Sexually transmitted infection positivity among female sexual assault victims was comparable to nonvictims. Adjusted for confounders, male victims had lower odds for a sexually transmitted infection than did nonvictims. Return rate of victims for treatment was high.
Supplemental digital content is available in the text.
Abstract
Background
Victims could become infected with sexually transmitted infections (STIs) during a sexual assault. Several guidelines recommend presumptive antimicrobial therapy for sexual assault victims (SAVs). We assessed the STI positivity rate and treatment uptake of female and male SAVs at the Amsterdam STI clinic.
Methods
Sexual assault victims answered assault-related questions and were tested for bacterial STI (chlamydia, gonorrhea, and syphilis), hepatitis B, and HIV during their initial visits. Sexual assault victim characteristics were compared with non-SAV clients. Backward multivariable logistic regression analysis was conducted to assess whether being an SAV was associated with a bacterial STI. The proportion of those returning for treatment was calculated.
Results
From January 2005 to September 2016, 1066 (0.6%) of 168,915 and 135 (0.07%) of 196,184 consultations involved female and male SAVs, respectively. Among female SAVs, the STI positivity rate was 11.2% versus 11.6% among non-SAVs (P = 0.65). Among male SAVs, the STI positivity rate was 12.6% versus 17.7% among non-SAVs (P = 0.12). In multivariable analysis, female SAVs did not have increased odds for an STI (odds ratio 0.94; 95% confidence interval, 0.77–1.13), and male SAVs had significantly lower odds for an STI (odds ratio, 0.60; 95% confidence interval, 0.36–0.98). Of SAVs requiring treatment, 89.0% (female) and 92.0% (male) returned.
Conclusions
The STI positivity rate among female SAVs was comparable with female non-SAVs, but male SAVs had lower odds for having a bacterial STI than did male non-SAVs, when adjusting for confounders. The return rate of SAV for treatment was high and therefore does not support the recommendations for presumptive therapy.
Approximately, 16.5% of women and 3.8% of men in the Netherlands have experienced vaginal or anal penetration, or oral sex without consent at least once in their lifetime.1 Before the age of 16, 8.1% of women and 2.5% of men experience this kind of sexual assault.1 Most earlier studies have shown a high positivity rate of sexually transmitted infections (STIs) at initial evaluation of sexual assault victims (SAVs).2 However, Beck-Sagué and Solomon3 argued that adolescents and adults frequently acquire STI through consensual sexual activity, whereas it is unclear whether victims are infected during an assault. Data on the STI positivity among female SAVs are scarce and even less is known about rates among male SAVs.2
The Centers for Disease Control and Prevention 2015 STD Treatment Guidelines recommend empirical presumptive antimicrobial therapy (before test results are available) targeting gonorrhea, chlamydia, and trichomoniasis at the initial evaluation of SAVs, in view of their high STI positivity rates and low rate of return for follow-up visits.2 In the Netherlands, there is no national guideline concerning STI testing and presumptive therapy for SAV.
The objective of this study was to assess the STI positivity rate and the follow-up rate in adolescent and adult female and male SAVs attending the STI clinic of Amsterdam, the Netherlands. In addition, we used data of both SAV and non-SAV clients to study whether being a victim of a sexual assault was associated with an STI diagnosis.
METHODS
Study Population and Procedures
The STI outpatient clinic of the Public Health Service of Amsterdam (GGD Amsterdam) annually performs around 45,000 free-of-charge and anonymous STI consultations. Before 2009, clients could walk in (“first-come, first-served” policy). Since 2009, clients had to apply for an appointment (online or by telephone): only high-risk clients and SAV received an appointment. Clients considered high-risk for STI included those reporting STI-related symptoms, those referred by a health care provider, those notified of an STI, men who have sex with men, commercial sex workers, clients who paid for sex (until 2015), clients younger than 25 years, clients reporting 3 or more sex partners (until 2015), clients of non–Western European and non–North American ethnicity, and/or sexual partners of people of these ethnicities. All behavior indicators refer to the 6 months before consultation. Demographics, detailed medical and sexual history, and test results were registered in an electronic patient database. Only in case of contact with an STI (proved with a notification card) or a positive Gram smear result, presumptive antibiotic treatment was given.
Since 2012, clients younger than 25 years without previously mentioned risk factors have only been offered chlamydia and gonorrhea testing.4 All other clients were tested for Chlamydia trachomatis, Neisseria gonorrhoeae, and syphilis. HIV testing was offered on indication before 2007. From 2007 onward, an opt-out strategy was adopted.
C. trachomatis was tested using nucleic acid amplification tests, and N. gonorrhoeae was tested using nucleic acid amplification tests or culture. Details on anatomical sites tested, laboratory tests used, and manufacturer details are presented in Supplemental Table 1, http://links.lww.com/OLQ/A235.
STI Clinic Procedure in Case of Sexual Assault
In this article, sexual assault refers to nonconsensual penetration of the mouth, anus, or vagina, because these acts are associated with exposure to STI.1,2 Sexual assault victims with a minimum age of 12 years were referred to the clinic by a health care provider (e.g., general practitioner or forensic physician) or by the police, or came of their own initiative. A professional asked all clients at the STI clinic whether their request for an STI test was related to a sexual assault. Sexual assault as reason for visit was only registered at the first STI consultation after an assault. If the clinic consultation took place within 72 hours after the assault, postexposure prophylaxis for HIV was considered according to a “risk of HIV exposure” assessment.5 Unless already vaccinated, all SAVs were offered a hepatitis B vaccination. Sexual assault victims were routinely tested for chlamydia, gonorrhea, syphilis, hepatitis B, and HIV. During consultation, questions were asked related to the sexual assault. From July 2013 onward, the time (≤7 days, >7 days) between the assault and STI consultation was registered.
Statistical Analysis
The anonymized medical records from the electronic patient database were analyzed in SPSS version 21.0 (IBM Corp, Armonk, NY). Sexual preference was determined by the sex of sexual partners in the preceding 6 months. Before 2011, ethnicity was self-reported. From 2011 onward, ethnicity was defined based on an algorithm combining country of birth index, mother, and father.6 Ethnicity was categorized into Dutch versus non-Dutch, consisting of 9 different groups (see Table 1 for these groupings). Number of sex partners was categorized in quartiles. Age was categorized in 10-year age groups. A bacterial STI diagnosis was defined as being diagnosed as having C. trachomatis, N. gonorrhoeae, and/or infectious syphilis. HIV status—based on self-reported HIV-positive status and HIV test result at consultation—was categorized into known positive, newly diagnosed positive, negative, and unknown.
TABLE 1.
Demographics, Sexual Behavior, and Diagnosed STIs Among 1066 Clinic Visits From Female Victims of a Sexual Assault and 165,742 Clinic Visits From Female Clients Who Were Not a Victim of Sexual Assault of the STI Clinic in Amsterdam, the Netherlands; January 2005 to September 2016
Whether someone was an SAV was registered per consultation, and all consultations, including those of clients with multiple consultations, were included in the analysis. For readability, this article will use the terms “SAV clients” and “non-SAV clients” instead of “consultations in which a sexual assault was (or was not) reported.” Characteristics of SAV were compared with non-SAV for men and women separately. χ2 Test or Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables were used. For SAV with a bacterial STI having to return for treatment, the proportion lost to follow-up was assessed. Univariable and multivariable logistic regression analyses were performed to analyze whether being a victim of a sexual assault was an independent determinant for a bacterial STI diagnosis. To correct for repeated measurements (clients with multiple consultations), we used generalized estimating equations (STATA 13.0 software; STATA Intercooled, College Station, TX). Multivariable model building was done using a backward stepwise procedure, including only those variables with a univariable P value of less than 0.25.7 All variables with a P < 0.05 were kept in the final multivariable model. The variable of interest—being victim of a sexual assault—was forced into the model. The variables “physical symptoms” and “being notified” were excluded from the multivariable analysis, because they are consequences of a possible STI, and not risk factors or causes. To correct for possible differences introduced into the clinical population in 2009 by the transition from a walk-in to an appointment-based clinic, univariable and multivariable subanalyses were performed for 2005–2008 and 2009–2016. P values of less than 0.05 were considered statistically significant.
RESULTS
Between January 2005 and September 2016, 168,915 consultations were performed among female and 196,184 consultations among male clients. Of these, 1066 (0.63%) and 135 (0.07%) consultations involved female and male SAVs, respectively (Fig. 1). Of 1066 consultations among female SAVs, 27 clients had 2, and 2 clients had 3 SAV consultations. Three male SAVs had 2, and 1 client had 3 SAV consultations.
Figure 1.
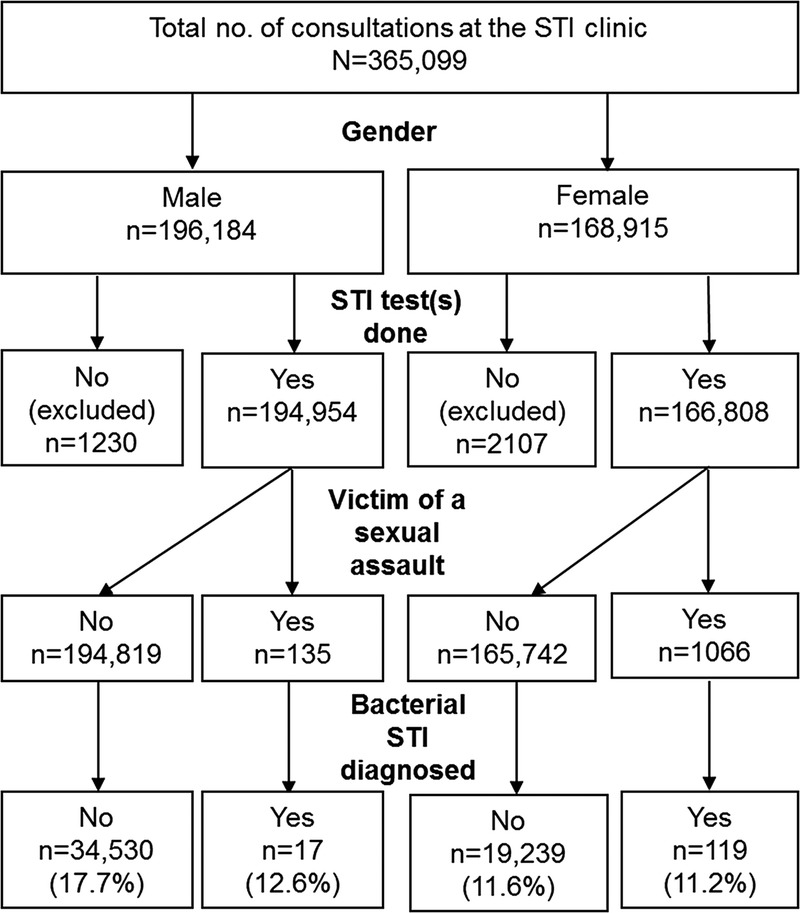
Flowchart of consultations at the STI clinic in Amsterdam, the Netherlands; January 2005 to September 2016.
Female SAVs
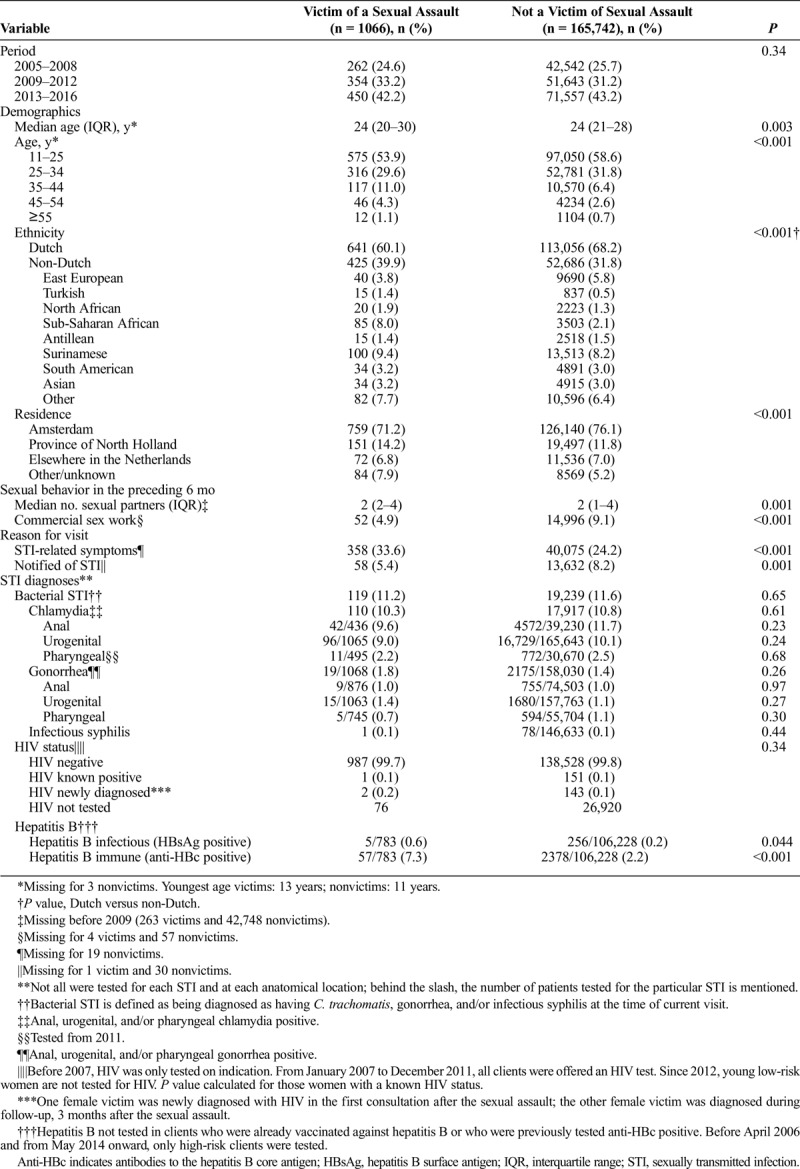
Compared with non-SAV female clients, SAVs were significantly older, less often Dutch, and more often of sub-Saharan African origin (Table 1). Sexual assault victims less often lived in Amsterdam, reported more sexual partners, more frequently reported STI-related symptoms, were less often notified of STI exposure, and reported commercial sex work in the preceding 6 months less often. The proportion of SAV consultations diagnosed as having a bacterial STI (n = 119; 11.2%) did not differ from non-SAVs (11.6%, P = 0.65). However, SAVs did more frequently test hepatitis B surface antigen and anti-hepatitis B core-antigen positive (P = 0.044 and P < 0.001). In the univariable logistic regression analysis—except from being an SAV—all other determinants (age, ethnicity, residence, HIV status, number of sexual contacts, and commercial sex work) were significantly associated with having a bacterial STI (Supplemental Table 2, http://links.lww.com/OLQ/A235). After adjusting for the previously mentioned variables in the multivariable logistic regression analysis, being an SAV was not associated with having a bacterial STI (odds ratio [OR], 0.94; 95% confidence interval [CI], 0.77–1.13). In the multivariable subanalyses, SAVs did not have higher odds for diagnosis with a bacterial STI (2005–2008: OR, 1.16 [95% CI, 0.79–1.69]; 2009–2016: OR, 0.84 [95% CI, 0.67–1.05]).
Of 119 female SAVs with a bacterial STI, presumptive antibiotic treatment was given to 10. Of the remaining 109, 97 (89%) returned to the clinic for treatment and 12 (11.0%) did not return: 8 could not be reached, 2 were treated by their general practitioner, 1 was treated at her local health service, and in 1 case, the clinic sent a prescription to the client's pharmacy.
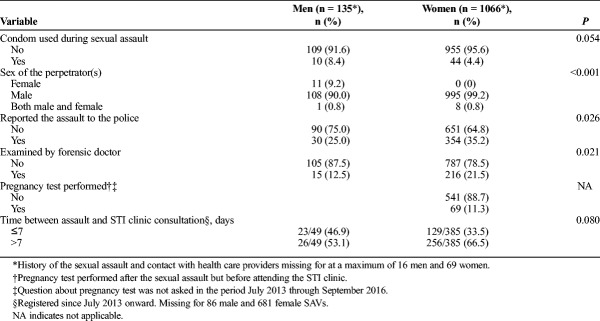
All the assailants of female SAVs were male, yet in 8 cases, a female was also involved (Table 2). For 4.4% of female SAVs, condoms were used during the sexual assault. A minority of SAVs reported the assault to the police or underwent forensic examination. The interval between the assault and STI consultation was known for 385 (36.1%) of 1066 consultations; 33.5% of the assaults had occurred in the preceding 7 days, and the STI positivity rate in this group was similar to SAVs assaulted more than 7 days before consultation (10.1% vs. 10.9%, P = 0.80).
TABLE 2.
Sexual Assault History and Contact With Health Care Providers After the Assault of 135 Clinic Visits From Male and 1066 From Female Clients Attending the STI Clinic in Amsterdam, the Netherlands; January 2005 to September 2016
Male SAVs
Compared with non-SAV male clients, male SAVs less often lived in Amsterdam, were less often Dutch and Surinamese, and were more often from sub-Saharan African, North African, or Asian decent (Table 3). In the 6 months preceding the STI clinic visit, 56% of the male SAVs and 39% of non-SAVs reported sexual contact with men only (P < 0.001). Male SAVs reported a lower number of sexual partners, were more often paid for sex in the preceding 6 months, and were less often notified of STI exposure. The bacterial STI positivity rate was not significantly different between male SAVs (n = 17; 12.6%) and non-SAVs (17.7%, P = 0.12). However, significantly fewer male SAVs had a urogenital chlamydia and anal gonorrhea diagnosis. In the univariable logistic regression analysis—except from being an SAV—all other variables (age, ethnicity, residence, HIV status, sex of sexual partner(s), number of sexual contacts, commercial sex work, and paying for sex) were significantly associated with having a bacterial STI (Supplemental Table 3, http://links.lww.com/OLQ/A235). After adjusting for the previously mentioned variables in multivariable analysis, being a male SAV was associated with a lower risk of having a bacterial STI (OR, 0.60; 95% CI, 0.36–0.98). In the multivariable subanalyses—although nonsignificant—male SAVs had lower odds of being diagnosed with having a bacterial STI (2005–2008: OR, 0.28 [95% CI, 0.07–1.11]; 2009–2016: OR, 0.68 [95% CI, 0.39–1.18]).
TABLE 3.
Demographics, Sexual Behavior, and Diagnosed STIs Among 135 Clinic Visits From Male Victims of a Sexual Assault and 194,819 Clinic Visits From Other Male Clients Who Were Not a Victim of Sexual Assault of the STI Clinic in Amsterdam, the Netherlands; January 2005 to September 2016

Of 17 male SAVs with a bacterial STI, presumptive antibiotic treatment was given to 3. Of the remaining 14, all but 1 (7.1%) returned to our clinic for treatment.
Most assailants were male, but in 11 cases, only female assailants were reported (Table 2). Among 8.4% of the male SAVs, condoms were used during the sexual assault. A minority of SAVs reported the assault to the police or underwent forensic examination. The period between the assault and STI consultation was known for 49 (36.3%) of 135 consultations; 46.9% of the assaults occurred in the preceding 7 days, and the STI positivity rate in this group was not significantly different from SAVs assaulted more than 7 days before consultation (17.4% vs. 23.1%, P = 0.73).
DISCUSSION
This study is based on 12 years of clinical data and compares characteristics and STI positivity rates among SAVs with non-SAV STI clinic clients. Among female SAVs, the bacterial STI positivity rate was comparable with that among non-SAV female STI clinic clients. Although most sexual assaults reported among male SAVs could have resulted in a higher risk of STI acquisition (no condom use and male assailants), the relatively large group of male SAVs included in this study had a significantly lower risk of testing STI positive than did non-SAV male clients. Because we do not routinely offer presumptive treatment to SAV, we were able to investigate SAV follow-up. For both female and male SAVs, the return rate for treatment was very high (89% and 92%, respectively). Based on this finding, we believe that presumptive therapy is not necessary, at least not in our or similar settings.
Sexually transmitted infection screening among both preadolescent and adolescent clients in the United States showed an STI positivity rate (including herpes, human papillomavirus, and condylomata) of 19.6% among girls and 6.3% among boys.8 Among 64 female and 1 male SAVs in an inner-city genitourinary medicine clinic in London, 2 cases (3.1%) with a bacterial STI (chlamydia) were detected.9 In a Norwegian sexual assault center, the chlamydia positivity rate (6.4%) among SAVs within 1 week after the assault was lower than that in a comparable clinical population (16% among 15- to 19-year and 12% among 20- to 24-year-olds).10 Among SAVs examined at a French Department of Forensic Medicine, chlamydia and gonorrhea were detected among 14.7% and 4.9%, respectively.11 Our study mainly focused on bacterial STI, and comparable bacterial STI positivity rates among SAV and non-SAV clients were found. Among female clients, a sexual assault was not a risk factor, whereas male SAVs had a lower risk of a bacterial STI than did non-SAVs.
A study from the United Kingdom stratifying for recent consensual sexual intercourse showed an STI positivity of 25.6% among those who had and 4.3% among those who had not had intercourse 3 months before the assault.12 The median number of sex partners in the present study (2 for female SAVs and 3 for male SAVs) also indicates that diagnosed STI could be unrelated to the assault. In addition to this possible effect of consensual sexual activity on contracting an STI, a longer period between the assault and the STI consultation might have influenced our results. The time between the assault and the STI clinic visit was only known for 36% of the consultations, and approximately one third of the female and half of the male SAVs were assaulted in the 7 days preceding the clinic visit.
In a review, Seña et al.2 observed that we do not know much about the STI positivity rate among male SAVs. In our study, male SAVs had a lower STI positivity rate (unadjusted; not significant). However, when adjusted for age, ethnicity, residence, HIV status, sex of sexual partner(s), number of sexual contacts, commercial sex work, and paying for sex, a significantly lower risk of bacterial STI was apparent. A British study showed that compared with female SAVs, male SAVs were more likely to access the routine walk-in genitourinary medicine clinic (compared with a specialized sexual assault clinic), perhaps because they are less likely to report a sexual assault to the police and therefore miss out on the forensic medical examiners referral pathway.13 Our findings are in agreement with theirs: only one quarter reported the assault to the police compared with 35% of women, and 1 in 8 was examined by a forensic doctor compared with 22% of women. These male SAVs might have experienced a lower threshold to accessing the STI clinic compared with a specialized sexual assault clinic. Possibly, non-Dutch SAVs also experienced a lower threshold, because SAVs—compared with non-SAVs—were more often non-Dutch.
Both sub-Saharan African female and male victims reported a sexual assault more often. During the study period, a considerable group of asylum seekers from sub-Saharan regions affected by civil war and military unrest were living in the Netherlands. In these conflicts, rape was used as a weapon, which might explain the overrepresentation of this population in our study.14 A US study among college students showed ethnic differences in the rate of reported sexual assaults.15 African American and European American women reported similar rates and Asian American women reported lower rates of sexual assault.
In our study, the return rate for a follow-up visit was relatively high compared with the 48.8% among preadolescent and adolescent US SAVs.8 This difference might be explained by the fact that SAVs in the United States study had to come for follow-up examinations and not for treatment. In other studies describing poor follow-up among recent SAVs, clients were treated presumptively, and a follow-up visit was indicated for repeat testing and examinations.16–18 In our study, all clients received testing at the initial visit and only had to return to the clinic for treatment if indicated. Possibly, clients who were diagnosed as having an STI were more willing to return to the clinic than those clients who had to return for additional testing. A different study population—SAV visiting a Dutch STI clinic—might also explain the difference in follow-up rates and treatment uptake. In addition, UK investigators in a study among female rape victims at a sexual assault clinic did not recommend presumptive treatment at initial evaluation.12 Their arguments against presumptive treatment were a low incidence of STI among female SAVs, the lack of a simple antibiotic regime that can eradicate all bacterial STIs, and the hindrance of presumptive therapy in effective partner notification and treatment.
Compared with women seen at a Dutch sexual assault support center, the SAVs in this study were relatively old (a mean of 24 years versus a median of 21 years), and this is probably due to the STI clinic policy of not providing services for children.19 Compared with non-SAV STI clinic clients, SAVs were more often of non-Dutch origin and often reported STI-related symptoms. As a result, the included group of SAV might not be representative of other SAV study populations. In addition, some STI clinic clients may not have disclosed being assaulted.
For STI clinic policy, it is very important to assess whether SAVs are at risk for STI and should receive dedicated STI care. We found comparable to lower STI positivity rates among SAV clients versus non-SAV STI clinic clients; we also observed a high follow-up rate. These findings do not support the Centers for Disease Control and Prevention guideline to provide presumptive antimicrobial therapy targeted against gonorrhea, chlamydia, and trichomoniasis at the initial evaluation.2 This guideline is based on a high STI positivity rate in combination with low follow-up return rates among SAV clients.
Although this article shows that STIs are frequently identified among female and male SAV STI clinic clients, their rates do not exceed those found among non-SAV clients. Still, it is important that STI clinics offer SAV clients low-threshold, priority access. With specialized counseling and dedicated STI care, STI clinics can play an important role for this group. Although difficult to prove in practice, future research should focus on the fraction of STI attributable to sexual assaults. In line with antibiotic stewardship, STI clinics should consider treating only diagnosed bacterial STI with antibiotics among SAVs.
Supplementary Material
Footnotes
Acknowledgments: The authors thank all the nurses of the STI clinic. Special thanks go to Anne-Wiek Ferwerda for providing the STI clinic information and protocols concerning sexual assault victims.
Conflicts of Interest and Sources of Funding: None declared.
Contributors: M.v.R., L.v.K., M.S.v.d.L., and H.d.V designed the study protocol. M.v.R. was responsible for the data collection. M.v.R., L.v.K., and M.S.v.d.L. performed the statistical analyses, and M.v.R. and L.v.K. drafted the manuscript. All authors commented on draft versions, and all approved the final version.
Previously presented: Information from this article has been presented as a poster at the STI & AIDS World Congress (July 12, 2017, Rio, Brazil; abstract numbers P3.228 and P3.229).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.de Haas S, van Berlo W, Bakker F, et al. Prevalence and characteristics of sexual violence in the Netherlands, the risk of revictimization and pregnancy: Results from a national population survey. Violence Vict 2012; 27:592–608. [DOI] [PubMed] [Google Scholar]
- 2.Seña AC, Hsu KK, Kellogg N, et al. Sexual assault and sexually transmitted infections in adults, adolescents, and children. Clin Infect Dis 2015; 61(Suppl 8):S856–S864. [DOI] [PubMed] [Google Scholar]
- 3.Beck-Sagué CM, Solomon F. Sexually transmitted diseases in abused children and adolescent and adult victims of rape: Review of selected literature. Clin Infect Dis 1999; 28(Suppl 1):S74–S83. [DOI] [PubMed] [Google Scholar]
- 4.van Rooijen MS, Koekenbier RH, Hendriks A, et al. Young low-risk heterosexual clients prefer a chlamydia home collection test to a sexually transmitted infection clinic visit in Amsterdam, the Netherlands: A cross-sectional study. Sex Transm Dis 2016; 43:710–716. [DOI] [PubMed] [Google Scholar]
- 5. Draaiboek Seksaccidenten (in Dutch). 2013. Available at: http://nlsitestatcom/rivm/rivm-nl/s?linkdocumenten_en_publicatiesprofessioneel_praktischdraaiboekeninfectieziektenlci_draaiboekenseksaccidenten&ns_type=pdf&ns_url=http://wwwrivmnl/dsresource?objectid=1a59bcbd-3ce4-447e-83f6-2ccdc6869c74&type=org&disposition=inline. Accessed June 26, 2017.
- 6.Alders M, CBS Classification of the population with a foreign background in the Netherlands. Available at: https://www.cbs.nl/nr/rdonlyres/d314ba81-b4a9-492f-8c9b-b50e7d3a3e5d/0/classificationforeign.pdf. Accessed January 29, 2018.
- 7.Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008; 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavril AR, Kellogg ND, Nair P. Value of follow-up examinations of children and adolescents evaluated for sexual abuse and assault. Pediatrics 2012; 129:282–289. [DOI] [PubMed] [Google Scholar]
- 9.Adlington R, Browne R. Management of patients seen post-sexual assault at a north London inner city genitourinary medicine clinic 2005–2008. Int J STD AIDS 2011; 22:286–287. [DOI] [PubMed] [Google Scholar]
- 10.Hagemann CT, Nordbo SA, Myhre AK, et al. Sexually transmitted infections among women attending a Norwegian Sexual Assault Centre. Sex Transm Infect 2014; 90:283–289. [DOI] [PubMed] [Google Scholar]
- 11.Jaureguy F, Chariot P, Vessieres A, et al. Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infections detected by real-time PCR among individuals reporting sexual assaults in the Paris, France area. Forensic Sci Int 2016; 266:130–133. [DOI] [PubMed] [Google Scholar]
- 12.Lacey HB. Sexually transmitted diseases and rape: The experience of a sexual assault centre. Int J STD AIDS 1990; 1:405–409. [DOI] [PubMed] [Google Scholar]
- 13.Thompson C. Review of 212 individuals attending a city centre genitourinary medicine clinic following acute sexual assault. J Clin Forensic Med 2006; 13:186–188. [DOI] [PubMed] [Google Scholar]
- 14.Supervie V, Halima Y, Blower S. Assessing the impact of mass rape on the incidence of HIV in conflict-affected countries. AIDS 2010; 24(18):2841–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Littleton HL, Grills-Taquechel AE, Buck KS, et al. Health risk behavior and sexual assault among ethnically diverse women. Psychol Women Q 2013; 37:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibb AM, McManus T, Forster GE. Should we offer antibiotic prophylaxis post sexual assault? Int J STD AIDS 2003; 14:99–102. [DOI] [PubMed] [Google Scholar]
- 17.Glaser JB, Schachter J, Benes S, et al. Sexually transmitted diseases in postpubertal female rape victims. J Infect Dis 1991; 164:726–730. [DOI] [PubMed] [Google Scholar]
- 18.Holmes MM, Resnick HS, Frampton D. Follow-up of sexual assault victims. Am J Obstet Gynecol 1998; 179:336–342. [DOI] [PubMed] [Google Scholar]
- 19.Bicanic I, Snetselaar H, De Jongh A, et al. Victims' use of professional services in a Dutch sexual assault centre. Eur J Psychotraumatol 2014; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.