Abstract
Background and study aims
Endoscopic submucosal dissection (ESD) has been developed as an option for treatment of esophageal, gastric and colorectal lesions. However, there is no consensus on the role of ESD in duodenal tumors.
Methods
This systematic review and meta-analysis compared ESD and endoscopic mucosal resection (EMR) in sporadic non-ampullary superficial duodenal tumors (NASDTs), including local experience. We conducted a search in PubMed, Scopus and the Cochrane library up to August 2017 to identify studies that compared both techniques reporting at least one main outcome (en-bloc/complete resection, local recurrence). Pooled outcomes were calculated under fixed and random-effect models. Subgroup analyses were conducted.
Results
A total of 753 patients presenting with 784 NASDTs (242 ESD, 542 EMR) in 14 studies were included. Tumor size (MD: 5.88, [CI95 %: 2.15, 9.62], P = 0.002, I 2 = 79 %) and procedure time (MD: 65.65, [CI95 %: 40.39, 90.92], P < 0.00001, I 2 = 88 %) were greater in the ESD group. En-bloc resection rate was significantly higher in Asian studies (OR: 2.16 [CI95 %: 1.15, 4.08], P = 0.02, I 2 : 46 %). ESD provided a higher complete resection rate (OR: 1.63 [I95 %: 1.06, 2.50], P = 0.03, I 2 : 59 %), but there was no risk difference in the risk of local recurrence (RD: – 0.03 [CI95 %: – 0.07, 0.01], P = 0.15, I 2 : 0 %) or delayed bleeding. ESD was associated with an increased number of intraoperative perforations [RD: 0.12 (CI95 %: 0.04, 0.20), P = 0.002, I 2 : 56 %] and emergency surgery for delayed perforations. The inclusion of eligible studies was limited to retrospective series with inequalities in comparative groups.
Conclusions
Duodenal ESD for NASDTs may achieve higher en-bloc and complete resections at the expense of a greater perforation rate compared to EMR. The impact on local recurrence remains uncertain.
Introduction
Endoscopic submucosal dissection (ESD) has been widely accepted and was developed treatment of esophageal, gastric, colonic and rectal lesions, allowing high en-bloc and curative resection rates with a satisfactory safety profile 1 2 3 . However, duodenal lesions are uncommon and there is no consensus on the role of ESD in the small bowel, where dissection may be much more challenging with a high incidence of adverse events even in experienced centers 4 5 . Indeed, there are no randomized studies or meta-analyses assessing ESD vs. endoscopic mucosal resection (EMR) outcomes and the European Society of Gastrointestinal Endoscopy does not recommend ESD in the duodenum 6 . Conversely, EMR has been reported to be an effective therapeutic option in sporadic non-ampullary duodenal tumors 7 , but resections in piecemeal fashion may lead to a non-negligible recurrence rate 8 . Thus, the duodenum seems to be the new barrier of ESD, as the usefulness and safety of this technique remain unclear. The aim of the current systematic review and meta-analysis was to comparatively assess the characteristics and outcomes of ESD and EMR procedures for non-ampullary superficial duodenal tumors (NASDTs) who underwent EMR and ESD procedures.
Methods
Search strategy
A literature search was conducted in MEDLINE (through PubMed), Scopus and the Cochrane Library up to August 6, 2017. The medical terms “((ESD OR endoscopic submucosal dissection) OR (EMR OR endoscopic mucosal resection)) AND (duodenal OR duodenum OR small bowel OR non-ampullary)” were used. Two review authors (EPCR, LQ) independently screened references and selected studies for inclusion, assessed eligibility and validity of each study and extracted data. Any disagreements were resolved by reviewing an article and settled by consensus. We also searched the references of included articles to identify other potentially relevant articles (citing reference search). A parallel manual search was also performed using Google Scholar. All human studies subjected to adult population (> 18 years old) and published in English were considered. All duplicate studies were removed.
First, titles and abstracts of papers were examined to exclude irrelevant articles. Next, the full text of all selected studies was screened according to inclusion and exclusion criteria. Efforts were made to contact the corresponding author if the study information was incomplete. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement has been used in the preparation of this manuscript. The current review and meta-analysis was prospectively registered in PROSPERO database (CRD42017073197) and approved by the local Ethics Committee.
Selection criteria
Eligibility criteria for the included studies relied on previously published guidelines for systematic reviews and were based on the PICO framework: P (Population – patients with non-ampullary duodenal neoplasms), I (Intervention – endoscopic resection by EMR and ESD), C (Comparative intervention – EMR/ESD), and O (Outcomes – at least one of the following main comparative outcomes: en-bloc resection, complete resection, recurrence). Secondary outcomes were bleeding and perforation. Meeting abstracts, reviews, editorials, opinions, letters and surveys were excluded. Studies reporting only on duodenal ESD or EMR without a comparative analysis were also excluded. Studies including exclusively submucosal tumors were not considered. However, studies including both superficial and subepithelial lesions were considered.
Data extraction and quality assessment
Data extraction was carried out using a standardized collection sheet. Study characteristics collected included year of publication, study period, primary country of the study, study design, number of patients and lesions, sporadic or non-sporadic status, mean age, sex distribution, tumor size, location and procedure time. Risk of bias (quality) assessment was independently assessed (EPCR, LQ) with Newcastle-Ottawa quality Assessment scale (NOS) according to the Cochrane Non-Randomized Studies Methods Working Group. Quality scores of studies range from zero to nine in three categories (selection, comparability, and outcome). We classified the study quality according to the study score into poor (0 – 3), moderate (4 – 6) and high (7 – 9). No study was excluded based on this score, but a sensitivity analysis to account for the effect of poor quality studies was planned.
Outcomes
Main outcomes included en-bloc resection, complete resection and recurrence rates. Intraoperative or delayed (post-procedure) adverse events (AEs) (bleeding and perforation) were the secondary outcomes. Lack of data and different definitions from distinct cohorts prevented formal meta-analyses for intraoperative bleeding.
Statistical analysis
All analyses were performed according to original treatment allocation (intention-to-treat analysis). To assess comparability of groups at the baseline, the mean differences (MD) and 95 % CIs were estimated using the inverse variance weighting, such as age, sex, tumor’s size, and follow-up times. When means and/or standard deviations were not reported in the original paper, they were estimated from reported medians, ranges and sample size 9 .
For binary outcome data, the odds ratio (OR) and 95 % CIs were used. En-bloc and complete resection outcomes were calculated under a fixed-effect model described by Mantel-Haenszel. As clinical heterogeneity of study participants, follow-up and definitions of bleeding and perforation were present among the studies selected for the meta-analysis, combined risk difference (RD) for the association of EMR/ESD and secondary outcomes or local recurrence was pooled under a random-effects model. The RD was used to evaluate AEs or tumor recurrence because they may not have occurred in some groups. Heterogeneity analysis was performed using the Tau and I 2 index. If I 2 > 50 %, potential sources of heterogeneity were identified by sensitivity analyses conducted by omitting one study at a time and investigating the influence on the overall pooled estimate. Potential publication biases were assessed by funnel-plot visual analysis to point out whether small studies had larger effect sizes than would be expected. A two-sided P value < 0.05 was considered statistically significant. Statistical analysis was performed with RevMan v.5.3 (Cochrane Library, Oxford, UK) and SPSS v.23 (IBM, SPSS, Ilinois, United States).
Results
Identification of eligible studies
The search identified a total of 602 articles and 28 full-text records were assessed foreligibility after screening and full text review. Finally, 14 studies 10 11 12 13 14 15 16 17 18 19 20 21 22 23 were included in the current meta-analysis. The flow-chart is shown in Fig. 1 .
Fig. 1 .
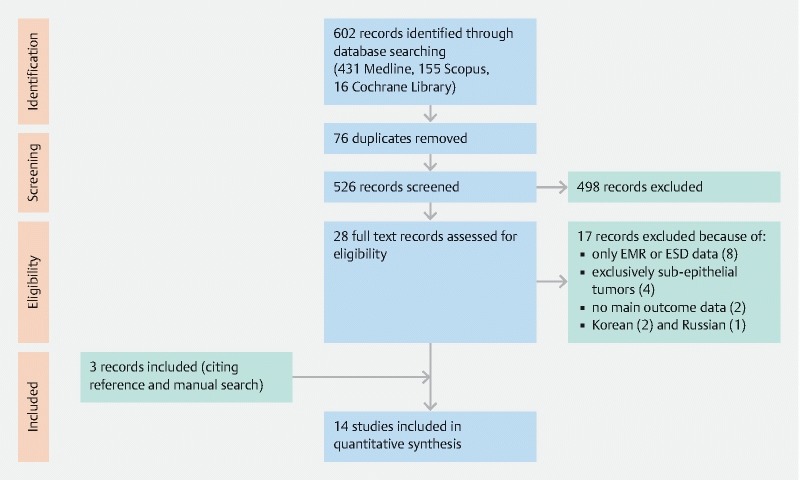
Flow diagram of search strategy of the systematic review.
Study characteristics
Characteristics of included studies are shown in Table 1 . A total of 753 patients presenting with 784 NASDTs (242 ESD, 542 EMR) were included. All studies had a retrospective design and were published between 2009 and 2017 in Eastern (n = 12) or Western countries (n = 2). Assessment of study quality based on NOS resulted in high (n = 5), moderate (n = 7) and low (n = 2) scores.
Table 1. Study characteristics of publications included in the systematic review and meta-analysis.
| Study | Cohort study design | Country | Patients | Age | Enrollment period | Lesions n (ESD/EMR) | Main outcome measures 1 |
| Pérez-Cuadrado-Robles (2018) 23 | Single-center, retrospective | Belgium | 150 | 66 (31 – 83) | 2005 – 2017 | 166 (37,129) | En-bloc and complete resection, local recurrence |
| Hoteya (2017) 10 | Single-center, retrospective | Japan | 129 | 61 ± 11.2 (range: 32 – 86) | 2005 – 2015 | 129 (74,55) | En-bloc and complete resection, local recurrence. |
| Teoh (2015) 22 | Multicenter, retrospective | Hong-Kong | 12 | – | 2010 – 2013 | 12 (6,6) | En-bloc resection |
| Nonaka (2015) 12 | Single-center, retrospective | Japan | 113 | 61.7 ± 11.9 | 2000 – 2013 | 121 (8,113) | En-bloc and complete resection, local recurrence |
| Park (2015) 11 | Multicenter, retrospective | Korea | 51 | 59.5 ± 12.5 | 2002 – 2013 | 51 (6,45) | En-bloc and complete resection, local recurrence |
| Inoue (2014) 17 | Single-center, retrospective | Japan | 59 | 58 | 1993 – 2011 | 63 (10,53) | En-bloc resection |
| Basford (2014) 21 | Multicenter, retrospective | United Kingdom | 34 | 69 (48 – 87) | 2005 – 2012 | 34 (13,21) | En-bloc resection, local recurrence |
| Matsumoto (2014) 13 | Single-center, retrospective | Japan | 44 | 65 ± 9 (35 – 79) | 2005 – 2013 | 46 (15,31) | En-bloc and complete resection, local recurrence |
| Yamamoto (2014) 14 | Single-center, retrospective | Japan | 47 | 65.8 ± 12.4 | 2006 – 2013 | 47 (30,17) | En-bloc and complete resection, local recurrence |
| Kakushima (2014) 18 | Single-center, retrospective | Japan | 23 | 68 (43 – 81) | 2002 – 2012 | 23 (13, 10) | En-bloc and complete resection, local recurrence |
| Seo (2014) 15 | Single-center, retrospective | Korea | 40 | 59.9 (39 – 83) | 2003 – 2012 | 40 (7, 33) | En-bloc and complete resection, local recurrence |
| Zhong (2012) 20 | Single-center, retrospective | China | 21 | 55 (29 – 72) | 2007 – 2011 | 21 (9, 12) | En-bloc and complete resection, local recurrence |
| Endo (2010) 19 | Single-center, retrospective | Japan | 16 | 66.5 (53 – 80) | 2005 – 2009 | 16 (5, 11) | En-bloc and complete resection, local recurrence |
| Honda (2009) 16 | Single-center, retrospective | Japan | 14 | 60.7 ± 12 | 2005 – 2008 | 15 (9, 6) | En-bloc resection |
ESD, endoscopic submucosal dissection; EMR, endoscopic mucosal resection
All studies but Teoh considered the secondary outcomes (intraoperative/delayed perforation and delayed bleeding).
Endoscopic resection of NASDTs was indicated based on tumor characteristics and suspected histology. Endoscopic treatment was only indicated in adenomas > 10 mm or confirmed adenocarcinomas in one study 12 while it was only indicated in lesions ≤ 20 mm presenting with high-grade dysplasia or mucosal cancer in another report 14 . Thus, in some studies, endoscopic resection was exclusively performed in suspected adenoma 12 19 , high-grade dysplasia or non-invasive carcinoma based on endoscopic findings or preoperative biopsies 10 14 , excluding lesions with a final nonadenomatous histology 15 16 17 20 21 . The reasons for choosing EMR or ESD were very heterogeneous among the different authors. The overall choice was based on tumor characteristics (macroscopic morphology), scope maneuverability and the feasibility of en-bloc resection by EMR 10 12 13 15 19 23 . In this regard, ESD appeared to be the chosen technique in depressed tumors 14 17 18 , and adenomas ≥ 20 mm in diameter was the main indication for piecemeal EMR 21 or ESD approach 23 . However, several series did not provide enough information in this regard 11 16 20 22 .
Although all the studies were of superficial lesions, three articles also included neuroendocrine tumors 11 13 22 . Additionally, only five authors 11 14 16 19 23 excluded pedunculated lesions. Indeed, the EMR outcomes were mixed with those of polypectomy technique (no submucosal injection) in four studies 12 15 18 20 , however in two cases 15 20 it was possible to separate and analyze the data consequently in the meta-analysis. The “injection of snaring” technique during EMR was carried out in all studies with or without a cap on the tip of the endoscope, but there were some reports also considering patients with “strip biopsy” technique 12 17 . In addition, a double-balloon enteroscope was used in two studies 16 19 to improve scope positioning and maneuverability. Regarding specific backgrounds, five studies only included sporadic NASDTs 11 15 19 21 23 and two studies 12 18 considered both sporadic lesions and familial polyposis syndrome (13 patients). Finally, the sporadic status was unknown in the remaining papers 10 13 14 16 17 20 22 .
Considering baseline patient characteristics, there was not significant differences in age in both groups (MD: 2.39, [CI 95 %: – 0.83, 5.61], P = 0.15, I 2 = 30 %). However, the mean differences in tumor size (MD: 5.88, [CI95 %: 2.15, 9.62], P = 0.002, I 2 = 79 %) and procedure time (MD: 65.65, [CI95 %: 40.39, 90.92], P < 0.00001, I 2 = 88 %) were statistically higher in ESD resections using a random effect model. Heterogeneity in size and procedure time was significant, but there was no change of pooled effect after sensitivity or subgroup analysis. Location was also a determining factor in guiding endoscopic resection, as lesions placed in distal duodenum have been described as easier to close 14 with a better scope maneuverability. From 19 % to 100 % of lesions were in distal duodenum (starting from D2) in the reviewed studies, but the pooled risk difference was similar between ESD and EMR (RD: 0.02 [CI 95 %: – 0.05, 0.10], P = 0.55, I 2 = 58 %).
En-bloc resection rate
The definition of en-bloc resection was homogeneous among all studies and all of them reported this outcome. The en-bloc resection rate was higher for EMR in four studies 11 15 22 23 and lower for ESD in the remaining papers. This outcome was not estimable in two studies with 100 % en-bloc resection rates 18 20 . Overall pooled OR was not different between ESD and EMR groups (OR: 1.31 [CI95 %: 0.84, 2.03] P = 0.23) with a heterogeneity of 49 % ( Fig. 2 ). However, considering only Eastern studies in sensitivity analysis, the combined effect showed a higher ESD en-bloc resection rate (OR: 2.16 [CI95 %: 1.15, 4.08], P = 0.02] with a similar heterogeneity. The random effect model was also performed with no differences in pooled effect.
Fig. 2.
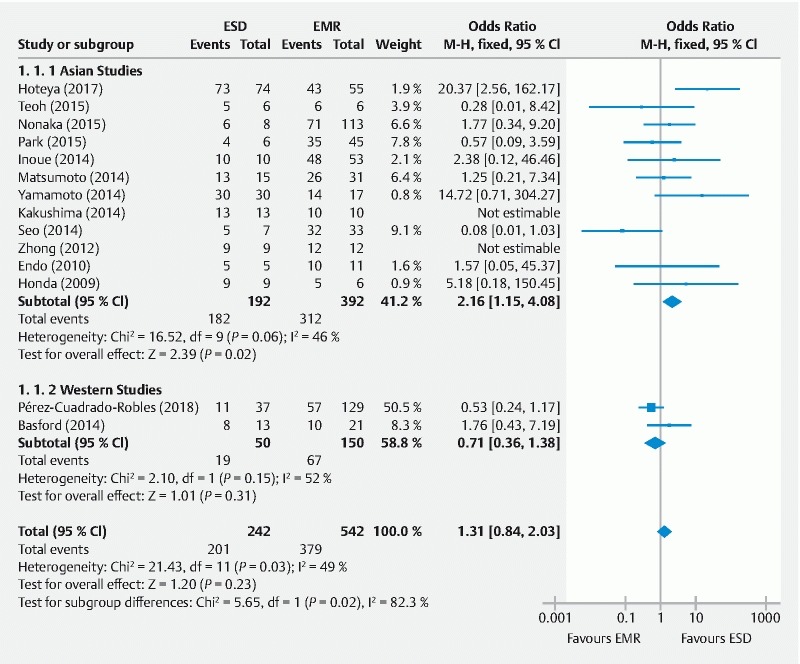
Forest plot for the association between the endoscopic resection technique and en-bloc resection (event/total) using a fixed-effects model and subgroup analysis in non-ampullary superficial duodenal tumors. ESD, endoscopic submucosal dissection; EMR, endoscopic mucosal resection.
Complete resection rate
Complete resection outcome was described by 10 authors. The definition was homogeneous even if histopathological assessment was poorly described in some series. The pooled OR favored ESD (OR: 1.63 [CI 95 %: 1.06, 2.50], P = 0.03) but heterogeneity among different papers was significant ( Fig. 3 ). Interestingly, subgroup analysis showed a higher pooled effect for ESD (OR: 2.77 [CI 95 %: 1.71, 4.76], P < 0.001) with much lower heterogeneity when considering Asian authors. Additionally, one study reported higher complete resection rates than en-bloc resection rates 11
Fig. 3.
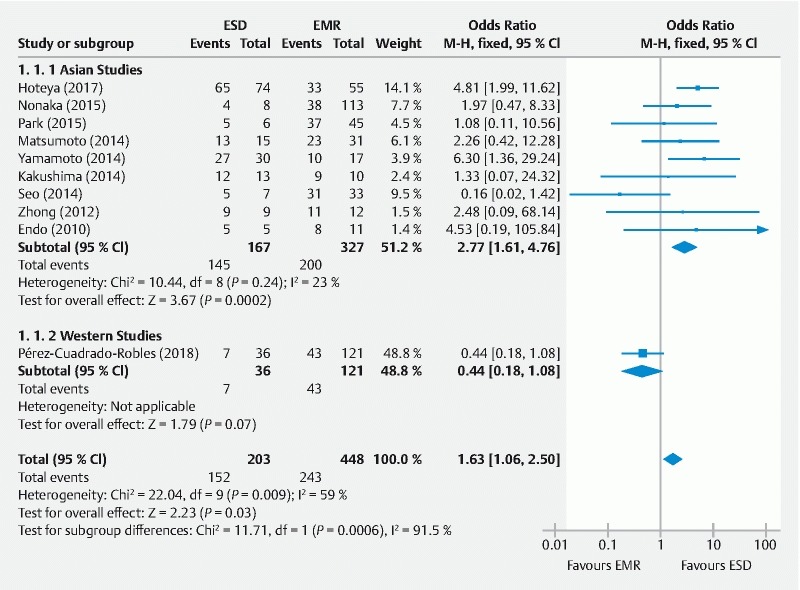
Forest plot for the association between the endoscopic resection technique and complete resection (event/total) using a fixed-effects model and subgroup analysis in non-ampullary superficial duodenal tumors. ESD, endoscopic submucosal dissection; EMR, endoscopic mucosal resection.
Local recurrence
Follow-up was described in all but three studies 16 17 22 and the median observation period ranged from 6 to 51 months with not established minimum follow-up in most cases. However, median follow-up time was longer with ESD 10 11 13 or EMR 14 procedures with no subgroup information in the remaining studies. Additionally, three authors reported a loss to follow-up of > 20 % of the population. Notably, Matsumoto 13 , Nonaka 12 and Park 11 described a loss to follow-up of 41 %, 33 % and 33 %, respectively. In addition, there was one study with follow-up still ongoing 19 , but it was considered for local recurrence pooled analysis. A follow-up biopsy was performed at the discretion of the endoscopist based on endoscopic findings in all studies, but this information was rarely reported. Thus, local recurrence was reported by 11 authors in 584 patients, with no risk difference in both groups (RD: – 0.03 [95 % CI: – 0.07, 0.01), P = 0.15) ( Fig. 4 ). Subgroup analysis was not performed as heterogeneity was 0 %.
Fig. 4.
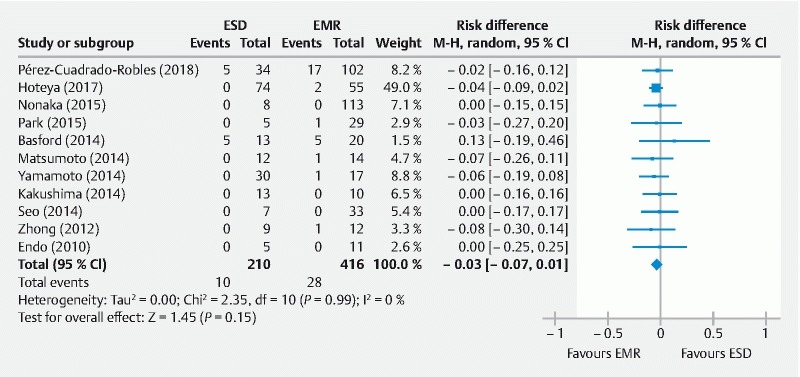
Forest plot for the risk difference (RD) in local recurrence rates (event/total) between endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) using a random-effects model in non-ampullary superficial duodenal tumors.
Delayed bleeding
Delayed bleeding was retained if melena or hematemesis requiring endoscopic hemostasis or further therapy was reported after the completion of the procedure 10 . Blood transfusion requirement 11 14 and a decrease in hemoglobin of 2 g 15 were also included in the definition. That outcome was not sufficiently explained in the methodology of five studies 13 14 16 18 19 . Finally, pooled risk of bleeding was similar in ESD or EMR groups ( Fig. 5 ). Endoscopic hemostatic therapy was successful in all reported cases.
Fig. 5.
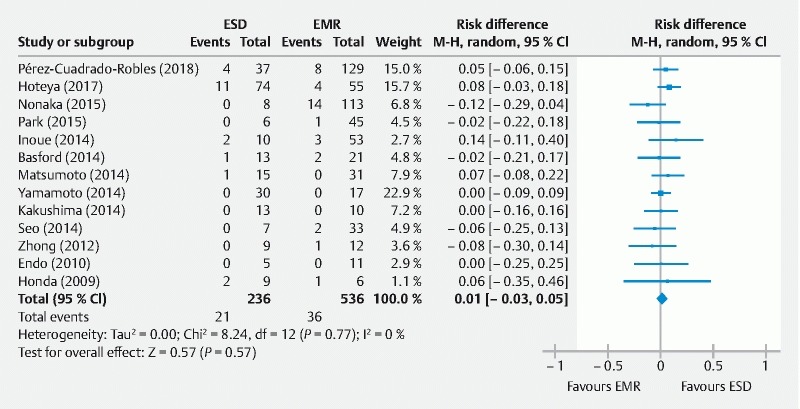
Delayed bleeding rates (event/total) for endoscopic submucosal dissection (ESD) versus endoscopic mucosal resection (EMR) for non-ampullary superficial duodenal tumors.
Perforation
All studies considered intraoperative and delayed perforation defined by free air on radiological examinations. However, that information was not available in one case 22 . The definition was not explained in six cases 13 16 18 19 20 21 and only two authors 11 23 made the difference between major and minor perforation based on whether intra-abdominal space was directly observed. Overall, there were more perforations in the ESD group, and the low RD was similar for intra-procedural and delayed perforation when subgroup analysis was carried out, but the difference wasnot statistically significant for delayed perforation ( Fig. 6 ). Although endoscopic treatment was successful for closing the perforation in most patients, emergency surgery was required in 0 % to 33 % after ESD. There was only one study reporting emergency surgery in a patient who underwent EMR 11 and the remaining interventions were carried out following ESD procedures.
Fig. 6.
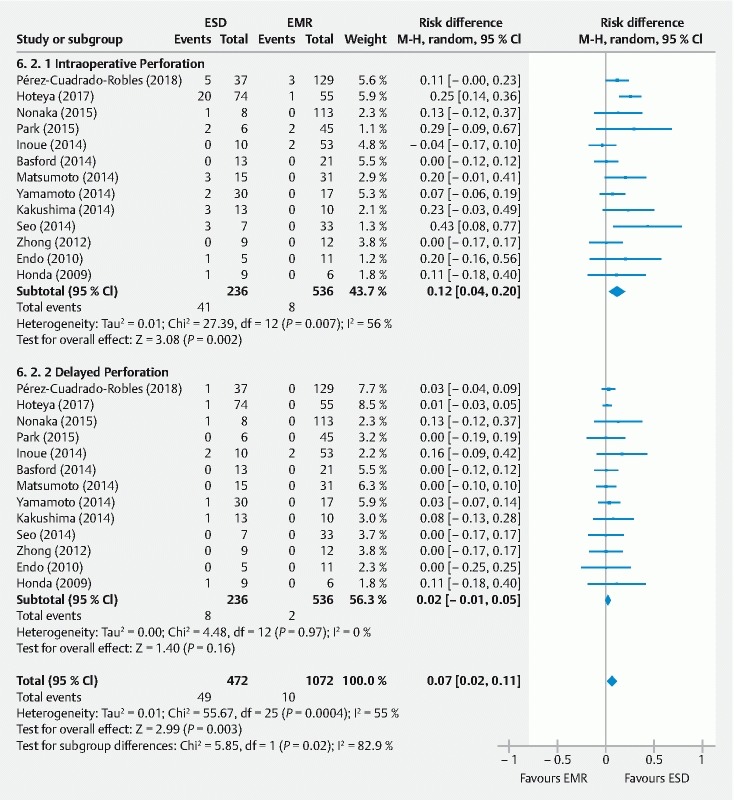
Intraoperative and delayed perforation rates (event/total) for endoscopic submucosal dissection (ESD) versus endoscopic mucosal resection (EMR) for non-ampullary superficial duodenal tumors.
Publication bias
Considering the main outcomes, the funnel plot was slightly asymmetrical, suggesting publication bias probably related to heterogeneity within different studies ( Fig. 7 ). To account for this possibility, we repeated our models pooling data from only high to moderate quality studies and found our results to be robust. However, there were different effects considering Western and Eastern studies. The funnel plot for secondary outcomes showed that the studies were reasonably well scattered with low risk of publication bias. When a single study involved in the meta-analysis was deleted each time, the results of pooled meta-analysis for bleeding and perforation remained unchanged, indicating that the results were stable.
Fig. 7.
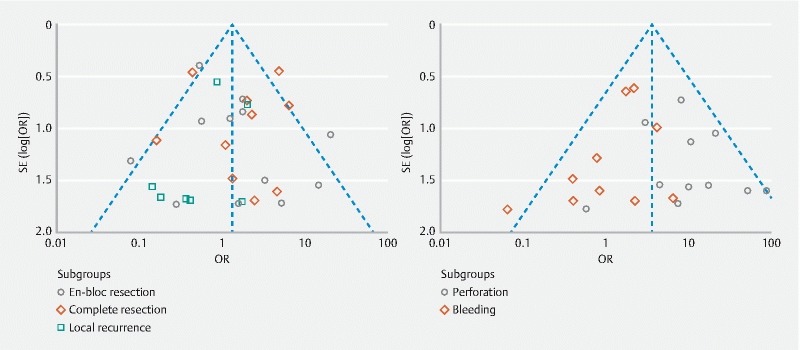
Funnel plots for main (left) and secondary outcomes (right). The shapes of the left funnel plot revealed a degree of asymmetry, which indicates publication bias may exist in main outcomes. Each point represents a separate study for the association of the endoscopic resection techniques with outcomes. OR: odds ratio; Log (OR): natural logarithm of OR; SE: standard error; SE (Log [OR]): standard error of Log (OR).
Discussion
To the best of our knowledge, the current meta-analysis represents the first systematic review comparatively assessing outcomes of ESD and EMR in sporadic NASDTs. The knowledge that ESD increases en-bloc and complete resection rates compared to EMR has led to major changes in clinical practice in the last decade 24 . However, there is not enough evidence supporting ESD in duodenal superficial tumors, where feasibility and safety are issues of major concern 25 . Recently, duodenal ESD has been reported as a safe procedure by using the pocket-creation method 26 or cooperative surgery 27 28 . In our meta-analysis, we excluded studies assessing duodenal ESD without comparative evaluation with EMR for the same population. Our study highlights the challenges of small retrospective series with heterogeneous lesions that often lack the sample size necessary to tease out important endoscopic and clinical outcomes. All included studies in the review had a retrospective design and the proportion of ESD/EMR lesions varied widely, probably reflecting a selection bias. In this sense, there were authors that considered as indications for ESD large lesions (> 10 – 20 mm) for which en-bloc resection by EMR was not possible, lesions suspected of noninvasive cancer 10 13 16 19 and depressed tumors 10 14 17 18 . Thus, the larger tumor size in the ESD group may have underestimated the ESD main outcomes and influenced the comparative analysis with EMR. Additionally, an elective hybrid-ESD approach was carried out in small lesions to increase the probability of en-bloc resection with a snare 16 , or challenging large tumors 21 23 . Finally, most authors agreed that the choice of the technique was made at the discretion of the endoscopist or during a consensus committee 12 .
En-bloc resection by EMR of lesions greater than 20 mm 12 or located near the pyloric ring may be difficult. The pooled meta-analysis favored ESD compared to EMR in Asian setting (OR: 2.16 [95 % CI: 1.15, 4.08], P = 0.02). Similarly, pooled results of the included studies suggest that complete resection may be higher in ESD approach. In subgroup analysis, OR slightly increased and low heterogeneity resulted. Although en-bloc and complete resection were significantly higher in ESD procedures when considering Asian studies, the results should be interpreted with caution, as the magnitudes of the effects were quite modest. Navaneethan 29 reported a pooled recurrence rate of 15 % after initial EMR with no increased risk based on whether polypectomy was en-bloc or piecemeal. Our systematic review and meta-analysis provides no support for the hypothesis that ESD is associated with a lower recurrence rate, but this could be because of insufficient power and follow-up duration 30 . Interestingly, most local recurrences were managed successfully by further endoscopic resection. Perforations may be associated with hybrid ESD/piecemeal approach, tumor size 10 , or the duodenal ESD technique itself 31 . Notably, intraoperative perforation was associated with ESD in our analysis. Emergency surgery was more frequently required after delayed perforation as previously described 25 . Sensitivity analysis for secondary outcomes did not change any meta-analysis result/effect substantially.
Strengths and limitations
Strengths of our review include a systematic and rigorous approach to identification of retrospective studies investigating the role of ESD and EMR in duodenum, as well as the comprehensive nature of our literature search in Western and Asian settings. The main limitation of the meta-analysis relies on the retrospective design and inequalities of comparative groups of included studies, presenting without cofactor adjustment. Long inclusion periods from tertiary centers with different learning curves, different patient populations and heterogeneous clinical settings may have also influenced the results.
Conclusions
In summary, ESD may achieve higher rates of en-bloc and complete resection compared to duodenal EMR but the impact on local recurrence is uncertain. Remarkably, the intraoperative perforation rate may be higher following ESD and leads to emergency surgery in delayed perforations. However, the validity of these meta-analyses is debatable and further prospective or controlled trials that include lesions of comparative characteristics for both techniques are still needed to elucidate the role of ESD in the duodenum.
Footnotes
Competing interests None
References
- 1.Bhatt A, Abe S, Kumaravel A et al. Indications and Techniques for Endoscopic Submucosal Dissection. Am J Gastroenterol. 2015;110:784–791. doi: 10.1038/ajg.2014.425. [DOI] [PubMed] [Google Scholar]
- 2.Deprez P H, Bergman J J, Meisner S et al. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy. 2010;42:853–858. doi: 10.1055/s-0030-1255563. [DOI] [PubMed] [Google Scholar]
- 3.ASGE Technology Committee . Maple J T, Abu Dayyeh B K et al. Endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:1311–1325. doi: 10.1016/j.gie.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Shibagaki K, Ishimura N, Kinoshita Y. Endoscopic submucosal dissection for duodenal tumors. Ann Transl Med. 2017;5:188. doi: 10.21037/atm.2017.03.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto H, Miura Y. Duodenal ESD: conquering difficulties. Gastrointest Endosc Clin N Am. 2014;24:235–244. doi: 10.1016/j.giec.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 7.Navaneethan U, Hasan M K, Lourdusamy V et al. Efficacy and safety of endoscopic mucosal resection of non-ampullary duodenal polyps: a systematic review. Endosc Int Open. 2016;4:E699–E708. doi: 10.1055/s-0042-107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakushima N, Kanemoto H, Tanaka M et al. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2014;21:12501–12508. doi: 10.3748/wjg.v20.i35.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hozo S P, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoteya S, Furuhata T, Takahito T et al. Endoscopic Submucosal Dissection and Endoscopic Mucosal Resection for Non-Ampullary Superficial Duodenal Tumor. Digestion. 2017;95:36–42. doi: 10.1159/000452363. [DOI] [PubMed] [Google Scholar]
- 11.Park S M, Ham J H, Kim B W et al. Feasibility of endoscopic resection for sessile nonampullary duodenal tumors: a multicenter retrospective study. Gastroenterol Res Pract. 2015;2015:692492. doi: 10.1155/2015/692492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nonaka S, Oda I, Tada K et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy. 2015;47:129–135. doi: 10.1055/s-0034-1390774. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto S, Yoshida Y. Selection of appropriate endoscopic therapies for duodenal tumors: an open-label study, single-center experience. World J Gastroenterol. 2014;14:8624–8630. doi: 10.3748/wjg.v20.i26.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto Y, Yoshizawa N, Tomida H et al. Therapeutic outcomes of endoscopic resection for superficial non-ampullary duodenal tumor. Dig Endosc. 2014;26:50–56. doi: 10.1111/den.12273. [DOI] [PubMed] [Google Scholar]
- 15.Seo J Y, Hong S J, Han J P et al. Usefulness and safety of endoscopic treatment for nonampullary duodenal adenoma and adenocarcinoma. J Gastroenterol Hepatol. 2014;29:1692–1698. doi: 10.1111/jgh.12601. [DOI] [PubMed] [Google Scholar]
- 16.Honda T, Yamamoto H, Osawa H et al. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21:270–274. doi: 10.1111/j.1443-1661.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- 17.Inoue T, Uedo N, Yamashina T et al. Delayed perforation: a hazardous complication of endoscopic resection for non-ampullary duodenal neoplasm. Dig Endosc. 2014;26:220–227. doi: 10.1111/den.12104. [DOI] [PubMed] [Google Scholar]
- 18.Kakushima N, Ono H, Takao T et al. Method and timing of resection of superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2014;26:35–40. doi: 10.1111/den.12259. [DOI] [PubMed] [Google Scholar]
- 19.Endo M, Abiko Y, Oana S et al. Usefulness of endoscopic treatment for duodenal adenoma. Dig Endosc. 2010;22:360–365. doi: 10.1111/j.1443-1661.2010.01014.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhong Y S, Shi Q, Wu H F et al. Endoscopic resection for the treatment of duodenal Brunner's adenoma. J Laparoendosc Adv Surg Tech A. 2012;22:904–909. doi: 10.1089/lap.2012.0250. [DOI] [PubMed] [Google Scholar]
- 21.Basford P J, George R, Nixon E et al. Endoscopic resection of sporadic duodenal adenomas: comparison of endoscopic mucosal resection (EMR) with hybrid endoscopic submucosal dissection (ESD) techniques and the risks of late delayed bleeding. Surg Endosc. 2014;28:1594–1600. doi: 10.1007/s00464-013-3356-y. [DOI] [PubMed] [Google Scholar]
- 22.Teoh A Y, Chiu P W, Chan S Y et al. Hospital Authority audit of the outcome of endoscopic resection of superficial upper gastro-intestinal lesions in Hong Kong. Hong Kong Med J. 2015;21:224–231. doi: 10.12809/hkmj144380. [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Cuadrado Robles E, Quénéhervé L, Margos W et al. En-bloc resection may not be the main goal in large duodenal adenomas. Endosc Int Open. 2018;6:E1008–E1014. doi: 10.1055/a-0577-7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barret M, Lepilliez V, Coumaros D et al. The expansion of endoscopic submucosal dissection in France: A prospective nationwide survey. United Europ Gastroenterol J. 2017;5:45–53. doi: 10.1177/2050640616644392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibagaki K, Ishimura N, Kinoshita Y. Endoscopic submucosal dissection for duodenal tumors. Ann Transl Med. 2017;5:188. doi: 10.21037/atm.2017.03.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miura Y, Shinozaki S, Hayashi Y et al. Duodenal endoscopic submucosal dissection is feasible using the pocket-creation method. Endoscopy. 2017;49:8–14. doi: 10.1055/s-0042-116315. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa D, Komatsu S, Dohi O et al. Laparoscopic and endoscopic co-operative surgery for non-ampullary duodenal tumors. World J Gastroenterol. 2016;21:10424–10431. doi: 10.3748/wjg.v22.i47.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irino T, Nunobe S, Hiki N et al. Laparoscopic-endoscopic cooperative surgery for duodenal tumors: a unique procedure that helps ensure the safety of endoscopic submucosal dissection. Endoscopy. 2015;47:349–351. doi: 10.1055/s-0034-1390909. [DOI] [PubMed] [Google Scholar]
- 29.Navaneethan U, Hasan M K, Lourdusamy V et al. Efficacy and safety of endoscopic mucosal resection of non-ampullary duodenal polyps: a systematic review. Endosc Int Open. 2016;4:E699–E708. doi: 10.1055/s-0042-107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marques J, Baldaque-Silva F, Pereira P et al. Endoscopic mucosal resection and endoscopic submucosal dissection in the treatment of sporadic nonampullary duodenal adenomatous polyps. World J Gastrointest Endosc. 2015;25:720–727. doi: 10.4253/wjge.v7.i7.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung J H, Choi K D, Ahn J Y et al. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy. 2013;45:133–135. doi: 10.1055/s-0032-1326178. [DOI] [PubMed] [Google Scholar]


