| Grip strength of affected hand (kg) | ||
| Median | 45 | 40 |
| Range | 13-68 | 19-63 |
| Isolated MCP joint contracture (no. [%]) | 51 (65%) | 53 (68%) |
| MCP and PIP joint contracture* (no. [%]) | 27 (35%) | 25 (32%) |
| Duration since first symptoms (yr) | ||
| Median | 5 | 5 |
| Mean | 6 | 7 |
| Range | 1-20 | 1-30 |
| Family history (no.) | 38 | 41 |
| Diabetes (no.) | 5 | 7 |
| URAM score | ||
| Median | 11 | 10 |
| Range | 0-29 | 0-33 |
| QuickDASH score | ||
| Median | 16 | 11 |
| Range | 0-59 | 0-63 |
Defined as a concomitant PIP joint contracture with a passive PIP extension lag of >5°.
Two patients in each group were lost to follow-up or were excluded after 2 years. In the PNF group, 1 patient died 1 year after treatment and the other patient did not wish to attend any follow-up visits. In the CCH group, 1 patient had moved and the other patient had received treatment in the same finger after a year due to a recurrence of the Dupuytren contracture and was thus excluded from further follow-up.
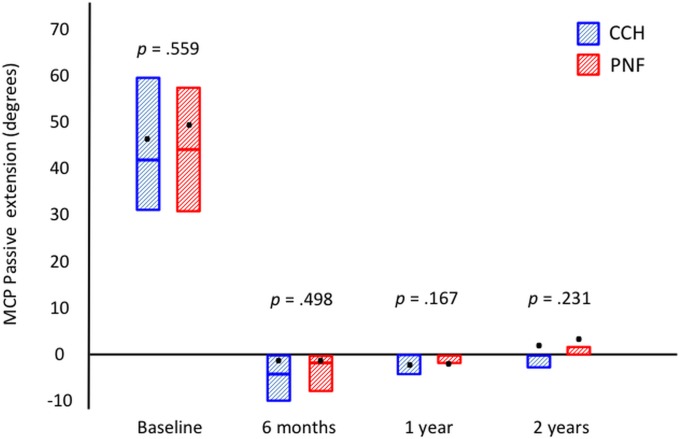
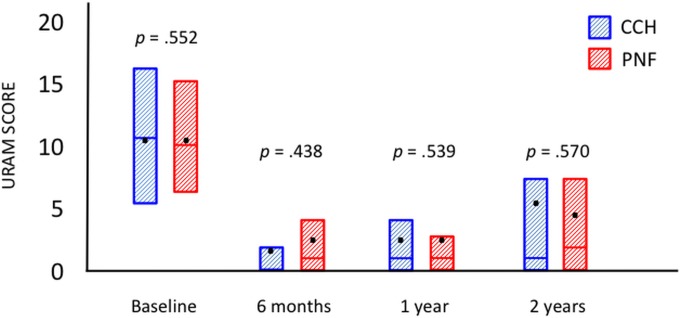
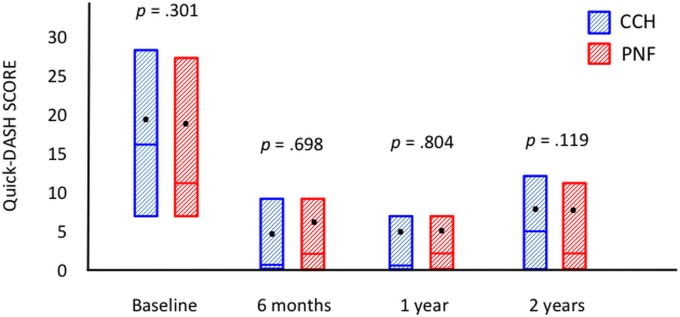
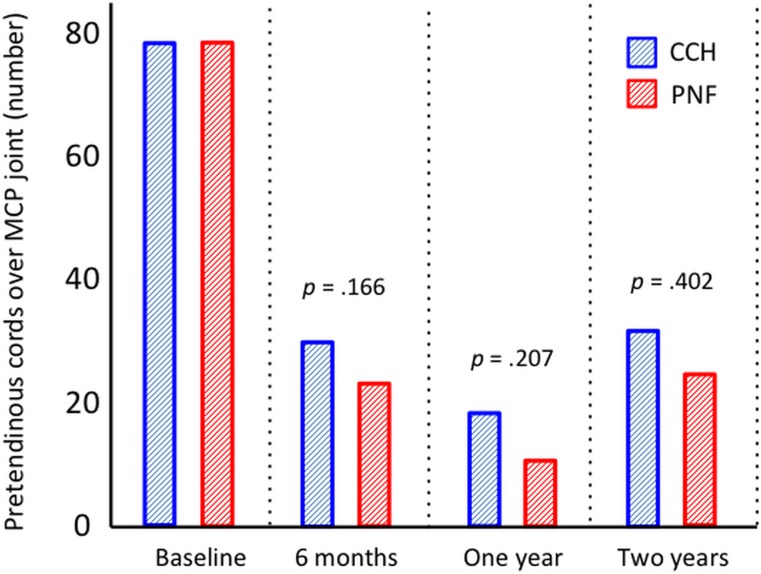
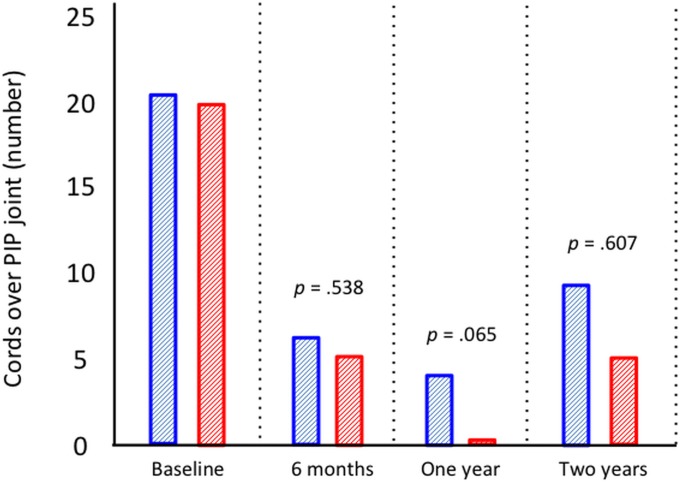
Seventy-six patients in each group were assessed at the time of follow-up at 2 years. Fifty-eight patients (76%) in the CCH group and 60 (79%) in the PNF group retained a straight finger, which was the primary outcome. The groups were also similar regarding all secondary outcomes (Table II). Both groups had a significant reduction in passive extension (Fig. 2), the URAM score (Fig. 3), and the QuickDASH score (Fig. 4) (p < 0.0001, p < 0.001, and p < 0.001, respectively; Wilcoxon rank test) compared with baseline, but there was no significant difference between the 2 treatment groups. The prevalence of Dupuytren cords decreased significantly for both the MCP (p < 0.0001) and the PIP (p < 0.001) joints (Figs. 5-A and 5-B), with an increase between 1 and 2 years.
Fig. 2.
Passive extension of the MCP joint at baseline and at the time of follow-up at 6 months and 1 and 2 years. The boxes represent the interquartile ranges; the bars within the boxes, the medians; and the dots, the means.
Fig. 3.
Patient scores on the URAM questionnaire at baseline and at the time of follow-up at 6 months and 1 and 2 years. The URAM score ranges from 0 (no disability) to 45 (most severe disability). The boxes represent the interquartile ranges; the bars within the boxes, the medians; and the dots, the means.
Fig. 4.
Patient scores on the QuickDASH questionnaire at baseline and at the time of follow-up at 6 months and 1 and 2 years. The QuickDASH score ranges from 0 (no disability) to 100 (most severe disability). The boxes represent the interquartile ranges; the bars within the boxes, the medians; and the dots, the means.
Figs. 5-A and 5-B Palpable cords at baseline and at the time of follow-up at 6 months and 1 and 2 years. A cord was defined as “continuous bulk of longitudinal subcutaneous tissue over the joint which tightens when the finger is passively extended.”
Fig. 5-A.
Pretendinous cords at the MCP joint level in all patients.
Fig. 5-B.
Cords over the PIP joint level in patients with concomitant PIP contracture.
TABLE II.
Results
| CCH | PNF | P Value | |
| MCP joint | |||
| Primary end point: reduction of MCP joint contracture to 0° to <5°* | |||
| 1 week | 70 (90%), n = 78 | 71 (91%), n = 78 | 0.786 |
| 6 months | 67 (89%), n = 75 | 68 (91%), n = 75 | 0.785 |
| 1 year | 70 (92%), n = 76 | 73 (94%), n = 78 | 0.721 |
| 2 years | 58 (76%), n = 76 | 60 (79%), n = 76 | 0.697 |
| Secondary end points | |||
| Recurrence of MCP joint contracture*† | |||
| 6 months | 0 (0%) | 0 (0%) | — |
| 1 year | 1 (1%) | 2 (3%) | 0.694 |
| 2 years | 10 (13%) | 9 (12%) | 0.806 |
| Passive MCP joint extension (°) | See Fig. 2 | See Fig. 2 | See Fig. 2 |
| Improvement in MCP joint extension from baseline‡ (°) | |||
| 1 week | 47 (38-60); 20-86 | 46 (40-57); 20-85 | 0.731 |
| 6 months | 44 (35-61); 8-90 | 45 (33-57); 6-87 | 0.580 |
| 1 year | 45 (35-60); 2-90 | 45 (35-58); 20-87 | 0.288 |
| 2 years | 42 (30-58); −28-90 | 40 (27-54); −8-87 | 0.568 |
| Active MCP joint range of motion‡ (°) | |||
| 1 week | 82 (74-88); 45-106 | 80 (76-88); 28-110 | 0.658 |
| 6 months | 82 (76-87); 52-100 | 82 (76-88); 43-100 | 0.740 |
| 1 year | 81 (72-86); 44-96 | 80 (76-86); 35-100 | 0.314 |
| 2 years | 77 (60-85); 18-94 | 78 (58-84); 16-94 | 0.313 |
| Patient-reported outcome measures | |||
| URAM score | See Fig. 3 | See Fig. 3 | See Fig. 3 |
| QuickDASH | See Fig. 4 | See Fig. 4 | See Fig. 4 |
| Patient-estimated effect of treatment (VAS)ठ| |||
| 1 week | 9 (8-10); 2-10 | 9 (8-9); 1-10 | 0.559 |
| 6 months | 9 (8-10); 0-10 | 9 (8-9); 4-10 | 0.588 |
| 1 year | 9 (7-10); −3-10 | 8 (7-10); −2-10 | 0.471 |
| 2 years | 8 (5-10); −4-10 | 8 (4-9); −7-10 | 0.337 |
| Patient-estimated satisfaction with treatment (VAS)‡# | |||
| 6 months | 10 (9-10); 1-10 | 10 (7-10); 2-10 | 0.609 |
| 1 year | 10 (9-10); 2-10 | 10 (8-10); 0-10 | 0.513 |
| 2 years | 9 (4-10); 0-10 | 9 (7-10); 0-10 | 0.571 |
| PIP joint with extension deficit of >5° | |||
| Reduction of PIP joint contracture to 0° to <5°* | |||
| 1 week | 9 (33%), n = 27 | 10 (40%), n = 25 | 0.618 |
| 6 months | 13 (50%), n = 26 | 9 (38%), n = 24 | 0.374 |
| 1 year | 12 (44%), n = 27 | 10 (40%), n = 25 | 0.746 |
| 2 years | 11 (41%), n = 27 | 9 (36%), n = 25 | 0.726 |
| Recurrence of PIP joint contracture*† | |||
| 6 months | 0 (0%) | 0 (0%) | — |
| 1 year | 3 (11%) | 1 (4%) | 0.336 |
| 2 years | 6 (22%) | 2 (8%) | 0.156 |
| Passive PIP joint extension‡ (°) | |||
| 1 week | 10 (4-16); 0-40 | 10 (0-18); −4-40 | 0.671 |
| 6 months | 6 (0-18); −2-52 | 10 (1-18); −6-52 | 0.651 |
| 1 year | 8 (0-23); −6-65 | 10 (0-20); −8-48 | 0.707 |
| 2 years | 10 (0-35); −6-62 | 14 (0-26); −8-48 | 0.339 |
| Improvement in PIP joint extension from baseline‡ (°) | |||
| 1 week | 17 (10-29); 6-52 | 12 (8-19); −6-38 | 0.164 |
| 6 months | 20 (10-28); 0-52 | 12 (1-21); −8-40 | 0.660 |
| 1 year | 15 (8-28); −8-56 | 14 (4-18); −20-38 | 0.336 |
| 2 years | 12 (3-23); −27-60 | 10 (3-18); −36-38 | 0.414 |
The values are given as the number of patients, with the percentage in parentheses, with or without the n value.
Recurrence = a loss of extension of the treated joint of ≥20°.
The values are given as the median, with the interquartile range in parentheses, followed by the range.
The patient’s response to the question, “How much straighter do you consider your finger to be after the treatment?” on a scale of 0 to 10, where 0 was defined as “unchanged” and 10 as “totally straight.”
The patient’s response to the question, “How satisfied are you with the result of the treatment?” on a scale of 0 to 10, where 0 was defined as “totally unsatisfied” and 10 as “totally satisfied”.
Discussion
This randomized controlled trial comparing CCH treatment with PNF for Dupuytren contracture revealed no significant differences between the 2 methods with regard to any outcome measurement at any time during the 2-year follow-up. The majority of the patients in both groups retained a straight finger throughout the trial and reported correspondingly good results on the patient-reported outcome measures that were used. However, both groups had a large increase in the number of recurrences as well as a trend toward an increase in URAM and QuickDASH scores between 1 and 2 years. These indications warrant longer follow-up, but the recurrence rates at 2 years in our trial clearly underscore those of previous reports15,20. Approximately one-third of the patients had a PIP joint contracture at baseline and, even though the Dupuytren cord was treated at the MCP joint level, 41% and 36% of the patients who had had a PIP joint contracture retained a straight PIP joint at 2 years in the CCH and PNF groups, respectively. Skov et al.14 compared PNF with CCH injection for the treatment of Dupuytren contracture at the PIP joint level and reported a significantly inferior outcome for CCH at 2 years whereas the clinical improvement in the PNF group was similar to our results. Another interesting observation is the prevalence of Dupuytren cords throughout our study, with the cord disappearing over time in >50% of our patients. Verjee et al.21 suggested that reduced tension in a cord might lead to myofibroblast apoptosis, whereby myofibroblast-rich nodules fail to persist, and our results lend support to this suggestion. Furthermore, we have reported that the morphological changes have the same ultrasonographic appearance after either CCH treatment or PNF22. This may suggest that the diminished tension in a locally treated ruptured Dupuytren cord, regardless of method, leads to the resorption of pathological collagen in some patients, and that a recurrent cord is formed de novo.
The strengths of this study include the fact that it was a randomized controlled trial; the absence of industry conflicts of interests, as it received institutional funding; the high internal validity given that a single hand surgeon and a single physiotherapist saw all of the patients; and the fact that 97% of the patients were assessed at 2 years. The limitations of this study include the low proportion of referred patients who were enrolled in the study, but this can be explained by the highly specific inclusion criteria (e.g., involvement of only 1 finger and no earlier treatment) in combination with the fact that our unit is the last referral unit for more complex cases and recurrences from all of the orthopaedic clinics in the region. Another limitation was the blinding process, with its heavy reliance on patient cooperation and its lower external validity.
In the U.S., the introduction of CCH has increased the percentage of Dupuytren contractures that are treated with minimally invasive techniques from 14% in 2007 to 39% in 2013, while the number of PNFs remained steady and the number of open surgical procedures declined throughout the study period3. Both CCH treatment and PNF are well-tolerated, minimally invasive treatment options for Dupuytren contracture, but there is a substantial difference in cost between the two. In this study, the total cost of treating 78 patients with CCH was calculated to be $110,000 USD compared with $41,000 USD for 78 patients treated with PNF—i.e., it was almost 3 times more expensive10. In our opinion, the substantially higher costs of CCH treatment must be justified by superior outcomes, which have not yet been reported.
Acknowledgments
Note: This investigation conforms with the University of Gothenburg Human Resource Protection Programme guidelines. The authors thank physiotherapist Marie Medbo for the follow-up of all of the patients throughout the study, and Lena Nyblom-Andersson for administration of patient data and logistics for the study.
Footnotes
Investigation performed at the Department of Hand Surgery, Sahlgrenska University Hospital, Gothenburg, Sweden
A commentary by Robert R. Slater Jr., MD, is linked to the online version of this article at jbjs.org.
Disclosure: No outside funding was received in support of this study. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/E777).
References
- 1.Elliot D. The early history of contracture of the palmar fascia. Part 1: the origin of the disease: the curse of the MacCrimmons: the hand of benediction: Cline’s contracture. J Hand Surg Br. 1988. August;13(3):246-53. [DOI] [PubMed] [Google Scholar]
- 2.Shih B, Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nat Rev Rheumatol. 2010. December;6(12):715-26. Epub 2010 Nov 9. [DOI] [PubMed] [Google Scholar]
- 3.Zhao JZ, Hadley S, Floyd E, Earp BE, Blazar PE. The impact of collagenase Clostridium histolyticum introduction on Dupuytren treatment patterns in the United States. J Hand Surg Am. 2016. October;41(10):963-8. Epub 2016 Aug 18. [DOI] [PubMed] [Google Scholar]
- 4.De Seze S, Debeyre N. [Treatment of Dupuytren’s disease by local hydrocortisone associated with straightening maneuvers; 70 treated cases]. Rev Rhum Mal Osteoartic. 1957. Jul-Aug;24(7-8):540-50. French. [PubMed] [Google Scholar]
- 5.Lermusiaux JL, Lellouche H, Badois JF, Kuntz D. How should Dupuytren’s contracture be managed in 1997? Rev Rhum Engl Ed. 1997. December;64(12):775-6. [PubMed] [Google Scholar]
- 6.Badois FJ, Lermusiaux JL, Massé C, Kuntz D. [Non-surgical treatment of Dupuytren disease using needle fasciotomy]. Rev Rhum Ed Fr. 1993. November 30;60(11):808-13. French. [PubMed] [Google Scholar]
- 7.Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009. September 3;361(10):968-79. [DOI] [PubMed] [Google Scholar]
- 8.Pess GM, Pess RM, Pess RA. Results of needle aponeurotomy for Dupuytren contracture in over 1,000 fingers. J Hand Surg Am. 2012. April;37(4):651-6. [DOI] [PubMed] [Google Scholar]
- 9.Baltzer H, Binhammer PA. Cost-effectiveness in the management of Dupuytren’s contracture. A Canadian cost-utility analysis of current and future management strategies. Bone Joint J. 2013. August;95-B(8):1094-100. [DOI] [PubMed] [Google Scholar]
- 10.Strömberg J, Ibsen-Sörensen A, Fridén J. Comparison of treatment outcome after collagenase and needle fasciotomy for Dupuytren contracture: a randomized, single-blinded, clinical trial with a 1-year follow-up. J Hand Surg Am. 2016. September;41(9):873-80. Epub 2016 Jul 27. [DOI] [PubMed] [Google Scholar]
- 11.Chen NC, Shauver MJ, Chung KC. Cost-effectiveness of open partial fasciectomy, needle aponeurotomy, and collagenase injection for Dupuytren contracture. J Hand Surg Am. 2011. November;36(11):1826-1834.e32. Epub 2011 Oct 5. [DOI] [PubMed] [Google Scholar]
- 12.Brazzelli M, Cruickshank M, Tassie E, McNamee P, Robertson C, Elders A, Fraser C, Hernandez R, Lawrie D, Ramsay C. Collagenase Clostridium histolyticum for the treatment of Dupuytren’s contracture: systematic review and economic evaluation. Health Technol Assess. 2015. October;19(90):1-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherman P, Jenmalm P, Dahlin LB. One-year results of needle fasciotomy and collagenase injection in treatment of Dupuytren’s contracture: a two-centre prospective randomized clinical trial. J Hand Surg Eur Vol. 2016. July;41(6):577-82. Epub 2015 Dec 1. [DOI] [PubMed] [Google Scholar]
- 14.Skov ST, Bisgaard T, Søndergaard P, Lange J. Injectable collagenase versus percutaneous needle fasciotomy for Dupuytren contracture in proximal interphalangeal joints: a randomized controlled trial. J Hand Surg Am. 2017. May;42(5):321-328.e3. [DOI] [PubMed] [Google Scholar]
- 15.Peimer CA, Blazar P, Coleman S, Kaplan FT, Smith T, Lindau T. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-year data. J Hand Surg Am. 2015. August;40(8):1597-605. Epub 2015 Jun 18. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG, Moher D, Schulz KF. Improving the reporting of randomised trials: the CONSORT statement and beyond. Stat Med. 2012. November 10;31(25):2985-97. Epub 2012 Aug 18. [DOI] [PubMed] [Google Scholar]
- 17.Beaudreuil J, Allard A, Zerkak D, Gerber RA, Cappelleri JC, Quintero N, Lasbleiz S, Bernabé B, Orcel P, Bardin T; URAM Study Group. Unité Rhumatologique des Affections de la Main (URAM) scale: development and validation of a tool to assess Dupuytren’s disease-specific disability. Arthritis Care Res (Hoboken). 2011. October;63(10):1448-55. [DOI] [PubMed] [Google Scholar]
- 18.Bernabé B, Lasbleiz S, Gerber RA, Cappelleri JC, Yelnik A, Orcel P, Bardin T, Beaudreuil J. URAM scale for functional assessment in Dupuytren’s disease: a comparative study of its properties. Joint Bone Spine. 2014. October;81(5):441-4. Epub 2014 Feb 22. [DOI] [PubMed] [Google Scholar]
- 19.Budd HR, Larson D, Chojnowski A, Shepstone L. The QuickDASH score: a patient-reported outcome measure for Dupuytren’s surgery. J Hand Ther. 2011. Jan-Mar;24(1):15-20; quiz 21. Epub 2010 Nov 24. [DOI] [PubMed] [Google Scholar]
- 20.van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012. February;129(2):469-77. [DOI] [PubMed] [Google Scholar]
- 21.Verjee LS, Midwood K, Davidson D, Essex D, Sandison A, Nanchahal J. Myofibroblast distribution in Dupuytren’s cords: correlation with digital contracture. J Hand Surg Am. 2009. December;34(10):1785-94. Epub 2009 Nov 11. [DOI] [PubMed] [Google Scholar]
- 22.Strömberg J, Vanek P, Fridén J, Aurell Y. Ultrasonographic examination of the ruptured cord after collagenase treatment or needle fasciotomy for Dupuytren’s contracture. J Hand Surg Eur Vol. 2017. September;42(7):683-8. Epub 2017 Jun 6. [DOI] [PubMed] [Google Scholar]