Abstract
Objective:
To compare the surgical outcomes of the Minimally Invasive Ponto Surgery (MIPS) technique with those of the linear incision technique with soft-tissue preservation for bone-anchored hearing systems (BAHS).
Design:
Sponsor-initiated multicenter, open, randomized, controlled clinical trial.
Setting:
Maastricht University Medical Centre, Ziekenhuisgroep Twente and Medisch Centrum Leeuwarden, all situated in The Netherlands.
Participants:
Sixty-four adult patients eligible for unilateral BAHS surgery.
Interventions Single-stage BAHS surgery with 1:1 randomization to the linear incision technique with soft-tissue preservation (control) or the MIPS (test) group.
Primary and Secondary Outcome Measurements:
Primary objective: compare the incidence of inflammation (Holgers Index ≥ 2) during 12 weeks’ follow-up after surgery. Secondary objectives: skin dehiscence, pain scores, loss of sensibility around the implant, soft-tissue overgrowth, skin sagging, implant extrusion, cosmetic results, surgical time, wound healing and Implant Stability Quotient measurements.
Results:
Sixty-three subjects were analyzed in the intention-to-treat population. No significant difference was found for the incidence of inflammation between groups. Loss of skin sensibility, cosmetic outcomes, skin sagging, and surgical time were significantly better in the test group. No statistically significant differences were found for dehiscence, pain, and soft-tissue overgrowth. A nonsignificant difference in extrusion was found for the test group. The Implant Stability Quotient was statistically influenced by the surgical technique, abutment length, and time.
Conclusion:
No significant differences between the MIPS and the linear incision techniques were observed regarding skin inflammation. MIPS results in a statistically significant reduction in the loss of skin sensibility, less skin sagging, improved cosmetic results, and reduced surgical time. Although nonsignificant, the implant extrusion rate warrants further research.
Keywords: BAHS, Bone conduction, Bone-anchored hearing, Hearing loss, Holgers index, Minimally invasive ponto surgery, MIPS, Soft tissue reactions, Surgical outcomes, Surgical technique, Tissue preservation
The bone-anchored hearing system (BAHS) has become an accepted treatment option for subjects suffering from various types of hearing loss, such as conductive or mixed hearing loss or single-sided deafness, who are unable to benefit from conventional therapies, such as air conduction hearing aids or reconstructive middle ear surgery (1,2). It consists of a retro-auricular-placed titanium implant mounted with a percutaneous abutment, to which a sound processor can be attached. The implant integrates with the skull through a process of osseointegration. The sound processor receives sound, converts it to vibrations, and uses the skull as a conductive material to transmit it to the cochlea directly, thereby bypassing the ear canal and middle ear (3,4).
One concern when it comes to the BAHS is the skin around the implant. For the BAHS, this soft-tissue status is commonly assessed using the five-grade Holgers Index (5). Periabutment inflammation, defined as a Holgers Index of 2 or above, is the most common complication of BAHS and it can be associated with pain and discomfort (6,7). Other complications related to BAHS include pain, numbness of the skin adjacent to the implant, soft-tissue overgrowth, and implant extrusion (6,8).
To improve outcomes, both the implant design and the surgical technique have evolved over the last few decades (9–11). The design of the skin-penetrating abutment has been refined from an angulated sharp design to curved or cylindrical alternatives (11,12). Soon after the introduction of the BAHS, it was hypothesized that adverse soft-tissue reactions occur as a result of skin movements adjacent to the skin-penetrating abutment (13). This led to the development of surgical techniques with soft-tissue reduction to minimize skin movements. In contrast to this hypothesis, van de Berg et al. (14) demonstrated that less invasive surgical techniques produced better results. Following this, a linear incision technique without any soft-tissue reduction was introduced, further improving outcomes, and it is currently the most advocated technique (9,10). However, raising a mucoperiosteal flap is associated with a degree of tissue damage and discomfort for the patient and it requires more surgical work such as suturing. As a result, there is a need to improve the surgical technique to further diminish adverse soft-tissue events. It is suggested that, by leaving the soft tissue and vascular supply surrounding the percutaneous abutment intact, the prerequisites for effective wound healing would be largely retained (10). To this end, surgeons have attempted a punch-only technique (15–17). This would obviate the need for an incision, reduce procedure time and clinical work load, with the aim of minimizing postoperative complications such as numbness, pain, swelling, infection and dehiscence, as well as possibly reducing costs. Until now, a standardized method and tools to perform the punch technique for BAHS have been lacking. Recently, the Minimally Invasive Ponto Surgery (MIPS) technique was introduced by Oticon Medical AB (Askim, Sweden) to address this problem (18,19). This surgical procedure is a punch-only technique performed with a specially designed surgical kit.
This is the first multicenter, randomized, controlled trial to compare the MIPS technique with the linear incision technique with soft-tissue preservation. In both groups, Ponto Wide implants (Oticon Medical AB) with mounted abutments were used, resulting in a design evaluating only the surgical technique. Here we report the surgical outcomes after 3 months’ follow-up.
METHODS
Study Design
The study protocol of this multicenter, randomized, controlled trial has previously been published (20). Maastricht University Medical Centre, Ziekenhuisgroep Twente, and Medisch Centrum Leeuwarden, all situated in The Netherlands, participated in the performance of the trial. Adult patients (above 18 yr) who were eligible to undergo a unilateral BAHS were asked to participate in this trial. The exclusion criteria were 1) a history of immunosuppressive disease, 2) use of systemic immunosuppressive medication, 3) bilateral BAHS placement, 4) relevant dermatological disease (e.g., psoriasis, severe eczema), and 5) participation in other studies. If a suitable site for a 4-mm implantation was not found or if the bone quality was assessed as being insufficient, the subject was regarded as early termination and excluded during surgery.
Randomisation and Blinding
Enrolled subjects were allocated consecutively to the test group (MIPS technique) or the control group (linear incision technique with soft-tissue preservation) in a 1:1 ratio stratified for sex. Subjects at each site were randomized independent of other centers using randomization software (Statistiska konsultgruppen, Gothenburg, Sweden). Blinding was not possible due to the type of intervention.
Procedures
ENT surgeons, experienced in the linear incision technique with soft-tissue preservation, performed all the surgeries and were given in-depth MIPS training before opening the trial for enrolment. The coordinating investigator and a surgical support team were present during the first few MIPS surgeries in the trial at every center. Based on patient preference, local or general anaesthesia was used. Abutment length was determined by measuring the skin thickness prior to the administration of local anesthetics. In both groups, a Ponto Wide 4-mm implant with a premounted abutment (9, 12, or 14 mm) was installed using an insertion torque setting of 40 to 50 Ncm (Oticon Medical, Askim, Sweden).
In the control group, the linear incision technique with soft-tissue preservation was performed (Fig. 1A)(10,20). In the test group, the MIPS technique was performed according to the manufacturer's instructions (Fig. 1B) (18–20). Detailed descriptions of the steps can be found in the protocol (20). Finally, in both groups, a healing cap was attached to the abutment and gauze drenched in antibiotic ointment (Terra-cortril, Pfizer Laboratories, New York, NY) was applied.
FIG. 1.
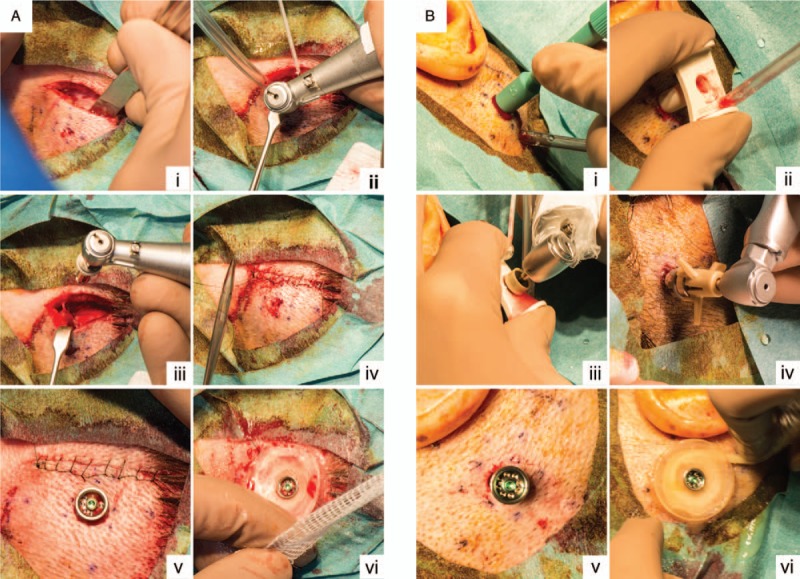
Surgical techniques. A, Linear incision technique with soft-tissue preservation. (i) Linear incision. (ii) Drilling procedure. (iii) Implant installation. (iv) Closing incision line. (v) Result after skin punch. (vi) Attachment of healing cap and application of dressing. B, Minimally Invasive Ponto Surgery. (i) Skin punch. (ii) Placement of the cannula. (iii) Drilling procedure. (iv) Implant installation with installation indicator. (v) Result. (vi) Attachment of healing cap and application of dressing.
Patients were assessed at inclusion, surgery, standard follow-up visits (9 d, 3 and 12 wk) and extraconsultations. The surgery procedure time (time from incision or punching to placement of healing cap) and total time in the operating theater were measured. The peri-abutment skin was graded on all postsurgery visits according to the Holgers Index (0 No irritation; 1 slight redness; 2 red and slightly moist tissue, no granuloma formation; 3 reddish and moist; sometimes granulation tissue; 4 removal of skin-penetrating implant necessary due to infection; 5 skin reactions assessed as Holgers ≥ 2 were defined as an incidence of inflammation).
A detailed description of procedures and assessments is available in the published protocol (20) and supplementary data (S1, see). In short, pain scores, the presence of skin dehiscence and sagging, soft-tissue height, soft-tissue overgrowth, and processor use were assessed on all follow-up visits, including extraconsultations. Skin sensibility and wound healing were assessed on standard follow-up visits. Implant Stability Quotient (ISQ) (Osstell AB, Gothenburg, Sweden) measurements were obtained directly after surgery and on all follow-up visits. At the 12-week follow-up, cosmetic results and the skin pocket were assessed. In addition, complications, adverse events (AE), serious adverse events, device deficiencies, and concomitant treatment were registered.
Outcomes
The outcomes have previously been described in detail (20). The primary end-point is the incidence of peri-abutment inflammation (Holgers Index ≥ 2) between surgery and the 12-week follow-up. Secondary outcomes include surgical procedure time, wound healing, the presence of dehiscence after surgery, soft-tissue overgrowth/height, loss of skin sensibility, pain, cosmetic results, ISQ measurements, and extrusion rate. Tertiary outcomes include the skin pocket size and total time of processor use. Intraoperative complications, postsurgical complications, AEs, serious adverse events, and device deficiencies were also noted.
Statistical Analysis
Sixty-two subjects were needed to ensure sufficient power (20). For the primary end-point, a χ2 test was performed. Holgers Index scores on standard visits were compared using the Mantel-Haenzel χ2 test. In overall terms, continuous variables were compared using the Mann–Whitney U test. Dichotomous variables were compared using the χ2 test or Fisher's exact test in the event of low counts. A two-way analysis of variance was conducted on the influence of anesthesia and surgical technique on the time spent in the operating theater. A mixed model was used to analyze ISQ High and ISQ Low. The extrusion rate was compared using the log-rank test. Statistical significance was assumed at 0.05. All analyses were performed with an intention-to-treat (ITT) population and a per-protocol (PP) population.
Missing data were mainly handled using a last observation carried forward method. Sensitivity analyses were performed for the incidence of inflammation (Holgers Index) and pain according to the following. For inflammation, the highest observed Holgers Index plus one was imputed in the sensitivity analysis, as well as a worst-case scenario using Holgers Index 4 scores. For the sensitivity analysis of pain scores, the highest possible value of 10 was imputed in a worst-case scenario.
Ethical Considerations
This study was performed in accordance with ISO 14155:2011 and the Declaration of Helsinki (21). The study was approved by the ethics committee at Maastricht University Medical Centre+ (NL50072.068.14) and is registered with ClinicalTrials.gov NCT02438618. Medisch Centrum Leeuwarden and Ziekenhuisgroep Twente were added as sites after acceptance of the amendment to extend the study to a multicenter study. The local ethics committees approved the execution of the protocol at these sites. All subjects provided written informed consent.
This study is sponsored by Oticon Medical AB (Askim, Sweden). The investigators had full access to all data. Monitoring was performed by the sponsor and TFS Develop (Zaltbommel, The Netherlands). Data analysis was conducted by Statistiska Konsultgruppen (Gothenburg, Sweden).
RESULTS
Patient Demographics
Between December 2014 and August 2016, 64 subjects were included (Fig. 2). Thirty-three subjects were randomized to the test group (52%) and 31 to the control group (48%). One subject was excluded during surgery due to the placement of a 3-mm implant, resulting in 63 subjects being analyzed in the ITT population. For the PP population, five subjects who experienced an implant extrusion were excluded. The patient characteristics were similar between the groups (Table 1). The primary outcome, secondary outcomes, and tertiary outcomes of the ITT population are presented in Table 2. The PP population results are presented in the supplementary data (S2–S3, see). Protocol deviations mainly included visits out of window.
FIG. 2.
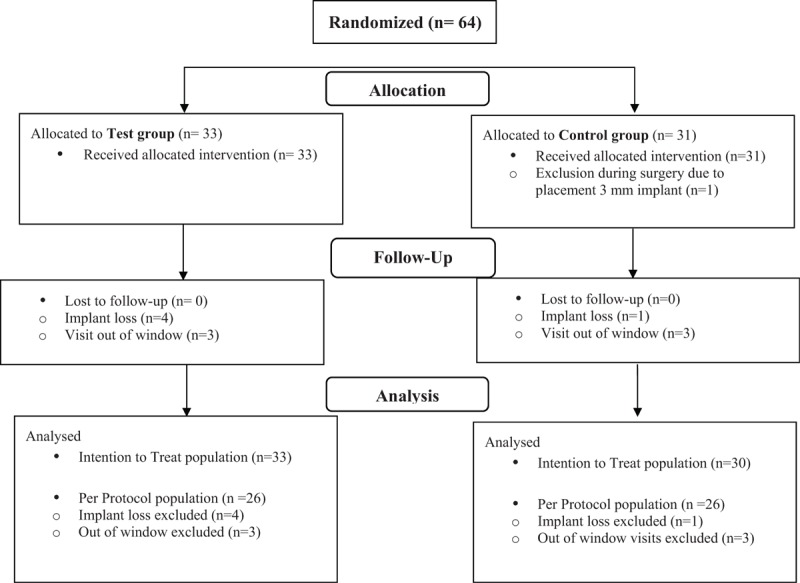
Subject flow chart. Sixty-four subjects were randomized. Twenty-nine subjects in each group were included in the per protocol analysis.
TABLE 1.
Baseline and surgery characteristics
| Baseline Characteristics | MIPS (n = 33) | Linear Incision (n = 30) |
| Age (yrs) | 50.3 (16.3) (44.5; 56.1) | 51.9 (16.1) (45.9; 57.9) |
| Sex | ||
| Male | 12 (36.4%) | 11 (36.7%) |
| Female | 21 (63.6%) | 19 (63.3%) |
| Type of hearing loss | ||
| Acquired conductive/mixed hearing loss | 26 (78.8%) | 25 (83.3%) |
| Single sided deafness | 6 (18.2%) | 5 (16.7%) |
| Congenital conductive hearing loss | 1 (3.0%) | 0 (0.0%) |
| Side scheduled for surgery | ||
| Right | 17 (51.5%) | 13 (43.3%) |
| Left | 16 (48.5%) | 17 (56.7%) |
| Smoking | ||
| No smoking | 26 (78.8%) | 22 (73.3%) |
| Smoking | 7 (21.2%) | 8 (26.7%) |
| Body mass index | 27.4 (6.4) (25.2; 29.7) | 28.3 (5.6) (26.2; 30.4) |
| Ethnicity | ||
| Caucasian | 33 (100.0%) | 30 (100.0%) |
| Surgery Characteristics | MIPS (n = 33) | Linear Incision (n = 30) |
| Type of anaesthesia | ||
| General | 16 (48.5%) | 17 (56.7%) |
| Local | 17 (51.5%) | 13 (43.3%) |
| Surgical time (min)a | 6.52 (2.84) 6.00 (2.00; 15.00) (5.51; 7.52) | 13.3 (3.5) 13.0 (8.0; 25.0) (12.0; 14.6) |
| Time in operation room (min)a/b | 44.2 (11.1) 45.0 (28.0; 72.0) (40.1; 48.3) | 50.8 (7.8) 50.5 (33.0; 69.0) (47.5; 54.1) |
| Skin thickness (mL) | 6.12 (1.80) (5.48; 6.76) | 6.03 (1.69) (5.40; 6.66) |
| Abutment length | ||
| 9 | 21 (63.6%) | 13 (43.3%) |
| 12 | 10 (30.3%) | 16 (53.3%) |
| 14 | 2 (6.1%) | 1 (3.3%) |
| Manual tightening performed | 12 (36.4%) | 8 (26.7%) |
| Concomitant medication during surgery | 28 (84.8%) | 23 (76.7%) |
| Intraoperative events | ||
| Drilling into vein | 2 (6.1%) | 2 (6.7%) |
| Dura mater exposed | 0 (0.0%) | 1 (3.3%) |
| Skin problems | 0 (0.0%) | 0 (0.0%) |
| Drilling into air pockets | 0 (0.0%) | 0 (0.0%) |
| Bleeding hematoma | 1 (3.0%) | 1 (3.3%) |
| Replacement suture | 0 (0.0%) | 0 (0.0%) |
Categorical variables: n (%) is presented. Continuous variables: mean (SD) (95% CI of mean) is presented.
aMedian (min; max) (95% CI) is presented.
bPatients with additional intervention during surgery were excluded.
MIPS indicates minimally invasive ponto surgery.
TABLE 2.
Outcomes
| Outcomes | |||
| Primary Outcome | MIPS (n = 33) | Linear Incision (n = 30) | P Value |
| Holgers index >= 2 at any time from surgery to 12 wk | 3 (9.1%) | 5 (16.7%) | 0.37 |
| Holgers index >= 2 at any time from surgery to 12 wk(Sensitivity analysis, highest observed Holgers index score plus one) | 3 (9.1%) | 5 (16.7%) | 0.37 |
| Holgers index >= 2 at any time from surgery to 12 wk(Sensitivity analysis, all implant losses have experienced Holgers index score of 4) | 7 (21.2%) | 5 (16.7%) | 0.65 |
| Secondary Outcomes | MIPS (n = 33) | Linear Incision (n = 30) | P Value |
| Wound dehiscence at 9 d | 16 (48.5%) | 22 (73.3%) | 0.078 |
| Extrusion rate | 4 (12.1%) | 1 (3.3%) | 0.19 |
| Loss of skin sensibility (mm)a | |||
| 9 d | 2.70 (6.13) 0.00(0.0; 25.0) (0.52; 4.87) | 13.5 (21.0) 4.5(0.0; 100.0) (5.6; 21.3) | 0.0050 |
| 3 wk | 0.375 (1.04) 0.0(0.0; 5.0) (0.00; 0.75) | 8.23 (17.25) 0.0(0.0; 70.0) (1.79; 14.68) | 0.013 |
| 12 wk | 0.14 (0.52) 0.0(0.0; 2.0) (0; 0.33) | 5.79 (13.75) 0.00(0.00; 60.00) (0.56; 11.02) | 0.0076 |
| No loss of sensibility (0 mm) | |||
| 9 d | 24 (72.7%) | 13 (43.3%) | |
| 3 wk | 27 (84.4%) | 18 (60.0%) | |
| 12 wk | 27 (93.1%) | 19 (65.5%) | |
| Soft tissue overgrowth | 0 (0.0%) | 0 (0.0%) | 1.00 |
| Mean skin level at 12 wk | 5.02 (1.42) (4.48; 5.56) | 5.08 (1.04) (4.68; 5.47) | 0.64 |
| Wound healing | |||
| 9 d | 7 (21.2%) | 5 (16.7%) | 0.89 |
| 3 wk | 22 (68.8%) | 20 (71.4%) | 0.82 |
| 12 wk | 29 (100.0%) | 29 (100.0%) | 1.00 |
| Pain scoringsa | |||
| Pain around implant | |||
| 9 d | 1.39 (1.87) 0.00(0.00;6.00) (0.73; 2.06) | 1.97 (2.61) 1.00(0.00:8.00) (0.99; 2.94) | 0.50 |
| 3 wk | 0.938 (1.22) 0.0(0.00;4.00) (0.50; 1.38) | 1.0 (1.61) 0.0(0.00;6.00) (0.04; 1.60) | 0.67 |
| 12 wk | 1.38 (2.23) 0.00(0.00;8.00) (0.53; 2.23) | 1.17 (2.04) 0.00(0.00;7.00) (0.40; 1.95) | 0.54 |
| Radiating pain | |||
| 9 d | 0.61 (1.66) 0.0(0.00;7.00) (0.02; 1.19) | 0.5 (1.57) 0.0(0.00;8.00) (0.0; 1.09) | 0.95 |
| 3 wk | 0.56 (1.39) 0.0(0.00;5.00) (0.06; 1.06) | 0.43 (1.36) 0.0(0.00;5.00) (0.0; 0.94) | 0.39 |
| 12 wk | 0.76 (1.86) 0.0(0.00;6.00) (0.05; 1.47) | 0.76 (1.8) 0.0(0.00;7.00) (0.04; 1.48) | 0.77 |
| Presence of headache | |||
| 9 d | 0.42 (1.39) 0.0(0.00;7.00) (0.0; 0.92) | 1.30 (2.39) 0.00(0.00;8.00) (0.41; 2.19) | 0.077 |
| 3 wk | 0.375 (1.476) 0.0(0.00;6.00) (0.0; 0.91) | 0.30 (1.32) 0.0(0.00;7.00) (0.0; 0.79) | 0.96 |
| 12 wk | 0.79 (2.1) 0.0(0.00;8.00) (0.00; 1.59) | 0.24 (0.83) 0.0(0.00;4.00) (0.08; 0.56) | 0.59 |
| Skin sagging at 12 wk | |||
| Quadrant 1 | 4 (13.8%) | 4 (14.3%) | 1.00 |
| Quadrant 2 | 7 (24.1%) | 19 (67.9%) | 0.0020 |
| Quadrant 3 | 1 (3.4%) | 1 (3.6%) | 1.00 |
| Quadrant 4 | 1 (3.4%) | 2 (7.1%) | 0.97 |
| Any quadrant | 8 (27.6%) | 20 (71.4%) | 0.0020 |
| Cosmetic resultsb | |||
| Observer scorings | |||
| Natural skin position | 2.72 (1.10) (2.31; 3.14) | 3.48 (1.38) (2.96; 4.01) | 0.025 |
| Extent of baldness | 2.24 (0.79) (1.94; 2.54) | 3.62 (1.35) (3.11; 4.13) | <0.0001 |
| Scarring | 2.41 (0.95) (2.05; 2.77) | 4.48 (1.79) (3.80; 5.16) | <0.0001 |
| Skin colour | 3.17 (1.23) (2.71; 3.64) | 3.86 (1.27) (3.38; 4.35) | 0.020 |
| Indentation | 2.34 (1.01) (1.96; 2.73) | 4.00 (1.63) (3.38; 4.62 | <0.0001 |
| Overall cosmetic score | 8.45 (0.74) (8.17; 8.73) | 7.17 (1.20) (6.72; 7.63) | <0.0001 |
| Subject scorings | |||
| Without processor (BAHS) | 8.42 (1.47) (7.83; 9.02) | 8.61 (1.29) (8.11; 9.11) | 0.75 |
| With processor attached | 7.41 (2.58) (6.39; 8.43) | 7.89 (1.83) (7.18; 8.60) | 0.73 |
| Tertiary Outcome | MIPS (n = 29) | Linear Incision (n = 29) | |
| Pocket size (normal position) (mm) | 0.207 (0.292) (0.096; 0.318) | 0.172 (0.251) (0.077; 0.268) | NP |
| Pocket size (maximum) (mm) | 0.672 (0.418) (0.513; 0.831) | 0.698 (0.440) (0.531; 0.866) | NP |
| Sound processor usage (h per wk) | 70.5 (37.2) (56.3; 84.6) | 90.3 (25.5) (80.3; 100.4) | NP |
Categorical variables: n (%). Continuous variables: mean (SD) (95% CI).
aMean (SD), median (min; max) (95% CI of the mean).
bCosmetic rating: observer outcomes (not including overall cosmetic score): 1–10. 1 being no difference with the healthy contralateral site, with 10 being the most negative difference with the healthy situation. Overall cosmetic and subject scorings: 1–10: 10 being the best cosmetic result and 1 being the most negative cosmetic result.
NP indicates not planned; MIPS, minimally invasive ponto surgery; BAHS, bone-anchored hearing systems.
Primary Outcome
The incidence of inflammation (Holgers Index ≥ 2) between surgery and 12 weeks showed no statistically significant difference between surgical techniques in the ITT population (p = 0.37) or PP population (p = 0.68) (Fig. 3A, Table 2 and Table S3, see). Five subjects experienced an episode of inflammation in the control group (16.7%) compared with three subjects in the test group (9.1%). Sensitivity analyses yielded similar results.
FIG. 3.
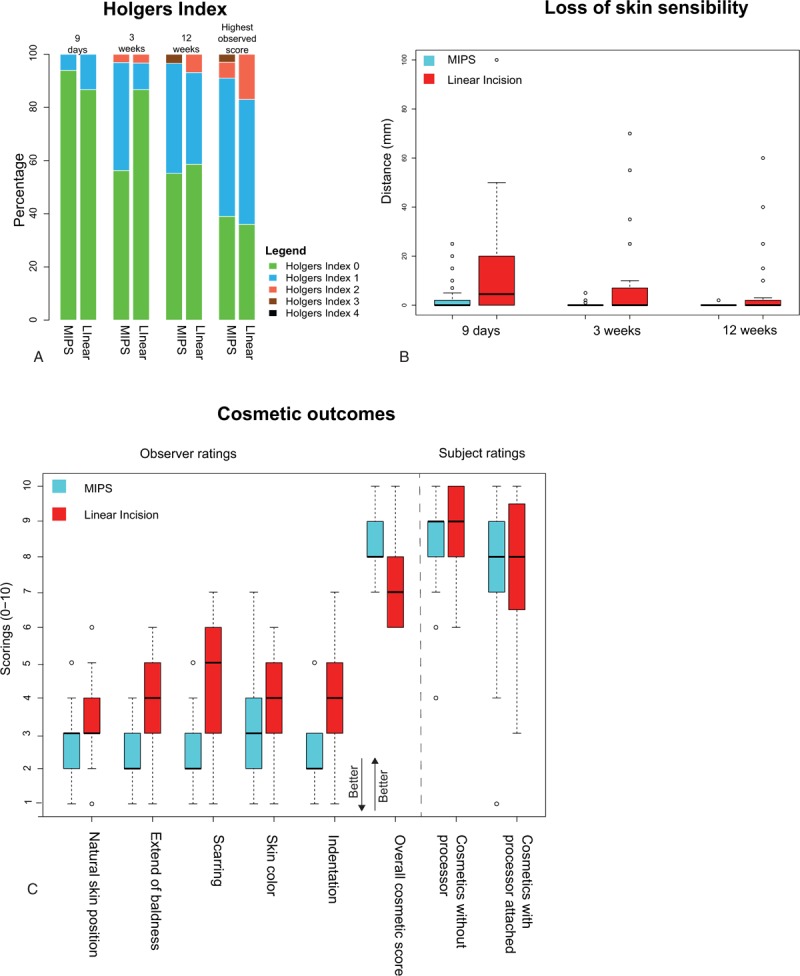
Primary outcome, loss of skin sensibility, and cosmetic aspects. A, Stacked bar chart for the Holgers Index scores on standard follow-up visits and the highest observed Holgers Index score. B, Box plots of loss of skin sensibility per treatment group on standard follow-up visits. C, Cosmetic outcomes at 12 weeks per treatment group. Cosmetic outcome specifics are described in Table 2.
Secondary and Tertiary End-points
Surgery
Surgery characteristics are presented in Table 1. Intraoperative events were few and comparable between the two groups (Table 2/S2, see). There were no conversions to linear incision for patients subjected to the MIPS surgery technique. The surgical procedure time was significantly shorter in the test group compared with the control group, with a mean time of 6.52 minutes (SD = 2.84) and 13.3 minutes (SD = 3.5) respectively (p < 0.0001). The time spent in the operating theater was significantly influenced by both the type of anesthesia (p < 0.0001) and the surgical technique (p = 0.0062). Adverse events during surgery, device deficiencies, and other device complaints are described in S5 (see).
Soft-tissue Outcomes
Wound healing at the implant-soft-tissue interface did not differ significantly between groups (Table 2). All wounds had healed after 12 weeks. At the 9-day follow-up, a slight dehiscence of the skin–abutment interface was observed in 14 subjects (48.5%) in the test group compared with 21 subjects (73.3%) in the control group (p = 0.078). An additional analysis of the Holgers Index ratings per visit revealed no difference at 9 days or 12 weeks (p = 0.33, p = 0.64) (Fig. 3A). At 3 weeks, a significantly larger number of cases with Holgers 1 scores were observed in the test group compared with the control group (40.6% versus 10%, p = 0.027). Skin sagging, mainly observed in the most cranial posterior quadrant, was present in 8 subjects (27.6%) and 20 subjects (71.4%) in the test and control group respectively (p = 0.002). There were no cases of soft-tissue overgrowth or significant differences in skin height between treatment groups.
Sensibility and Pain
Loss of sensibility was significantly less in the test group compared with the control group on all follow-up visits (Table 2, Fig. 3B). At 9 days, the mean loss of sensibility was 2.70 mm (SD = 6.13) and 13.5 mm (SD = 21.0) for the test and control group respectively (p = 0.005, Table 2, Fig. 3B). At 12 weeks, the maximum area affected was 2 mm in the test group and 60 mm in the control group. No significant differences in pain scores for pain around the BAHS, radiating pain or headache related to the BAHS were observed (Table 2).
Cosmetic Results
Natural skin position, extent of baldness, scarring, skin color, and indentation, as well as overall observer scores, were all significantly better in the test group compared with the control group (Table 2, Fig. 3C). Subject satisfaction scores relating to cosmetic results, with or without the processor attached, did not differ significantly between groups.
Implant Loss
During the 12-week follow-up period, four implants in the test group were extruded (12.1%) compared with one in the control group (3.3%) (Fig. 4A). Implant loss occurred between 25 days and 90 days postsurgery. A nonsignificant p value of 0.19 was found using the log-rank test (hazard ratio = 3.89, 95% CI = 0.4; 34.8).
FIG. 4.
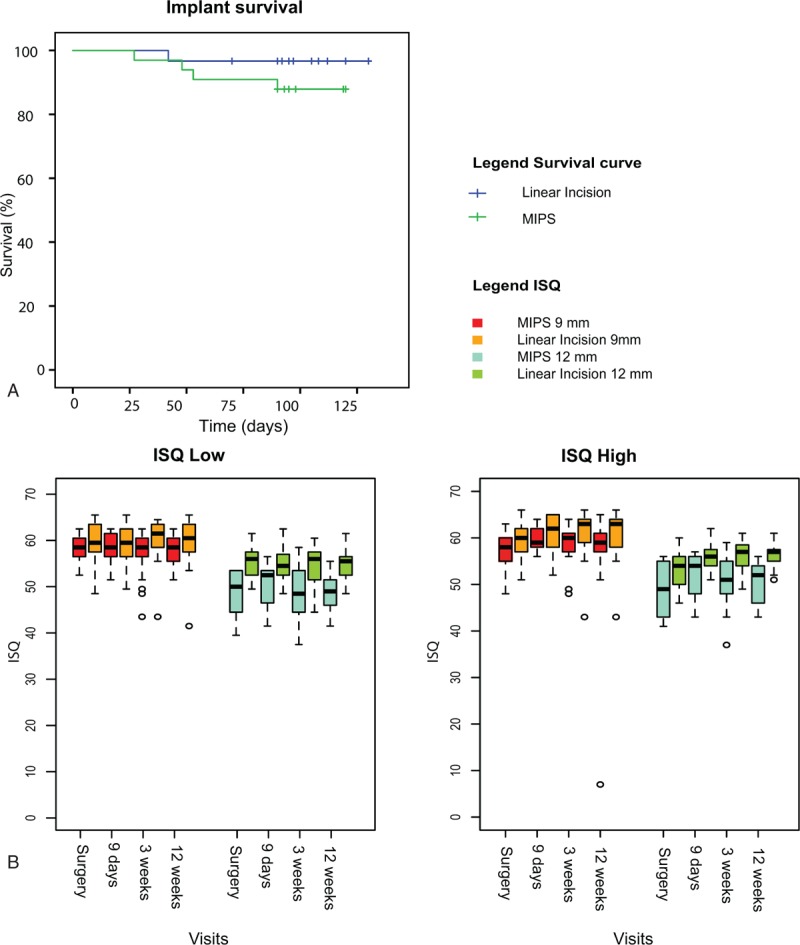
Extrusion rate and ISQ values. A, Kaplan–Meier survival plot for bone conduction hearing implants displayed per surgical technique. B, Boxplots of ISQ measurements at surgery and on standard follow-up visits. ISQ measurements are displayed for ISQ low and ISQ high per abutment length and surgical technique. ISQ indicates implant stability quotient.
Implant Stability Quotient
ISQ high and low on standard visits are presented in Figure 4B. Both ISQ high and ISQ low were significantly influenced by the surgical treatment (p = 0.014, p = 0.007) and abutment length (p < 0.001, p < 0.001). In overall terms, ISQ high was influenced by time (p < 0.002), but ISQ low was not (p = 0.38). The model is presented in S4 (see). In the test group, ISQ high was 2.35 points (95% CI = −4.21, −0.49) lower and ISQ low was 2.7 points lower (95% CI = −4.65, −0.76) compared with the control group. No obvious association was observed between the initial ISQ and implant loss (S6, see).
Serious Adverse Events, Adverse Events, and Device Complaints
Serious adverse events, adverse events, device complaints, and device deficiencies are presented in the supplementary data (S5, see). Common and expected complications related to BAHS were observed in both groups. Thirty-one subjects (91.4%) in the test group and 25 subjects (83.3%) in the control group had at least one reported AE, with a total of 168 AEs.
DISCUSSION
Principal Findings
This randomized, controlled, clinical trial compared the outcomes between surgery and the 12 week follow-up of two surgical procedures for the installation of BAHS: a new minimally invasive surgical technique (test) and the linear incision technique with soft-tissue preservation (control). No significant differences in the incidence of inflammation (Holgers ≥ 2) were found between procedures. However, MIPS surgery resulted in a significantly better outcome in terms of sensibility, surgical time, time spent in the operating theater, and cosmetic results. In addition, significantly less skin sagging and a tendency toward less dehiscence were observed in the test group. The test group exhibited significantly lower ISQ values and the extrusion rate was nonsignificantly higher.
Surgery
Few intraoperative events or adverse events were observed, with no clear differences between surgical techniques, thereby underscoring the reliability of both the linear incision technique and the punch-only approach. The minimally invasive nature of the MIPS procedure, the reduced surgical time, together with an efficient drilling sequence, make it a suitable technique to be performed under local anesthesia. Several surgeons have indicated that MIPS seems deceptively easy and that there is a learning curve (19). Similar experiences were observed in dental surgery when the flapless placement of dental implant systems was introduced. There is a need for appropriate training, as there seems to be a learning curve to achieve treatment success (22,23).
Soft-tissue Outcomes
Although no difference in the incidence of inflammation was observed between groups, on the 3-week follow-up visit, more mild skin reactions (Holgers = 1) were observed in the test group. In addition, for both techniques, a slight dehiscence with nonepithelialized skin was often observed at 9 days and it had typically disappeared by 3 weeks. It is possible that the observed mild skin reaction at 3 weeks in the MIPS group reflects a difference in the temporal course of the healing process compared with the linear incision technique where, in contrast to MIPS, a flap is raised. Moreover, we think that skin sagging could be influenced by the surgical manipulation of the skin and positioning during surgery. The fact that skin sagging was less prevalent using the MIPS technique corroborates this hypothesis. Despite these differences in soft-tissue outcomes, wound healing was comparable between techniques in the first 3 months. The long-term data for these patients will be published when available. To acquire a greater understanding of the mechanisms underpinning healing and soft-tissue reactions, additional tools such as quantitative polymerase chain reaction and microbiota were applied in this study and will supplement the findings reported here. It is to be hoped that this will enable an understanding of the temporal course of the tissue response.
Other Outcomes
The presence and the extent of loss of sensibility and cosmetic appearance have historically been an under-reported adverse patient outcome after BAHS implantation using the tissue reduction technique. Recent studies with a tissue preservation approach have shown improvements (9,10). In this study, numbness was even less prevalent after MIPS surgery compared with a linear incision approach. As a result, this could now be regarded as irrelevant in relation to MIPS. Cosmetic scoring results with the processor mounted on the BAHS were lower compared with the results without a mounted processor. As demonstrated in our results, it could be advantageous to use a scoring system that includes cosmetic results with and without the processor.
Adverse Events
In this study, we meticulously gathered possible AEs and this is the most likely explanation for the relatively large number of observed AEs. In overall terms, we found no clear differences in AEs between techniques. Difficulty sleeping on the implant side of the head was frequently spontaneously mentioned. This has previously never been described. To facilitate skin preservation techniques, abutment lengths have increased. The abutment Inadvertently sticks out further from the skull, which may explain this complaint. The severity of this complaint needs to be evaluated before it can be compared or put in context with other complaints such as pain, loss of skin sensibility, or inflammation.
ISQ
The mixed model revealed an association between the ISQ and abutment length, as well as the time after surgery. This is in line with previous findings (9,24–26). Interestingly, the ISQ was significantly influenced by the surgical technique. Both ISQ high and low were approximately 2.5 points lower in the test group compared with the control group. In comparison, abutment length influenced ISQ values by 6 to 12 points. Associations between the ISQ and surgical technique have previously not been reported for BAHS, although an effect of this kind has been reported for dental implants (27). Compared with the effect the abutment length has on ISQ values, the difference in the ISQ between the test and control group is small and the clinical relevance is probably insignificant. Furthermore, we found no relationship between the primary ISQ values and the extrusion rate (S6, see).
Extrusion
Studies published in recent years report high implant survival rates, even when using punch-only techniques, with loss rates between 0 and 5.8% being reported (9,15,17,25). We observed a survival rate of 88% for MIPS. During the last few decades, BAHS surgical tools have undergone only minor adjustments. For MIPS, a new drilling protocol with guided drilling via the cannula was developed to facilitate a flapless approach. These drills are more efficient and require less manual pressure, resulting in different tactile feedback to the surgeon compared with the classical systems (28). Several explanations have been postulated throughout the introduction of flapless approaches for dental implants and they could also be applicable to MIPS (29,30). Diminished visibility may lead to an angulated drilling/implant placement or incomplete insertion. Reduced access for external irrigation may lead to thermal damage (31,32). An in-vitro study comparing heat generation for flap and flapless drilling showed that the temperature was slightly higher for the flapless procedure in dental implants (33). Extrusion is a concern associated with MIPS and a possible association with cooling and implant positioning requires further study. Training, following instructions and caution all seem to be relevant factors for success.
Strengths and Limitations
One of the main strengths of this study is the multicenter, randomized, controlled design, with a large sample size of 63 subjects. In this study, we re-evaluated outcome measurements to increase reliability. In addition, adverse events were gathered in conjunction with regular follow-up visits and extraconsultations. We think that the setup of this study allows for a better estimation of complications such as inflammation due to the stringency applied to calculate and use a cumulative percentage of all visits, including extra visits. To allow for a correct comparison, no differences in implant type were allowed. Several limitations are relevant to this study. Healing, dehiscence, and the Holgers Index possibly influence each other, warranting some caution when interpreting the observed soft-tissue outcomes. A standardized, well-defined outcome measurement set would improve BAHS-related outcomes. The recently established AuroNet could aid in the creation of a standardized outcome set of this kind (34). Although all the surgeons were trained before the first MIPS surgery in the trial, experience between techniques differed. As this technique and instruments are different, a learning curve effect could also play a role. In our study population, due to chance all subjects were of Caucasian origin, limiting the general applicability of this data to a more mixed ethnic situation. Scar formation and BAHS-related skin complications have been shown to be affected by ethnicity (35). The previously observed higher rate of complications in African Americans (35) might even benefit more from improved outcomes.
Perspective
Encouraging outcomes, particularly patient-centered outcomes such as sensibility loss and cosmetic appearance, were observed for MIPS compared with the linear incision with soft-tissue preservation. Despite the fact that a nonsignificant statistical difference was found, extrusion remains a concern related to MIPS. Although reduced cooling might be an aggravating factor, an assumption like this requires careful evaluation and follow-up.
Longer term 22-month follow-up results from this study are expected to become available in the second half of 2018. Therein, data will be provided on the incidence of soft tissue inflammation, long-term processor usage, and changes in quality of life over time within subjects. At this point in time, a well-founded recommendation on surgical technique can hopefully be made.
As presented in our results, the relationship between the ISQ and extrusion is not straightforward, warranting further clinical data on an association, or lack thereof, between the ISQ and biomechanical stability. Both the inter- and intrarater reliability of the Holgers Index and biological validity would benefit from further study. As part of this clinical study, samples were taken for bacteria and tissue status to further investigate a subset of research questions (20). These data will hopefully shed further light on the correlations (or lack thereof) between clinical parameters, the Holgers index, the ISQ, and the biological tissue responses.
CONCLUSION AND RECOMMENDATION
No significant differences between MIPS and the linear incision technique were observed in terms of skin inflammation in the first 3 months. MIPS results in a statistically significant reduction in the loss of skin sensibility, less skin sagging, improved cosmetic results, and reduced surgical time. Although nonsignificant, the implant extrusion rate warrants further research.
Supplementary Material
Acknowledgments
The authors acknowledge the following people for their contribution to the investigation: Arpita Singh, Jan Leder (Oticon Medical AB), Marc van Hoof, Danielle Bollen, Afra Bruinen, Lucien Anteunis(†) (MUMC+), and Joanne Schelhaas (Pento Audiologisch Centrum Twente). The authors acknowledge the valuable feedback on this work by Professor P Thomsen (University of Gothenburg).
Footnotes
T.C. and M.L.J. contributed equally to this work.
Sponsor details: Oticon Medical AB, Askim Sweden (Oticonmedical.com)
This study is supported by a research grant from Oticon Medical AB (Askim, Sweden). T.C. is supported by a research grant from Oticon Medical AB (Askim, Sweden). M.L.J., S.J., and M.H. are paid employees of Oticon Medical. M.L.J. is supported by the research group of Professor P Thomsen (University of Gothenburg) and the resources provided by the Swedish Medical Research Council (K2015-52X-09495-28-4), ALFGBG-448851), the IngaBritt and Arne Lundberg Foundation and the Area of Advance Materials supported by the Swedish Government.
REFERENCES
- 1.Crowson MG, Tucci DL. Mini review of the cost-effectiveness of unilateral osseointegrated implants in adults: Possibly cost-effective for the correct indication. Audiol Neurotol 2016; 21:69–71. [DOI] [PubMed] [Google Scholar]
- 2.Monksfield P, Jowett S, Reid A, Proops D. Cost-effectiveness analysis of the bone-anchored hearing device. Otol Neurotol 2011; 32:1192–1197. [DOI] [PubMed] [Google Scholar]
- 3.Stenfelt S, Goode RL. Bone-conducted sound: Physiological and clinical aspects. Otol Neurotol 2005; 26:1245–1261. [DOI] [PubMed] [Google Scholar]
- 4.Tjellström A, Lindström J, Hallén O, Albrektsson T, Brånemark PI. Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol 1981; 2:304–310. [PubMed] [Google Scholar]
- 5.Holgers KM, Tjellström A, Bjursten LM, Erlandsson BE. Soft tissue reactions around percutaneous implants: A clinical study of soft tissue conditions around skin-penetrating titanium implants for bone-anchored hearing aids. Am J Otol 1988; 9:56–59. [PubMed] [Google Scholar]
- 6.Kiringoda R, Lustig LR. A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol 2013; 34:790–794. [DOI] [PubMed] [Google Scholar]
- 7.Dun CA, Faber HT, de Wolf MJ, Mylanus EA, Cremers CW, Hol MK. Assessment of more than 1,000 implanted percutaneous bone conduction devices: Skin reactions and implant survival. Otol Neurotol 2012; 33:192–198. [DOI] [PubMed] [Google Scholar]
- 8.Verheij E, Bezdjian A, Grolman W, Thomeer HG. A systematic review on complications of tissue preservation surgical techniques in percutaneous bone conduction hearing devices. Otol Neurotol 2016; 37:829–837. [DOI] [PubMed] [Google Scholar]
- 9.den Besten CA, Bosman AJ, Nelissen RC, Mylanus EAM, Hol MK. Controlled clinical trial on bone-anchored hearing implants and a surgical technique with soft tissue preservation. Otol Neurotol 2016; 37:504–512. [DOI] [PubMed] [Google Scholar]
- 10.Hultcrantz M. Outcome of the bone-anchored hearing aid procedure without skin thinning: A prospective clinical trial. Otol Neurotol 2011; 32:1134–1139. [DOI] [PubMed] [Google Scholar]
- 11.Nelissen RC, Stalfors J, de Wolf MJ, et al. Long-term stability, survival, and tolerability of a novel osseointegrated implant for bone conduction hearing: 3-year data from a multicenter, randomized, controlled, clinical investigation. Otol Neurotol 2014; 35:1486–1491. [DOI] [PubMed] [Google Scholar]
- 12.Mowinckel MS, Møller MN, Wielandt KN, Foghsgaard S. Clinical outcome of a wide-diameter bone-anchored hearing implant and a surgical technique with tissue preservation. Otol Neurotol 2016; 37:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tjellström A. Percutaneous implants in clinical practice. CRC Crit Rev Biocompatibility 1985; 1:205–228. [Google Scholar]
- 14.van de Berg R, Stokroos RJ, Hof JR, Chenault MN. Bone-anchored hearing aid: A comparison of surgical techniques. Otol Neurotol 2010; 31:129–135. [DOI] [PubMed] [Google Scholar]
- 15.Gordon SA, Coelho DH. Minimally invasive surgery for osseointegrated auditory implants: A comparison of linear versus punch techniques. Otolaryngol Head Neck Surg 2015; 152:1089–1093. [DOI] [PubMed] [Google Scholar]
- 16.Goldman Ra, Shaia WT, Georgolios A. The punch method for bone-anchored hearing aid placement. Otolaryngol Head Neck Surg 2012; 147 (2 suppl):78. [DOI] [PubMed] [Google Scholar]
- 17.Dumon T, Medina M, Sperling NM. Punch and Drill: Implantation of bone anchored hearing device through a minimal skin punch incision versus implantation with dermatome and soft tissue reduction. Ann Otol Rhinol Laryngol 2015; 125:199–206. [DOI] [PubMed] [Google Scholar]
- 18.Johansson M, Holmberg M. Design and clinical evaluation of MIPS—A new perspective on tissue preservation. White Pap Oticon Medical, Askim, Sweden 2015; October, Rep No M524252. [Google Scholar]
- 19.Johansson ML, Stokroos RJ, Banga R, et al. Short-term results from seventy-six patients receiving a bone anchored hearing implant installed with a novel minimally invasive surgery technique. Clin Otolaryngol 2017; 42:1043–1048. [DOI] [PubMed] [Google Scholar]
- 20.Calon TGA, van Hoof M, van den Berge H, et al. Minimally Invasive Ponto Surgery compared to the linear incision technique without soft tissue reduction for bone conduction hearing implants: study protocol for a randomized controlled trial. Trials 2016; 17:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WMA. Ethical Principles for Medical Research Involving Human Subjects. WMA Declar Helsinki 2013; 1–5. (June 1964). [Google Scholar]
- 22.Becker W, Goldstein M, Becker BE, Sennerby L. Minimally invasive flapless implant surgery: A prospective multicenter study. Clin Implant Dent Relat Res 2005; 7 suppl 1:S21–S27. [DOI] [PubMed] [Google Scholar]
- 23.Moraschini V, Velloso G, Luz D, Barboza EP. Implant survival rates, marginal bone level changes, and complications in full mouth-rehabilitation with flapless computer-guided surgery: A systematic review. Int J Oral Maxillofac Surg 2015; 44:892–901. [DOI] [PubMed] [Google Scholar]
- 24.Høgsbro M, Agger A, Johansen LV. Successful loading of a bone-anchored hearing implant at two weeks after surgery: Randomized trial of two surgical methods and detailed stability measurements. Otol Neurotol 2015; 36:e51–e57. [DOI] [PubMed] [Google Scholar]
- 25.Nelissen RC, den Besten CA, Mylanus EA, Hol MK. Stability, survival, and tolerability of a 4.5-mm-wide bone-anchored hearing implant: 6-month data from a randomized controlled clinical trial. Eur Arch Otorhinolaryngol 2016; 273:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelissen RC, Wigren S, Flynn MC, Meijer GJ, Mylanus EA, Hol MK. Application and interpretation of resonance frequency analysis in auditory osseointegrated implants: A review of literature and establishment of practical recommendations. Otol Neurotol 2015; 36:1518–1524. [DOI] [PubMed] [Google Scholar]
- 27.Manzano-Moreno FJ, Herrera-Briones FJ, Bassam T, Vallecillo-Capilla MF, Reyes-Botella C. Factors affecting dental implant stability measured using the ostell mentor device: A systematic review. Impant Dent 2015; 24:565–577. [DOI] [PubMed] [Google Scholar]
- 28.Johansson ML, Omar O. Comparative experimental study on a new drilling system for minimally invasive implantation of bone-anchored hearing aid systems. 10th World Biomater Congr (WBC), Montr Canada. 2016. [Google Scholar]
- 29.Chrcanovic BR, Albrektsson T, Wennerberg A. Flapless versus conventional flapped dental implant surgery: a meta-analysis. PLoS One 2014; 9:e100624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Bruyn H, Atashkadeh M, Cosyn J, van de Velde T. Clinical outcome and bone preservation of single TiUnite Implants installed with flapless or flap surgery. Clin Implant Dent Relat Res 2011; 13:175–183. [DOI] [PubMed] [Google Scholar]
- 31.Sclar AG. Guidelines for flapless surgery. J Oral Maxillofac Surg 2007; 65 (7 suppl):20–32. [DOI] [PubMed] [Google Scholar]
- 32.Katsoulis J, Avrampou M, Spycher C, Stipic M, Enkling N, Mericske-Stern R. Comparison of implant stability by means of resonance frequency analysis for flapless and conventionally inserted implants. Clin Implant Dent Relat Res 2012; 14:915–923. [DOI] [PubMed] [Google Scholar]
- 33.Jeong SM, Yoo JH, Fang Y, Choi BH, Son JS, Oh JH. The effect of guided flapless implant procedure on heat generation from implant drilling. J Craniomaxillofac Surg 2014; 42:725–729. [DOI] [PubMed] [Google Scholar]
- 34.Tysome JR, Hill-Feltham P, Hodgetts WE, et al. The Auditory Rehabilitation Outcomes Network: An international initiative to develop core sets of patient-centred outcome measures to assess interventions for hearing loss. Clin Otolaryngol 2015; 40:512–515. [DOI] [PubMed] [Google Scholar]
- 35.Zeitler DM, Herman BS, Snapp HA, Telischi FF, Angeli SI. Ethnic disparity in skin complications following bone-anchored hearing aid implantation. Ann Otol Rhinol Laryngol 2012; 121:549–554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


