Figure 4.
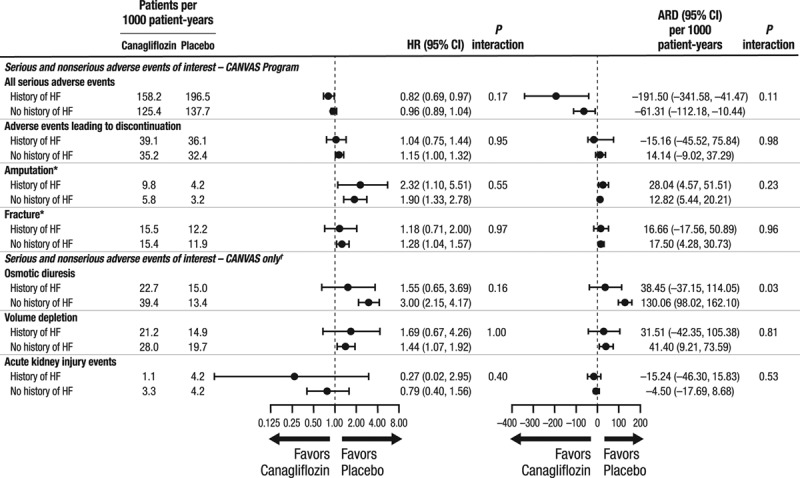
Proportional and absolute effects of canagliflozin compared with placebo on key safety outcome in patients with and without a history of heart failure at baseline. *Based on ITT dataset, whereas all other analyses based on on-treatment dataset. †For these adverse events, the annualized incidence rates are reported based on the CANVAS study alone through January 7, 2014, because, after this time, only serious adverse events or adverse events leading to discontinuation were collected. In the CANVAS-R study, only serious adverse events or adverse events leading to discontinuation were collected for these events. ARD indicates absolute risk difference over 5 years; CANVAS, Canagliflozin Cardiovascular Assessment Study; ITT, intent-totreat; CANVAS-R, Canagliflozin Cardiovascular Assessment Study–Renal; CI, confidence interval; HF, heart failure; and HR, hazard ratio.
