Abstract
Purpose
Preclinical data support a key role for the PI3K pathway in estrogen receptor–positive breast cancer and suggest that combining PI3K inhibitors with endocrine therapy may overcome resistance. This preoperative window study assessed whether adding the PI3K inhibitor pictilisib (GDC-0941) can increase the antitumor effects of anastrozole in primary breast cancer and aimed to identify the most appropriate patient population for combination therapy.
Patients and Methods
In this randomized, open-label phase II trial, postmenopausal women with newly diagnosed operable estrogen receptor–positive, human epidermal growth factor receptor 2 (HER2)–negative breast cancers were recruited. Participants were randomly allocated (2:1, favoring the combination) to 2 weeks of preoperative treatment with anastrozole 1 mg once per day (n = 26) or the combination of anastrozole 1 mg with pictilisib 260 mg once per day (n = 49). The primary end point was inhibition of tumor cell proliferation as measured by change in Ki-67 protein expression between tumor samples taken before and at the end of treatment.
Results
There was significantly greater geometric mean Ki-67 suppression of 83.8% (one-sided 95% CI, ≥ 79.0%) for the combination and 66.0% (95% CI, ≤ 75.4%) for anastrozole (geometric mean ratio [combination:anastrozole], 0.48; 95% CI, ≤ 0.72; P = .004). PIK3CA mutations were not predictive of response to pictilisib, but there was significant interaction between response to treatment and molecular subtype (P = .03); for patients with luminal B tumors, the combination:anastrozole geometric mean ratio of Ki-67 suppression was 0.37 (95% CI, ≤ 0.67; P = .008), whereas no significant Ki-67 response was observed for pictilisib in luminal A tumors (1.01; P = .98). Multivariable analysis confirmed Ki-67 response to the combination treatment of patients with luminal B tumors irrespective of progesterone receptor status or baseline Ki-67 expression.
Conclusion
Adding pictilisib to anastrozole significantly increases suppression of tumor cell proliferation in luminal B primary breast cancer.
INTRODUCTION
There is increasing evidence that aberrant signaling through the PI3K-mTOR signaling pathway plays a critical role in endocrine resistance.1 The PI3K-mTOR pathway is the most frequently altered pathway in estrogen receptor (ER)–positive breast cancer.2,3 Aberrant activation can occur through various mechanisms, including activating mutations or amplification of the PI3K catalytic subunits p110α (PIK3CA) and p110β (PIK3CB), the effectors AKT1, AKT2, or PDK1, or upstream receptor tyrosine kinases such as human epidermal growth factor receptor 2 (HER2), EGFR, or FGFR1, or through loss of the negative regulators PTEN or INPP4B.1-4
Surprisingly, activating mutations of PIK3CA have been shown to be associated with improved patient outcome in ER-positive breast cancer.5,6 Conversely, PIK3CA mutations have not been shown to be predictive of response to endocrine treatment or mTOR-targeted therapies.7,8 However, preclinical studies suggest that PIK3CA mutations are predictive of sensitivity to PI3K inhibitors but do not explain all of the sensitivity observed.9 Molecular profiling of ER-positive cancers demonstrated substantial overlap in gene signatures of PI3K activity between PIK3CA mutant and wild-type tumors, suggesting that other mechanisms aside from mutational activation of PIK3CA may drive signaling through the pathway8 and emphasizing the challenges of patient stratification in a pathway with multiple regulatory nodes and extensive crosstalk with other signaling networks.10 These data highlight the need for comprehensive molecular profiling of ER-positive cancers to identify biomarkers of response to PI3K inhibitors and to characterize patients most likely to benefit from this therapy.
Multiple lines of preclinical and clinical investigation demonstrate that inhibition of the PI3K-mTOR pathway can improve the efficacy of endocrine treatment.4,11-14 The OPPORTUNE (Randomised Phase II Window Study of Short-Term Preoperative Treatment With the PI3K Inhibitor GDC-0941 Plus Anastrozole Versus Anastrozole Alone in Patients With ER-Positive Primary Breast Cancer) trial was designed to assess whether addition of the pan-PI3K inhibitor pictilisib (PIC; GDC-0941) could increase the antiproliferative effects of short-term, preoperative treatment with anastrozole (ANA) in ER-positive primary breast cancer. Short-term preoperative studies are a validated strategy for evaluating the impact of targeted therapies alongside endocrine agents by using the nuclear proliferation marker Ki-67 as a surrogate end point of treatment benefit.15-20 Access to tumor tissue before and after treatment enables comprehensive analysis of biomarker changes, thus providing critical insights into the optimal patient population, biomarker responses, and potential mechanisms of resistance.
PATIENTS AND METHODS
Study Design
OPPORTUNE was an open-label, randomized phase II trial performed in 10 academic medical centers in the United Kingdom. The study had two primary aims: to detect an increase in Ki-67 suppression with PIC in ER-positive patients and to assess the treatment effects in subgroups defined by PI3K mutations, luminal A/B subtypes, and baseline Ki-67 scores. The main analysis of the overall treatment effects was planned with 70 evaluable patients and is reported here. A second, more comprehensive biomarker analysis will be performed with 141 patients to provide more power for subgroup analyses.
Patients were eligible if they were postmenopausal and had histologically diagnosed ER-positive, HER2-negative invasive breast cancer. ER positivity was defined as ≥ 1% of tumor cells positive on immunohistochemistry (IHC) or an Allred IHC score of ≥ 3. All patients had operable breast cancer ≥ 1 cm in diameter; adequate hematologic, hepatic, and renal function; and baseline fasting plasma glucose of < 7.8 mmol/L. Prior treatment of breast cancer or use of hormone replacement therapy was not permitted. Patients with inflammatory cancer or distant metastases were excluded. In addition, patients with significant pulmonary dysfunction, cardiac disease, or diabetes mellitus were excluded. The trial was approved by the United Kingdom Medicines and Healthcare Products Regulatory Agency and the London City East Research Ethics Committee (11/LO/1559). All patients provided written informed consent.
Randomization
Patients were randomly assigned (2:1, favoring the combination) to receive treatment with ANA or ANA + PIC. Computer-generated permuted blocks were used, and stratification was by center and histologic grade, as assessed on the diagnostic core biopsy. Participants and investigators were aware of assignment but the investigators who measured the biomarkers were blinded.
Treatment
ANA was given at a dose of 1 mg once per day. PIC was initially administered at 340 mg once per day; from August 2012 onward, PIC was reduced to 260 mg once per day according to safety data from other studies that indicated a lower rate of mucosal and skin toxicity at 260 mg. Five evaluable patients received PIC 340 mg; the remaining patients received PIC 260 mg. Study treatment was given for 14 days, followed by surgical resection and adjuvant therapy as appropriate for each patient according to local practice guidelines.
At least two core-cut tumor biopsies were taken at baseline and at the end of treatment. The last dose of study medication was required within 2 to 4 hours before the end-of-treatment biopsy. Formalin-fixed paraffin-embedded cores were placed into 10% buffered formalin within 10 minutes and fixed for ≥ 6 hours before further processing. Snap frozen cores were to be placed in liquid nitrogen within 10 minutes.
All tumor core biopsies were reviewed centrally. IHC was performed on 3- to 4-μm sections after heat-mediated antigen retrieval. Antibodies for Ki-67 (Clone 30-9, Ventana Medical Systems, Tucson, AZ), cleaved caspase-3 (Clone Asp175, Cell Signaling Technology, Danvers, MA), progesterone receptor (PgR; Clone 1E2, Ventana), and PTEN (Clone 138G6, Cell Signaling Technology) were used. Ki-67 and caspase-3 IHC results were recorded independently by two investigators who were blinded regarding treatment allocation and each other’s assessment. At least 1,000 invasive cancer cells were counted for Ki-67 analysis; Ki-67 was scored as the percentage of positively stained cells. A cutoff of 14% was selected to define high and low baseline Ki-67 expression, but alternative cutoffs (10% and 20%) were also evaluated.21,22 For caspase-3, at least 3,000 cells were assessed. PgR was assessed centrally and regarded as positive if Allred score was ≥ 3. PTEN was classified as positive if any cytoplasmic and/or nuclear expression was observed in tumor cells and negative if no immune reactivity was observed, with the stroma serving as a positive internal control.
Molecular breast cancer subtype was defined by using the NanoString PAM50 algorithm23 (Data Supplement). PIK3CA mutations were assessed by targeted next-generation sequencing by using the Ampliseq Comprehensive Cancer panel assay with the Ampliseq Library Kit 2.0 according to the manufacturer’s instructions (Ion Torrent, Life Technologies, Gaithersburg, MD).
Statistical Analysis
The sample size was based on the two primary aims. The first analysis was planned for 70 evaluable patients providing 80% power at the 5% significance level (one-sided) to detect an effect size of 0.77 between ANA and ANA + PIC. Effect size was defined as (MANA + PIC – MANA)/σpooled, where MANA + PIC and MANA are geometric mean Ki-67 suppression values and σpooled is equal to the square root of [(σANA + PIC2 + σANA2)/2]. The study was also planned to detect a 20% difference in RKi-67-day 15 and RΔKi-67 response rates between arms. The proportion of responders in the combination group was assumed to be 60% under the null hypothesis and 80% under the alternative hypothesis; the test statistic used is the one-sided Z test with pooled variance giving a power of 86%.
All analyses regarding Ki-67 changes were performed on a per-protocol population, defined as all patients who completed 2 weeks of treatment and for whom tumor biopsy specimens were available for assessment of biologic response.
The primary end point was change in Ki-67. Primary Ki-67 analysis was based on estimating the mean Ki-67 suppression in each group and the geometric mean ratio of proportional changes between groups. Geometric means were used because of the approximate lognormal distribution of the data. Values at day 15 were expressed as geometric mean proportions of the baseline and transformed into mean suppression (defined as one minus the geometric means of the proportional changes).
Secondary Ki-67 analyses were geometric mean end-of-treatment Ki-67 expression, individual end-of-treatment antiproliferative response (RKi-67 day 15) defined as Ln(Ki-67day 15) ≤ 2, and individual antiproliferative response (RΔKi-67) defined as a ≥ 50% decrease in Ki-67 expression.16,24 Secondary end points were safety, tolerability, and changes in the apoptosis marker caspase-3; caspase-3 analyses included geometric mean change in caspase-3 between day 15 and baseline and individual apoptotic response (RΔcasp3) defined as a ≥ 50% increase in caspase-3 IHC.
The ratio of the geometric means of the proportional changes and the end-of-treatment Ki-67 expression between groups was analyzed by using t tests. RKi-67 day 15 and RΔKi-67 response rates and one-sided 95% CIs were calculated separately for each arm and compared by using Fisher’s exact test. The relative risk and the associated 95% CI were calculated based on a Mantel-Haenszel heterogeneity χ2 test.
Safety data were reported for all patients who received at least one dose of the study treatment. The worst grade of any adverse event (AE) during trial treatment was reported and compared between trial arms by using Fisher’s exact test.
Multivariable linear regression models based on univariate analyses were conducted to determine which molecular parameters were predictive and/or prognostic for the disease. Ln(Ki-67day 15/Ki-67baseline) was modeled as a dependent variable. On the basis of univariable analyses, treatment, molecular subtype, PgR status, and their interaction with treatment were included. No demographic or stratifying factors were associated with outcome or improved the model fit. PIK3CA mutation and baseline Ki-67 expression showed little effect on outcome and were not included in the final model (Data Supplement).
RESULTS
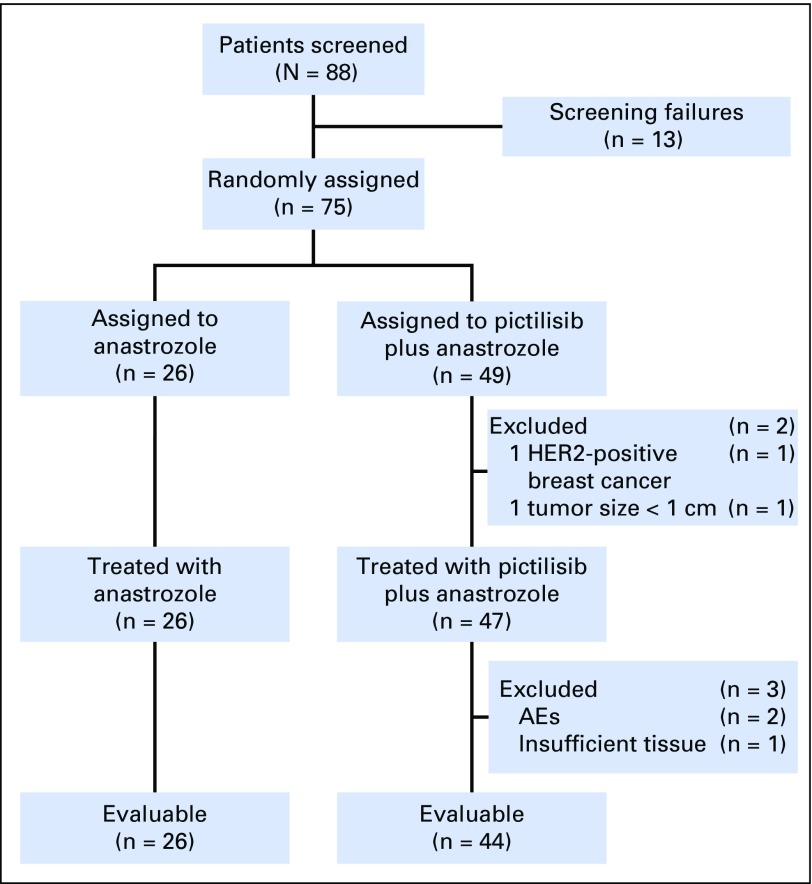
Between January 2012 and September 2014, 75 patients were randomly assigned (Fig 1). Two patients were excluded because of violations of key eligibility criteria. Assessment of the treatment effects was possible for 70 patients who successfully completed the protocol; two patients in the combination arm discontinued treatment early because of AEs and one patient had insufficient tissue for analysis. Baseline distributions of patient and tumor characteristics were similar in the treatment arms (Table 1); 62% of patients had luminal B tumors and 63% had a baseline Ki-67 score of > 14%.
Fig 1.
Trial CONSORT diagram. AE, adverse event; HER2, human epidermal growth factor receptor 2.
Table 1.
Patient Demographic and Tumor Characteristics at Baseline
| Characteristic | ANA Alone (n = 26) | ANA + PIC (n = 44) | ||
|---|---|---|---|---|
| No. (%) | Mean (range) | No. (%) | Mean (range) | |
| Age (years) | 68.7 (48.9-85.4) | 67.5 (49.2-83.0) | ||
| Size of primary tumor, cm | 20.2 (10-36) | 20.7 (10-50) | ||
| Tumor grade | ||||
| 1-2 | 22 (84.6) | 37 (84.1) | ||
| 3 | 4 (15.4) | 7 (15.9) | ||
| PgR status | ||||
| Positive | 16 (64.0) | 29 (95.1) | ||
| Negative | 9 (36.0) | 2 (4.9) | ||
| Molecular subtype | ||||
| Luminal A | 6 (31.6) | 14 (41.2) | ||
| Luminal B | 13 (68.4) | 20 (58.8) | ||
| Ki-67 (% positive tumor cells) | 25.0 (4.4-68.7) | 22.6 (2.7-67.8) | ||
| 0-14 | 9 (34.6) | 17 (38.6) | ||
| >14 | 17 (65.4) | 27 (61.4) | ||
| PIK3CA mutation status | ||||
| Wild-type | 14 (60.8) | 24 (60.0) | ||
| Mutation | 9 (39.1) | 16 (40.0) | ||
| Kinase domain mutation | 5 (21.7) | 9 (22.5) | ||
| Helical domain mutation | 3 (13.0) | 6 (15.0) | ||
NOTE. Kinase domain mutations include H1047R/Y, H1048R, and G1049D/R; helica -domain mutations include E524K and E545K.
Abbreviations: ANA, anastrozole; PgR, progesterone receptor; PIC, pictilisib.
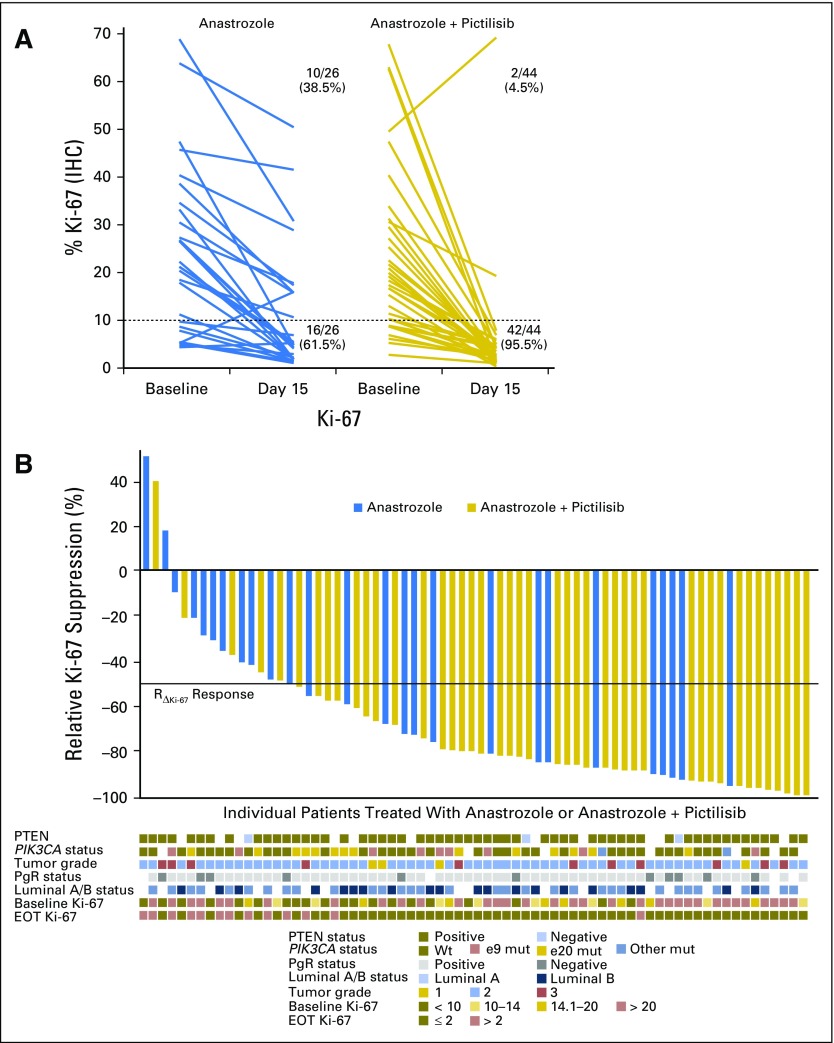
Tumor Ki-67 expression decreased in all but three patients from baseline to day 15 (Fig 2A-B); mean percentage suppression of Ki-67 was 83.8% (95% CI, ≥ 79.0%) for patients treated with ANA + PIC and 66.0% (95% CI, ≤ 75.4%) for patients treated with ANA (Table 2 and Fig 3A). The ratio (ANA + PIC:ANA) of mean Ki-67 suppression was 0.48 (95% CI, ≤ 0.72; P = .004). The mean end-of-treatment Ki-67 expression was 2.9% (95% CI, ≤ 3.7%) for ANA + PIC and 6.1% (95% CI, ≥ 4.1%) for ANA (P = .005).
Fig 2.
Individual Ki-67 changes from baseline to day 15. (A) Individual changes in percentage Ki-67 expression from baseline to day 15; the number and percentage of patients achieving an end-of-treatment (EOT) Ki-67 score of > 10% or ≤ 10% are provided for each group. (B) Individual relative Ki-67 suppression sorted from low to high; relative Ki-67 suppression is defined as Ln(Ki-67day 15) – Ln(Ki-67baseline); results are displayed on their original scale by back transformation. Individual PTEN status, PIK3CA status, progesterone receptor (PgR) status, luminal A/B status, tumor grade, baseline and EOT Ki-67 expression are indicated by colored boxes under each patient. IHC, immunohistochemistry; mut, mutant; Wt, wild-type.
Table 2.
Antiproliferative Response to ANA or ANA + PIC
| Response | ANA (n = 26)% (95% CI) | ANA + PIC (n = 44)% (95% CI) | Relative Risk (combination/ANA) | P |
|---|---|---|---|---|
| Geometric mean Ki-67 suppression | 66.0 (≤ 75.4) | 83.8 (≥ 79.0) | 0.48* (≤ 0.72) | .004 |
| Geometric mean EOT Ki-67 expression | 6.1 (≥ 4.1) | 2.9 (≤ 3.7) | 0.48* (≤ 0.73) | .005 |
| RΔKi-67 response rate | 53.9 (≤ 69.9) | 86.4 (≥ 77.8) | 1.60 (≥ 1.17) | .003 |
| RKi-67 day 15 response rate | 61.5 (≤ 77.2) | 90.9 (≥ 83.8) | 1.48 (≥ 1.13) | .003 |
NOTE. Geometric mean Ki-67 suppression is defined as Ln(Ki-67day15) – Ln(Ki-67baseline); the ratio (combination/ANA) of geometric mean Ki-67 suppression is provided with 95% CI. Geometric mean end-of-treatment (EOT) Ki-67 expression is defined as Ln(Ki-67day 15); individual EOT antiproliferative response RKi-67 day 15 is defined as Ln(Ki-67day 15) ≤ 2; individual antiproliferative response RΔKi-67 is defined as a ≥ 50% fall in Ki-67 expression between baseline and day 15.
Abbreviations: ANA, anastrozole; PIC, pictilisib.
Geometric mean ratio of Ki-67 proportional changes between the groups.
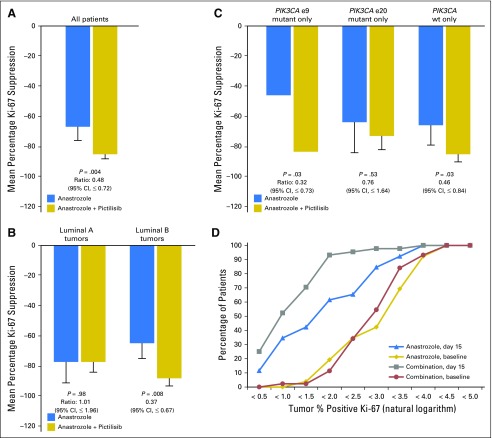
Fig 3.
Antiproliferative response to study treatment. (A) Antiproliferative response expressed as the geometric mean Ki-67 suppression from baseline to day 15. (B) Antiproliferative response by luminal subtype. (C) Antiproliferative response by PIK3CA mutation status; e9: exon 9 domain mutations (helical domain); e20: exon 20 domain mutations (kinase domain). (D) Antiproliferative response at day 15 compared with baseline. The cumulative proportion (by percentage) of patients who had tumors with percentage positive Ki-67 (expressed as the natural logarithm) less than the value on the x-axis is illustrated at baseline and at day 15 for each treatment arm. Error bars indicate 95% CI. wt, wild-type.
Individual RΔKi-67 response rates were 86.4% (95% CI, ≥ 77.%) for ANA + PIC and 53.9% (95% CI, ≤ 69.9%) for ANA (P = .003; Table 2). By using the definition that patients with Ln(Ki-67day 15) ≤ 2 represented an end-of-treatment response, 90.9% (95% CI, ≥ 83.8%) of patients treated with ANA + PIC were responders compared with 61.5% (95% CI, ≤ 77.2%) of patients treated with ANA (P = .003).
Predefined subset analyses investigated potential interactions of PI3K mutations, luminal A/B subtypes, and baseline Ki-67 scores with Ki-67 response. Given the limited power of these analyses, results must be considered exploratory and interpreted with caution.
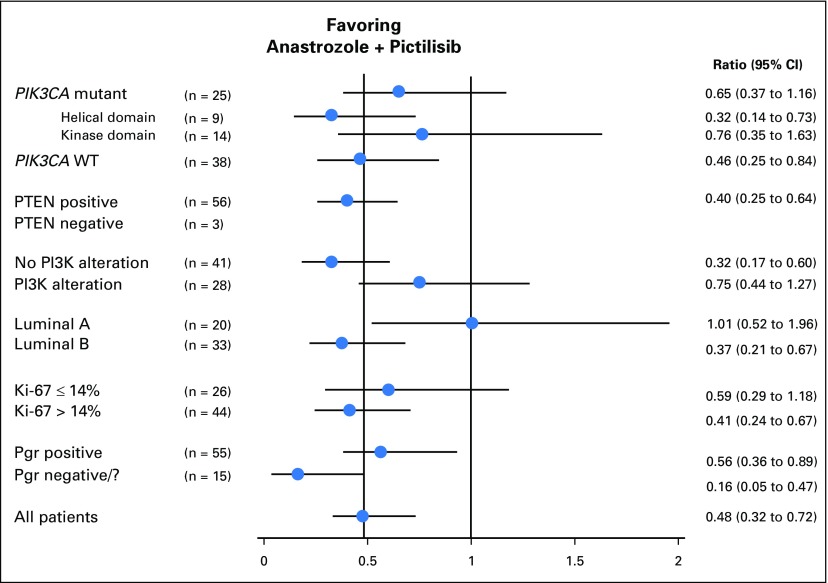
At least one PIK3CA mutation was detected in 25 tumors (39.7%), including 14 helical domain and nine kinase domain mutations. There was no significant correlation between PIK3CA mutations and the activity of PIC (Figs 3C and 4); interestingly, the small number of helical domain mutants showed a relatively poor response to ANA but a good response to ANA + PIC (mean Ki-67 suppression, 46.2% v 82.7%; ratio [ANA + PIC:ANA], 0.32 [95% CI, ≤ 0.73]; P = .03; Fig 3C).
Fig 4.
Ratio (combination:ANA) of geometric mean of Ki-67 proportional changes in prespecified subgroups. Pgr, progesterone; WT, wild type.
Subgroup analysis showed that patients with PAM50 luminal B tumors had a significantly higher antiproliferative response with ANA + PIC compared with ANA (mean Ki-67 suppression, 86.5% v 63.6%; ratio [ANA + PIC:ANA], 0.37; 95% CI, ≤ 0.67; P = .008), whereas adding PIC had no apparent benefit for luminal A tumors (ratio, 1.01; P = .98; Figs 3B and 4). Multivariable analysis confirmed significant interaction between treatment effect and molecular subtype (P = .03), confirming the hypothesis that Ki-67 suppression is higher with ANA + PIC treatment than with ANA for patients with luminal B tumors irrespective of PgR status or baseline Ki-67 expression. Patients with PR-negative luminal B cancers showed the greatest antiproliferative effect from treatment with ANA + PIC (ratio, 0.12). Furthermore, combined treatment also appeared to be more effective in PgR-negative luminal A cancers. There was no difference between baseline and end-of-treatment apoptosis levels between treatment groups (geometric mean caspase-3 change, –10.4% for ANA + PIC and –13.9% for ANA; ratio, 0.96; P = .90).
Treatment-related AEs were consistent with those previously described for PIC and ANA with more AEs in the PIC-treated group (Table 3). No pulmonary toxic effects associated with PIC were identified. Reducing PIC dose from 340 mg to 260 mg reduced the skin toxicity significantly (grade 3, 38% v 3.3%; P =.013). At a PIC dose of 260 mg, grade 3 AEs were asymptomatic hyperglycemia and rash in one patient each. Treatment was discontinued in two patients receiving 340 mg PIC because of hypersensitivity reaction and rash. AEs were rapidly reversible, and all patients received subsequent standard therapy as planned.
Table 3.
Adverse Events in the Safety Population
| Most Common Adverse Events* | ANA Alone (n = 26) | ANA + PIC (340 mg) (n = 8) | ANA + PIC (260 mg) (n = 39) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1/2 | G3 | G1/2 | G3 | G1/2 | G3 | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Fatigue | 6 | 23 | 1 | 4 | 7 | 88 | 0 | 6 | 26 | 0 | ||
| Rash | 0 | 0 | 2 | 25 | 3 | 38† | 3 | 8 | 1 | 3 | ||
| Diarrhea | 1 | 4 | 0 | 4 | 50 | 0 | 20 | 52 | 0 | |||
| Dysgeusia | 1 | 4 | 0 | 2 | 25 | 0 | 4 | 10 | 0 | |||
| Dyspepsia | 0 | 0 | 1 | 13 | 0 | 7 | 18 | 0 | ||||
| Anorexia | 1 | 4 | 0 | 2 | 25 | 0 | 5 | 13 | 0 | |||
| Nausea | 3 | 12 | 0 | 7 | 88 | 0 | 16 | 41 | 0 | |||
| Vomiting | 0 | 0 | 2 | 25 | 0 | 5 | 13 | 0 | ||||
| Stomatitis | 0 | 0 | 1 | 13 | 0 | 2 | 5 | 0 | ||||
| Hyperglycemia | 0 | 0 | 0 | 0 | 3 | 8 | 1 | 3 | ||||
| Creatinine | 0 | 0 | 3 | 38 | 0 | 3 | 8 | 0 | ||||
| Arthralgia | 5 | 19 | 0 | 1 | 13 | 0 | 1 | 3 | 0 | |||
| Headache | 4 | 16 | 0 | 1 | 13 | 0 | 3 | 8 | 0 | |||
| Hot flashes | 6 | 23 | 0 | 0 | 0 | 2 | 5 | 0 | ||||
NOTE. The safety population includes all patients who received at least one dose of the study drug.
Abbreviations: ANA, anastrozole; G, grade; PIC, pictilisib.
Included are all adverse events with an incidence of 10% or more in either group.
Fisher’s exact P = .013 between PIC 340 mg and 260 mg.
DISCUSSION
OPPORTUNE is the first trial of a PI3K inhibitor in ER-positive early-stage breast cancer. The study successfully met the primary end point, demonstrating that adding PIC to ANA significantly increased the antiproliferative response. Both mean Ki-67 suppression and the percentage of tumors with significant Ki-67 reduction were substantially higher for ANA + PIC compared with ANA. Most importantly, the end-of-treatment Ki-67 suppression was also significantly higher for ANA + PIC. This is particularly relevant because only end-of-treatment Ki-67 expression but not baseline expression has been associated with improved recurrence-free survival (RFS).16 In the IMPACT (Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen) trial, 5-year RFS rates were 85%, 75%, and 60%, respectively, for the lowest, middle, and highest tertiles of Ki-67 expression after 2 weeks of preoperative endocrine therapy.16 End-of-treatment Ki-67 expression seems to integrate the prognostic value of baseline proliferation and the predictive value of responding to endocrine therapy, thus making it an excellent predictor of outcome in this setting.16
We also investigated the interaction between antiproliferative response and PIK3CA mutations. In keeping with other series,2,6 approximately 40% of tumors carried a mutation in the PIK3CA gene, 84% of these in one of the hotspots in the helical and kinase domains. Baseline Ki-67 expression was comparable between wild-type and mutant samples (23.3%, 20.7%, and 25.5% for wild-type, helical, or kinase mutations), confirming previously reported results.25,26 There was no association between PIK3CA mutation status and antiproliferative response to ANA, in keeping with other studies suggesting that PIK3CA mutations have limited impact on endocrine therapy.8,25,26 Our data suggest that PIK3CA mutations are also not associated with increased antiproliferative response for ANA + PIC. This is consistent with results from trials of PIC or everolimus in metastatic breast cancer.7,27,28 Interestingly, tumors with helical or kinase domain mutations appeared to respond differently (Ki-67 suppression ratio: helical, 0.32 [95% CI, ≤ 0.73]; kinase, 0.76 [95% CI, ≤ 1.63]). A similar observation was reported in a neoadjuvant trial of everolimus in ER-positive breast cancer in which helical domain mutations were also associated with an increased benefit.24 This observation may merit testing in future studies.
Preplanned subset analyses suggest that the additional antiproliferative effects of PIC may largely be limited to luminal B tumors, whereas luminal A tumors demonstrated no additional effect unless they were PgR negative. The latter result has to be seen in the context that negative PgR status and luminal B subtype are closely associated. Multivariable analysis also suggested that the impact of molecular subtype was independent of baseline proliferation. This is relevant because the PAM50 classification includes genes from the ER response pathway as well as cell cycle progression. Consequently, there is a link between PAM50 subtypes and Ki-67 expression, but overlap is incomplete. In the OPPORTUNE trial, the discordance rate between baseline Ki-67 expression and PAM50 classification was 21% by using a cutoff of 14%, and there was only a weak interaction between baseline Ki-67 and response to PIC (Data Supplement). Overall, these findings are supportive of an association between luminal B subtype, insensitivity to endocrine treatment, and antiproliferative response to treatment with PIC, which has implications for future trial design. However, given the limited power, additional confirmation from the final analysis should be awaited before definitive conclusions can be drawn.
As expected, the rate of apoptosis was low, with the majority of tumors containing < 1% apoptotic cells. No differences were observed between treatment groups, but the strong correlation between Ki-67 and apoptosis scores found in this and other trials16 could mask an effect of PI3K inhibition on apoptosis as observed in preclinical studies.12
Although the OPPORTUNE trial showed an increased response with ANA + PIC in early breast cancer, the FERGI (A Phase II, Double-Blind, Placebo-Controlled, Randomized Study of GDC-0941 or GDC-0980 With Fulvestrant Versus Fulvestrant in Advanced or Metastatic Breast Cancer in Patients Resistant to Aromatase Inhibitor Therapy) trial failed to demonstrate a significant benefit of adding PIC to fulvestrant in metastatic disease. This may have been related to the lower dose of PIC, with 23.6% of patients requiring dose modifications and 34% discontinuing PIC.28 Because the OPPORTUNE trial did not allow dose modifications and excluded patients who discontinued treatment before surgery, results might reflect the potential of PI3K inhibitors if a sufficient dose can be maintained. Alternative strategies to specifically target the alpha subunit of PI3K, which may have a wider therapeutic index than pan-PI3K inhibitors, might overcome these limitations.
There are a few caveats with respect to the data presented here. First, the study was not sufficiently powered for detailed subset analyses, and there is a risk of false-positive findings. Second, baseline PgR status was imbalanced between treatment arms with fewer PgR-negative tumors in the combination arm; this limits the study’s ability to verify results from the recent FERGI study subset analysis, which suggested that only PgR-positive patients benefit from PI3K. Third, not all patients in the combination arm received the same dose of PIC. However, mean Ki-67 suppression was comparable for patients treated with 340 mg (68.8%) and 260 mg (76.7%). Finally, although previous studies have clearly established an association between Ki-67 response and RFS, it is unclear to what degree the same applies for combinations of endocrine treatment with other agents. Results of the OPPORTUNE trial therefore must be interpreted with caution in terms of potential long-term benefits.
Overall, the OPPORTUNE trial is, to the best of our knowledge, the first study to demonstrate that addition of the pan-PI3K inhibitor PIC significantly increases the antiproliferative response to ANA in ER-positive early-stage breast cancers. PIK3CA mutations were not predictive of response to PIC, although patients with exon 9 mutation showed a particularly poor response to ANA that was reversed by the addition of PIC. Luminal B and PgR-negative cancers may enrich for tumors, with the most antiproliferative effect with ANA + PIC.
Acknowledgment
We thank all participating patients and staff at OPPORTUNE centers and the independent trial steering committee for their oversight of the trial.
Footnotes
Supported by the National Institute for Health Research and Cancer Research UK funding (to Barts/Brighton Experimental Cancer Medicine Centre for the OPPORTUNE trial). Recruitment in the United Kingdom was supported by the National Institute for Health Research Cancer Research Network and the UK Experimental Cancer Medicine Centre network. Additional funding and study medication were provided by Genentech, South San Francisco, CA. Next-generation sequencing analysis was supported by Grant No. CG-12-07 from the National Breast Cancer Foundation of Australia (M.T.).
Presented in part at the 37th Annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 9-13, 2014.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: ISRCTN26131497.
See accompanying editorial on page 1970
AUTHOR CONTRIBUTIONS
Conception and design: Peter Schmid, Sarah E. Pinder, Steven Gendreau, Mika Derynck, Arnie Purushotham, Alastair Thompson
Provision of study materials or patients: Peter Schmid, Duncan Wheatley, Jane Macaskill, Charles Zammit, Jennifer Hu, Robert Price, Nigel Bundred, Sirwan Hadad, Alice Shia, Arnie Purushotham, Alastair Thompson
Collection and assembly of data: Peter Schmid, Sarah E. Pinder, Duncan Wheatley, Jane Macaskill, Charles Zammit, Jennifer Hu, Robert Price, Nigel Bundred, Sirwan Hadad, Alice Shia, Louise Lim, Patrycja Gazinska, Natalie Woodman, Darren Korbie, Matt Trau, Paul Mainwaring, Steven Gendreau, Mark R. Lackner, Mika Derynck, Timothy R. Wilson, Hannah Butler, Gemma Earl, Peter Parker, Arnie Purushotham, Alastair Thompson
Data analysis and interpretation: Peter Schmid, Sarah E. Pinder, Shah-Jalal Sarker, Paul Mainwaring, Steven Gendreau, Mark R. Lackner, Mika Derynck
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Randomized Preoperative Window-of-Opportunity Study of the PI3K Inhibitor Pictilisib Plus Anastrozole Compared With Anastrozole Alone in Patients With Estrogen Receptor–Positive Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Peter Schmid
Employment: Genentech (I)
Research Funding: Genentech, AstraZeneca
Sarah E. Pinder
Honoraria: Roche
Consulting or Advisory Role: Genomic Health
Travel, Accommodations, Expenses: Roche, Genomic Health (I)
Duncan Wheatley
Honoraria: Roche
Speakers’ Bureau: Roche
Jane Macaskill
No relationship to disclose
Charles Zammit
No relationship to disclose
Jennifer Hu
No relationship to disclose
Robert Price
No relationship to disclose
Nigel Bundred
No relationship to disclose
Sirwan Hadad
No relationship to disclose
Alice Shia
No relationship to disclose
Shah-Jalal Sarker
No relationship to disclose
Louise Lim
No relationship to disclose
Patrycja Gazinska
No relationship to disclose
Natalie Woodman
No relationship to disclose
Darren Korbie
No relationship to disclose
Matt Trau
No relationship to disclose
Paul Mainwaring
Honoraria: Astellas Pharma, Janssen Oncology, Pfizer, MSD Oncology
Consulting or Advisory Role: Astellas Pharma, Janssen Oncology, Genentech, Novartis, Pfizer
Speakers’ Bureau: Astellas Pharma, Janssen Oncology, Genentech, MSD Oncology
Patents, Royalties, Other Intellectual Property: Nanotechnology patents not related to current article
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology, Genentech
Steven Gendreau
Employment: Genentech
Stock or Other Ownership: Roche, Genentech
Mark R. Lackner
Employment: Genentech
Stock or Other Ownership: Genentech
Mika Derynck
Employment: Genentech
Stock or Other Ownership: Genentech
Patents, Royalties, Other Intellectual Property: Use of PI3K inhibitors in breast cancer; use of pertuzumab in cancer
Travel, Accommodations, Expenses: Genentech
Timothy R. Wilson
Employment: Genentech
Stock or Other Ownership: Genentech
Hannah Butler
No relationship to disclose
Gemma Earl
No relationship to disclose
Peter Parker
Stock or Other Ownership: FASTBASE Solutions
Research Funding: Teva
Patents, Royalties, Other Intellectual Property: Amplified FRET; Coincidence Detection
Arnie Purushotham
No relationship to disclose
Alastair Thompson
No relationship to disclose
REFERENCES
- 1.Miller TW, Balko JM, Arteaga CL: Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer J Clin Oncol 29:4452–4461,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Network : Comprehensive molecular portraits of human breast tumours Nature 490:61–70,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephens PJ Tarpey PS Davies H, etal: The landscape of cancer genes and mutational processes in breast cancer Nature 486:400–404,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorpe LM, Yuzugullu H, Zhao JJ: PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting Nat Rev Cancer 15:7–24,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loi S Haibe-Kains B Majjaj S, etal: PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer Proc Natl Acad Sci USA 107:10208–10213,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabine VS Crozier C Brookes CL, etal: Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study J Clin Oncol 32:2951–2958,2014 [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN Piccart-Gebhart MJ Rugo HS, etal: Correlation of molecular alterations with efficacy of everolimus in hormone receptor–positive, HER2- negative advanced breast cancer: Results from BOLERO-2 J Clin Oncol 201331:(suppl; abstr LBA509) [Google Scholar]
- 8.López-Knowles E Segal CV Gao Q, etal: Relationship of PIK3CA mutation and pathway activity with antiproliferative response to aromatase inhibition Breast Cancer Res 16:R68,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien C Wallin JJ Sampath D, etal: Predictive biomarkers of sensitivity to the phosphatidylinositol 3′ kinase inhibitor GDC-0941 in breast cancer preclinical models Clin Cancer Res 16:3670–3683,2010 [DOI] [PubMed] [Google Scholar]
- 10.Miller TW Rexer BN Garrett JT, etal: Mutations in the phosphatidylinositol 3-kinase pathway: Role in tumor progression and therapeutic implications in breast cancer Breast Cancer Res 13:224,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghayad SE Vendrell JA Ben Larbi S, etal: Endocrine resistance associated with activated ErbB system in breast cancer cells is reversed by inhibiting MAPK or PI3K/Akt signaling pathways Int J Cancer 126:545–562,2010 [DOI] [PubMed] [Google Scholar]
- 12.Crowder RJ Phommaly C Tao Y, etal: PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer Cancer Res 69:3955–3962,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulay A Rudloff J Ye J, etal: Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer Clin Cancer Res 11:5319–5328,2005 [DOI] [PubMed] [Google Scholar]
- 14.Baselga J Campone M Piccart M, etal: Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer N Engl J Med 366:520–529,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowsett M Ebbs SR Dixon JM, etal: Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: Influence of hormonal status and HER-2 in breast cancer—A study from the IMPACT trialists J Clin Oncol 23:2477–2492,2005 [DOI] [PubMed] [Google Scholar]
- 16.Dowsett M Smith IE Ebbs SR, etal: Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer J Natl Cancer Inst 99:167–170,2007 [DOI] [PubMed] [Google Scholar]
- 17.Ellis MJ Tao Y Luo J, etal: Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics J Natl Cancer Inst 100:1380–1388,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polychronis A Sinnett HD Hadjiminas D, etal: Preoperative gefitinib versus gefitinib and anastrozole in postmenopausal patients with oestrogen-receptor positive and epidermal-growth-factor-receptor-positive primary breast cancer: A double-blind placebo-controlled phase II randomised trial Lancet Oncol 6:383–391,2005 [DOI] [PubMed] [Google Scholar]
- 19.Hadad S Iwamoto T Jordan L, etal: Evidence for biological effects of metformin in operable breast cancer: A pre-operative, window-of-opportunity, randomized trial Breast Cancer Res Treat 128:783–794,2011 [DOI] [PubMed] [Google Scholar]
- 20.Macaskill EJ Bartlett JM Sabine VS, etal: The mammalian target of rapamycin inhibitor everolimus (RAD001) in early breast cancer: Results of a pre-operative study Breast Cancer Res Treat 128:725–734,2011 [DOI] [PubMed] [Google Scholar]
- 21.Yerushalmi R Woods R Ravdin PM, etal: Ki67 in breast cancer: Prognostic and predictive potential Lancet Oncol 11:174–183,2010 [DOI] [PubMed] [Google Scholar]
- 22.Maisonneuve P Disalvatore D Rotmensz N, etal: Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes Breast Cancer Res 16:R65,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker JS Mullins M Cheang MC, etal: Supervised risk predictor of breast cancer based on intrinsic subtypes J Clin Oncol 27:1160–1167,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baselga J Semiglazov V van Dam P, etal: Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer J Clin Oncol 27:2630–2637,2009 [DOI] [PubMed] [Google Scholar]
- 25.Loi S Michiels S Baselga J, etal: PIK3CA genotype and a PIK3CA mutation-related gene signature and response to everolimus and letrozole in estrogen receptor positive breast cancer PLoS One 8:e53292,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis MJ Lin L Crowder R, etal: Phosphatidyl-inositol-3-kinase α catalytic subunit mutation and response to neoadjuvant endocrine therapy for estrogen receptor positive breast cancer Breast Cancer Res Treat 119:379–390,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treilleux I Arnedos M Cropet C, etal: Predictive markers of everolimus efficacy in hormone receptor positive (HR+) metastatic breast cancer (MBC): Final results of the TAMRAD trial translational study J Clin Oncol 31,2013. (suppl; abstr 510) [Google Scholar]
- 28.Krop I Johnston S Mayer IA, etal: The FERGI phase II study of the PI3K inhibitor pictilisib (GDC-0941) plus fulvestrant vs fulvestrant plus placebo in patients with ER+, aromatase inhibitor (AI)-resistant advanced or metastatic breast cancer—Part I results 37th Annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 9–13,2014. (abstr S2-02) [Google Scholar]