Abstract
Background
It is unknown whether postanoxic cortical and subcortical myoclonus are distinct entities with different prognoses.
Methods
In this retrospective cohort study of 604 adult survivors of cardiac arrest over 8.5 years, we identified 111 (18%) patients with myoclonus. Basic demographics and clinical characteristics of myoclonus were collected. EEG reports, and, when available, raw video EEG, were reviewed, and all findings adjudicated by 3 authors blinded to outcomes. Myoclonus was classified as cortical if there was a preceding, time-locked electrographic correlate and otherwise as subcortical. Outcome at discharge was determined using Cerebral Performance Category.
Results
Patients with myoclonus had longer arrests with less favorable characteristics compared to patients without myoclonus. Cortical myoclonus occurred twice as often as subcortical myoclonus (59% vs 23%, respectively). Clinical characteristics during hospitalization did not distinguish the two. Rates of electrographic seizures were higher in patients with cortical myoclonus (43%, vs 8% with subcortical). Survival to discharge was worse for patients with myoclonus compared to those without (26% vs 39%, respectively), but did not differ between subcortical and cortical myoclonus (24% and 26%, respectively). Patients with cortical myoclonus were more likely to be discharged in a comatose state than those with subcortical myoclonus (82% vs 33%, respectively). Among survivors, good functional outcome at discharge was equally possible between those with cortical and subcortical myoclonus (12% and 16%, respectively).
Conclusions
Cortical and subcortical myoclonus are seen in every sixth patient with cardiac arrest and cannot be distinguished using clinical criteria. Either condition may have good functional outcomes.
Findings previously considered to predict poor outcome following cardiac arrest1 have been challenged and associations with mortality are increasingly seen as self-fulfilling prophecies.2 Postanoxic myoclonus is seen in up to 20% of patients who remain comatose after resuscitation, and historically has been considered a poor prognostic sign.1 This is particularly felt to be true if beginning within the first 24 hours after arrest, or if myoclonus was generalized and persistent.3,4 However, cases of myoclonic status epilepticus (MSE) with good outcome call this assertion into question.5-9 Much of the literature does not reliably distinguish between cortical and subcortical myoclonus, and the term MSE is not used consistently.10 Here we investigate the hypothesis that the phenotype of patients with cortical myoclonus is distinct from those with subcortical myoclonus, carrying implications for treatment and prognostication.
Methods
Patient cohort
From a consecutive series of 604 cardiac arrest adult patients (aged ≥18) who survived resuscitation, were admitted to an intensive care unit between October 2007 and April 2016, and remained unresponsive after return of spontaneous circulation (ROSC), we identified 111 patients (18%) who had clinical myoclonus within the first 4 days postarrest.
Data collection
The hospital's institutional review board approved the review of charts and collection of data and approval was received from an ethical standards committee. Charts were reviewed and the following characteristics recorded: age, sex, date and time of arrest, arrest site (in vs out of hospital), if the arrest was witnessed and bystander cardiopulmonary resuscitation (CPR) performed, initial rhythm, time to ROSC, completion of targeted temperature management (TTM), target temperature (33 vs 36°C), whether life-sustaining therapies were withdrawn (WLST), and cerebral performance category (CPC) prearrest and at discharge. Date and time of clinical myoclonus onset (+/− offset) and myoclonus semiology (categorized into large-amplitude whole body, segmental, or multifocal11 as well as whether stimulus-induced or spontaneous) were recorded.
EEG was recorded continuously with 21 electrodes using digital EEG bedside monitoring system (Xltek; Natus Medical, Oakville, Canada; sampling rate 200–512 Hz). Electrode placement followed the International 10–20 system and impedances were kept <10 kOhm. EEG reports were reviewed for all patients and myoclonus was categorized as cortical (if there was a time-locked electrographic correlate documented as preceding the clinical myoclonus) or subcortical (if no electrographic correlate preceded the movement).11 Patients were categorized as having MSE if the myoclonus was cortical and occurred at least once every 10 seconds for >10 minutes, or at least once per minute for >30 minutes.12 For 38 patients with myoclonus, raw video EEG was available and 2 epilepsy-trained neurologists (MGH and JC) reviewed and categorized myoclonus while blinded to patient outcome. Agreement between the documented report and re-review of raw video EEG was 95%. In cases of disagreement, a consensus was reached by 3 authors (ASR, MGH, and JC), again blinded to outcome. EEG patterns were characterized using 2012 American Clinical Neurophysiology Society criteria.12 Events consisting of generalized epileptiform discharges at a frequency of 3 Hz or greater, or clearly evolving discharges reaching greater than 4 Hz, were considered electrographic seizures.12 Antiepileptic drugs (AEDs) and continuous IV infusions (cIVs) required to control cortical myoclonus were recorded.
Jerk-locked EEG back-averaging
EEG recordings free of artifact and containing an EMG channel consisting of 2 electrodes 2 cm apart from each other, over the muscle of interest, were clipped and exported in standard European Data Format (.edf) into MNE-python.13
In order to optimize the signal/noise ratio, raw EMG signal was processed as follows:
60-Hz notch to the remove power line signal and 1-Hz high pass filters to remove slow shifts
Computation of the first derivative to disentangle myogram signal and movement artifact
Derivative 30-Hz high pass filtering to remove jerk movement artifact
We selected by visual analysis the processed signal showing the best signal/noise ratio and then chose an arbitrary cutoff allowing the detection of the majority of jerk onsets. These steps were critical and visually controlled by simultaneously displaying the 3 processed EMG signals with the detected jerk. If needed, a temporal offset was applied to ensure that the triggers were placed exactly at the onset of jerk EMG artifacts. A sample of this analysis is provided in figure e-2 (links.lww.com/CPJ/A26).
Following literature recommendations,14 we ensured that we could obtain at least 50 events.
EEG signal was filtered (60 Hz notch and 0.5 Hz high pass filters), segmented from −300 to +300 ms relative to the onset of the jerks, averaged, baseline corrected from −300 to −200 ms, and digitally transformed to an average reference. Jerk time-locked evoked potentials obtained were plotted simultaneously with the averaged EMG to show their temporal relations. The script is available on Github (github.com/neuroicu).
Clinical management
All postarrest patients were managed according to American Heart Association guidelines.15 At our institution, all patients who have not returned to their neurologic baseline after ROSC are considered candidates for TTM. Target temperature is 33°C barring a clear contraindication (such as bradycardia or bleeding resulting in hemodynamic instability). Cases meeting these contraindications are offered TTM with target temperature of 36°C. Continuous video EEG is placed during cooling and remains on for at least 72 hours. No seizure prophylaxis is used, but seizures, including electrographic ones, are aggressively treated using continuous anesthetic agents if necessary, while periodic discharges alone are not routinely treated. Cardiac arrest patients are all managed in an intensive care unit and closely followed by a neurointensivist or neurology consult team.
Statistical analysis
Categorical variables were compared using Fisher exact test or Pearson χ2, as appropriate. Binary logistic regression analysis was applied to identify independent predictors of myoclonus subtype, and those with p < 0.1 were entered into a multivariate analysis. Significance for the multivariate model was set at a p value <0.05. Bonferroni correction was applied as appropriate for multiple comparisons. SPSS (version 23, Chicago, IL) was used for all statistical analyses.
Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Of the 111 patients with myoclonus, solely subcortical myoclonus was present in 23% (n = 25), solely cortical in 59% (n = 65), both semiologically distinct subcortical and cortical in 5% (n = 6). In 14% (n = 15), we were unable to unequivocally determine the subtype of myoclonus. For all further analyses on myoclonus subtype, only patients with clearly subcortical or cortical myoclonus were used (n = 90).
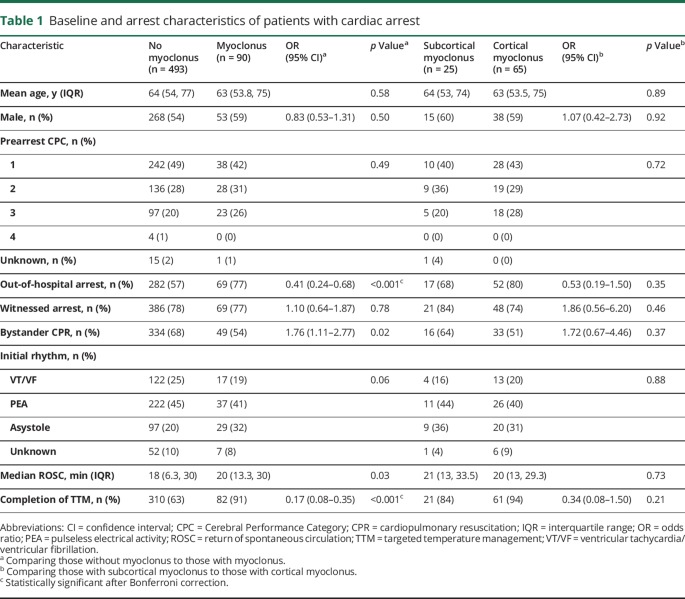
Compared to 493 patients without myoclonus, patients with myoclonus were more likely to have an outside of hospital arrest, and more likely to complete TTM (table 1). Among the entire cohort of those with cardiac arrest, patients who completed TTM were more likely to have had an arrest outside of the hospital (odds ratio [OR] 1.8; 95% confidence interval [CI] 1.2–2.5), less likely to have had a witnessed arrest (OR 0.6; 95% 0.39–0.98), and less likely to have had bystander CPR (OR 0.47; 95% CI 0.32–0.70), with longer ROSC (median 20 vs 10 minutes, p < 0.001). No differences in arrest characteristics were found in those with cortical vs subcortical myoclonus.
Table 1.
Baseline and arrest characteristics of patients with cardiac arrest
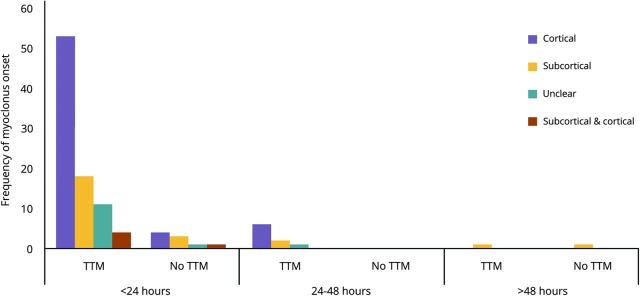
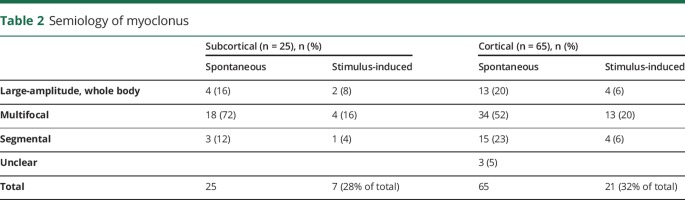
Myoclonus onset was overwhelmingly (90%) within the first 24 hours after ROSC (figure 1), which did not differ between subcortical vs cortical myoclonus. Description of semiology was available for 87 of 90 patients (table 2), but did not aid in distinguishing between subcortical and cortical origin. The rate of electrographic seizures in patients with cortical myoclonus was higher than with subcortical myoclonus (43% vs 8%, respectively, p = 0.03). Of the 65 patients who had cortical myoclonus, 77% (n = 50) met criteria for MSE. Cortical myoclonus was controlled clinically in 77% (n = 50), requiring an average of 1.7 ± 0.8 AEDs and 1.0 ± 0.6 cIVs (figure e-1A, links.lww.com/CPJ/A26). In 44% (n = 29), the cortical myoclonus remained controlled after weaning cIVs with patients most frequently being on multiple non cIV–AEDs such as levetiracetam, valproic acid, or benzodiazepines (figure e-1B). Initial EEG pattern and pattern at the time of clinical control of cortical myoclonus appear in table e-1. Subcortical myoclonus was more successfully treated with 80% being clinically controlled (figure e-1B), and 28% (n = 7) experiencing resolution of myoclonus without any AEDs.
Figure 1. Onset of postanoxic myoclonus.
Frequency of myoclonus onset is grouped into 24-hour epochs from time of return of spontaneous circulation. TTM = targeted temperature management.
Table 2.
Semiology of myoclonus
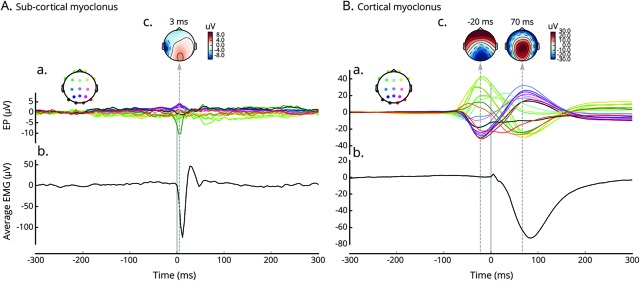
Given observed difficulties in distinguishing the 2 using EEG in 15% of patients, we prospectively applied jerk-locked EEG back-averaging in 2 patients with postanoxic myoclonus to EEG recorded together with EMG leads. Figure 2 shows the results of back-averaging in subcortical (A) and cortical (B) myoclonus.
Figure 2. Jerk-locked EEG back-averaging.
Results of jerk-locked EEG back-averaging performed on 2 exemplary recordings from −300 to 300 ms surrounding onset of the jerk detected on EMG lead. Evoked potentials time-locked to the jerk (EP; A) are plotted along the averaged EMG (Avg-EMG; C) to reveal their temporal relation. Each individual channel line is color-coded to display the sensor positions. Topoplots display the topographies of the detected EP (B). (A) 59-year-old man with out-of-hospital asystolic arrest (return of spontaneous circulation [ROSC] 15 minutes) who underwent targeted temperature management (TTM) to 33°C. On postarrest day 1, he developed facial myoclonus, uncontrolled despite levetiracetam and valproic acid. Recording demonstrated subcortical myoclonus. EMG lead was placed on the left platysma muscle (maximum myoclonic activity) and averaging was computed from 285 events with sampling rate of 200 Hz. No time-locked evoked potential appears before the onset of the myoclonus. Noncerebral muscle artifact is seen on EEG in the left temporal region. (B) A 63-year-old man with out-of-hospital asystolic arrest (ROSC 20 minutes) who underwent TTM to 33°C. On postarrest day 1, he developed jaw myoclonus, uncontrolled despite levetiracetam and midazolam. Recording demonstrated cortical myoclonus. EMG lead was placed on the left chin and averaging was computed from 365 events with sampling rate of 256 Hz. (A) Clear biphasic time-locked evoked potential appears around −70 ms before the onset of the myoclonus and peaks at −20 ms.
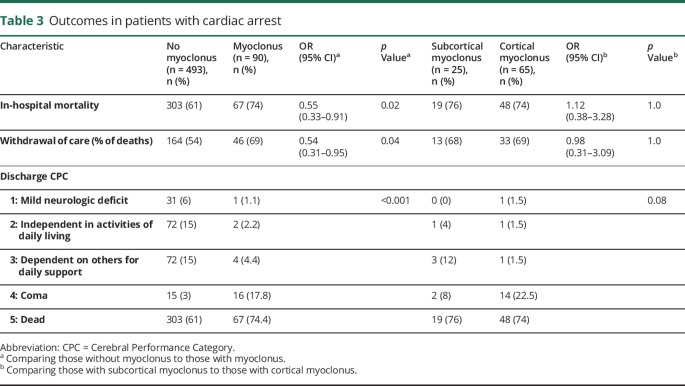
In-hospital mortality was higher among patients with myoclonus (74%, vs 61% without myoclonus, OR 1.8; 95% CI 1.1–3.0), and patients without myoclonus were more likely to be discharged in an independent state (CPC 1–2) (54%, vs 13%, OR 7.9; 95% CI 2.27–27.5). Mortality rates and rates of WLST were similar between patients with cortical vs subcortical myoclonus (table 3). Among survivors, patients with cortical myoclonus were more likely to be discharged in a comatose state (82% survivors, vs 33% with subcortical myoclonus, OR 9.3; 95% CI 1.1–76.7). However, neither cortical nor subcortical myoclonus precluded a good functional outcome, with 12% and 16% of survivors, respectively, being discharged independent in activities of daily living. Neither the timing of myoclonus onset nor semiology was associated with outcome.
Table 3.
Outcomes in patients with cardiac arrest
Discussion
In this study, we characterize early postanoxic myoclonus by reviewing clinical details regarding semiology and timing, as well as presence or absence of EEG correlates. Prior literature has rarely distinguished between cortical and subcortical myoclonus,9,16 and the AAN prognostic criteria do not distinguish between the two or require EEG for diagnosis.1 When the distinction has been made, the literature often has applied a less specific definition of cortical myoclonus, labeling it as cortical if epileptiform activity was present on the EEG, without distinguishing whether electrographic activity was time-locked and preceding the myoclonic jerk.8,9,17 Here we comprehensively studied myoclonus and categorized patients based on continuous video-EEG recordings and detailed behavioral assessments. Further, we show the feasibility of a jerk-locked EEG back-averaging to categorize myoclonus in these patients.
We identified 18% of patients with clinical myoclonus, confirming rates seen in prior studies.8,9,16,18 Clinical features such as timing of onset and semiology were inadequate to distinguish between the two. Some patients had semiologically distinct subcortical and cortical myoclonus. Further, we found that, while cortical myoclonus confers a higher risk of electrographic seizures (43%), almost every 10th patient with subcortical myoclonus also had seizures, further emphasizing the need for vigilance in EEG monitoring even in patients with subcortical myoclonus. Cortical myoclonus was more likely to occur in patients who completed TTM, a marker in our cohort of longer arrest with less favorable characteristics. Patients with subcortical myoclonus showed a similar tendency, though this likely did not reach significance due to our small numbers. In-hospital mortality between patients with and without myoclonus, and between patients with cortical and subcortical myoclonus, did not differ. While in 14% of cases the subtype of myoclonus could not be identified retrospectively, we prospectively show the feasibility of using jerk-locked back-averaging to aid in distinguishing cortical from subcortical myoclonus for future studies.
In the present study, we show a higher rate of survival in patients with postanoxic myoclonus than prior reports,14,16 with 30% alive at discharge. This may reflect the aggressiveness of our institution and family preference to abstain from WLST. This is further supported by the observation that WLST occurred at comparable rates to other studies within our entire cardiac arrest cohort2,9,19 despite having less favorable arrest characteristics, including longer ROSC times and fewer patients with a shockable rhythm.6,9 Good short- and long-term functional outcome was possible, but rare, among patients with clinical evidence of myoclonus, which supports several isolated reports in the literature.5-9 Cortical myoclonus carried a worse functional prognosis, with patients more likely to be discharged in a comatose state (82% vs 33%), while survival did not differ between subtypes of myoclonus. These differences may reflect distinct injury patterns, which may shed light on the underlying generators of cortical vs subcortical myoclonus.
There are several limitations to this study. First, in clinical practice, distinctions between cortical and subcortical postanoxic myoclonus are typically based on EEG findings.9,14 Following clinical practice, we chose to distinguish these two groups using EEG criteria. However, more advanced methods such as jerk-locked back-averaging20,21 and coherence analysis22,23 have been applied in other disease processes to detect premyoclonic cortical potentials not apparent on scalp EEG. Given the limitations of the chosen methodology, it is conceivable that some patients with cortical myoclonus may have been misclassified as subcortical. It is unclear how often postanoxic patients with myoclonus in clinical practice as well as in our study are misclassified as subcortical myoclonus. Given the observed difference in outcomes that we observed, it is paramount that future studies verify our findings using jerk-locked back-averaging or EEG-EMG coherence studies. Second, because the data on clinical myoclonus were retrospectively collected, we relied on medical record documentation, which at times lacked detail. In particular, we lacked specific reasons for WLST decisions, and presence of myoclonus may have factored into counseling of families. However, since neurologists or neurointensivists are consulted on all patients with cardiac arrest at our hospital, descriptions of semiology were fairly thorough and movements were almost always captured on continuous video EEG. Third, raw EEG with video was only available for 34% of our patients; however, within that cohort, there was 95% agreement between the EEG report and our re-review of the original file. Fourth, while in our hospital seizures are aggressively treated, there is no standardized management protocol for myoclonus in our hospital, leading to variations in treatment and difficulty in assessing the effectiveness of individual AEDs and cIVs. Finally, long-term outcomes were not available for all patients, and outcome at discharge may not have accurately represented the potential of patients to recover neurologic function with rehabilitation.
Though there is no clear diagnostic or treatment algorithm for myoclonus,10 we propose accurate distinction of myoclonus subtypes using back-averaging with a dedicated EMG channel. If a patient has concomitant status epilepticus, existing algorithms for treatment of status epilepticus should be used, preferentially using valproic acid instead of phenytoin given the benefit of valproic acid for myoclonus and the potential for phenytoin to worsen subcortical myoclonus.24 Further treatment of either cortical or subcortical myoclonus should involve sequential use of valproic acid, levetiracetam, and benzodiazepines, all of which treat both cortical and subcortical myoclonus.11 While paralytic trials have been suggested to remove myogenic artifact from EEG recordings, the mere presence of a cerebral discharge without knowing its relationship to the myogenic jerk does not imply a physiologic cause and effect.25 Here we show that jerk-locked back-averaging of the EEG, a technique that has been used to detect an EEG correlate that otherwise might be missed,14 can better delineate any temporal or spatial relationship of the cortical discharge to the myoclonic jerk.
Cortical myoclonus is twice as common as subcortical myoclonus, and both can be associated with good outcome. Clinical features cannot reliably distinguish between the two, and EEG alone may not be adequate to diagnose, but jerk-locked EEG back-averaging clearly can. Cortical myoclonus is associated more often with discharge in a comatose state. Going forward, the literature should more systematically and prospectively distinguish between subcortical and cortical myoclonus in studies of treatment and outcome.
TAKE-HOME POINTS
→ Postanoxic myoclonus occurs more often in patients with longer arrests and with nonshockable rhythms.
→ Clinical criteria including semiology, timing of onset, and location of myoclonus do not determine whether there is a time-locked electrographic correlate to the myoclonus.
→ Patients with cortical myoclonus are more likely to be discharged in a comatose state than those with subcortical myoclonus.
Author contributions
Alexandra S. Reynolds: study concept and design, acquisition of data, statistical analysis, analysis and interpretation of data, drafting the manuscript. Benjamin Rohaut: study concept and design, analysis and interpretation of data, drafting and revising the manuscript for content. Manisha G. Holmes: study concept and design, analysis and interpretation of data, revising the manuscript for content. David Robinson: revising the manuscript for content, acquisition of data. William Roth: revising the manuscript for content, acquisition of data. Angela Velazquez: revising the manuscript for content, acquisition of data, study coordination. Caroline Couch: revising the manuscript for content, acquisition of data. Alex Presciutti: revising the manuscript for content, acquisition of data. Daniel Brodie: revising the manuscript for content. Vivek K. Moitra: revising the manuscript for content. LeRoy E. Rabbani: revising the manuscript for content. Sachin Agarwal: revising the manuscript for content, acquisition of data. Soojin Park: revising the manuscript for content. David Roh: revising the manuscript for content. Jan Claassen: study concept and design, analysis and interpretation of data, revising the manuscript for content, study supervision and coordination.
Study funding
No targeted funding reported.
Disclosure
A.S. Reynolds receives support from the NIH/NINDS. B. Rohaut receives research support from Assistance Publique–Hôpitaux de Paris, Institut National de la Santé et de la Recherche Médicale (INSERM), Amicale des Anciens Internes en Médecine et Syndicat des Chefs de Clinique & Assistants des Hôpitaux de Paris (AAIHP-SCCAHP), and Philippe Foundation. M.G. Holmes, D. Robinson, W. Roth, A. Velazquez Novas, C. Couch, and A. Presciutti report no disclosures. D. Brodie is currently the co-chair of the Trial Steering Committee for the VENT-AVOID trial sponsored by ALung Technologies, was previously on the medical advisory board of ALung Technologies and Kadence (Johnson & Johnson), and serves as Associate Editor-in-Chief of ECLS Journal. V.K. Moitra, L.E. Rabbani, and S. Agarwal report no disclosures. S. Park has received a consultant honorarium from Arbor Pharmaceuticals; serves as Academic Editor for PLOS One; and receives research support from NIH/NIEHS. D. Roh reports no disclosures. J. Claassen serves on a scientific advisory board for SAGE; serves on the editorial boards of Neurocritical Care and Annals of Neurology; and receives research support from the DANA Foundation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Wijdicks EFM, Hijdra A, Young GB, Bassetti CL, Wiebe S; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;67:203–210. [DOI] [PubMed] [Google Scholar]
- 2.Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation 2016;102:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wijdicks EF, Parisi JE, Sharbrough FW. Prognostic value of myoclonus status in comatose survivors of cardiac arrest. Ann Neurol 1994;35:239–243. [DOI] [PubMed] [Google Scholar]
- 4.Thömke F, Marx JJ, Sauer O, et al. Observations on comatose survivors of cardiopulmonary resuscitation with generalized myoclonus. BMC Neurol 2005;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossetti AO, Oddo M, Liaudet L, Kaplan PW. Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology 2009;72:744–749. [DOI] [PubMed] [Google Scholar]
- 6.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol 2010;67:301–307. [DOI] [PubMed] [Google Scholar]
- 7.Lucas JM, Cocchi MN, Salciccioli J, et al. Neurologic recovery after therapeutic hypothermia in patients with post-cardiac arrest myoclonus. Resuscitation 2012;83:265–269. [DOI] [PubMed] [Google Scholar]
- 8.Bouwes A, van Poppelen D, Koelman JHTM, et al. Acute posthypoxic myoclonus after cardiopulmonary resuscitation. BMC Neurol 2012;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seder DB, Sunde K, Rubertsson S, et al. Neurologic outcomes and postresuscitation care of patients with myoclonus following cardiac arrest. Crit Care Med 2015;43:965–972. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds AS, Claassen J. Treatment of seizures and postanoxic status epilepticus. Seminars in neurology. Thieme Med Publishers 2017;37:033–039. [DOI] [PubMed] [Google Scholar]
- 11.Levy A, Chen R. Myoclonus: pathophysiology and treatment options. Curr Treat Options Neurol 2016;18:21. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 13.Gramfort A, Luessi M, Larson E, et al. MEG and EEG data analysis with MNE-Python. Front Neurosci 2013;26:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibasaki H, Hallett M. Electrophysiological studies of myoclonus. Muscle Nerve 2005;31:157–174. [DOI] [PubMed] [Google Scholar]
- 15.Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S465–S482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmer J, Rittenberger JC, Faro J, et al. Clinically distinct electroencephalographic phenotypes of early myoclonus after cardiac arrest. Ann Neurol 2016;80:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aicua Rapun I, Novy J, Solari D, Oddo M, Rossetti AO. Early Lance-Adams syndrome after cardiac arrest: prevalence, time to return to awareness, and outcome in a large cohort. Resuscitation 2017;115:169–172. [DOI] [PubMed] [Google Scholar]
- 18.Sadaka F, Doerr D, Hindia J, Lee KP, Logan W. Continuous electroencephalogram in comatose postcardiac arrest syndrome patients treated with therapeutic hypothermia: outcome prediction study. J Intens Care Med 2015;30:292–296. [DOI] [PubMed] [Google Scholar]
- 19.Grossestreuer AV, Gaieski DF, Abella BS, et al. Factors associated with post-arrest withdrawal of life-sustaining therapy. Resuscitation 2017;110:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibasaki H, Yamashita Y, Tobimatsu S, Neshige R. Electroencephalographic correlates of myoclonus. Adv Neurol 1986;43:357–372. [PubMed] [Google Scholar]
- 21.Celesia GG, Parmeggiani L, Brigell M. Dipole source localization in a case of epilepsia partialis continua without premyoclonic EEG spikes. Electroencephalogr Clin Neurophysiol 1994;90:316–319. [DOI] [PubMed] [Google Scholar]
- 22.Brown P, Farmer SF, Halliday DM, Marsden J, Rosenberg JR. Coherent cortical and muscle discharge in cortical myoclonus. Brain 1999;122:461–472. [DOI] [PubMed] [Google Scholar]
- 23.Panzica F, Canafoglia L, Franceschetti S, et al. Movement-activated myoclonus in genetically defined progressive myoclonic epilepsies: EEG-EMG relationship estimated using autoregressive models. Clin Neurophysiol 2003;114:1041–1052. [DOI] [PubMed] [Google Scholar]
- 24.Frucht S, Fahn S. The clinical spectrum of posthypoxic myoclonus. Mov Disord 2000;15(suppl 1):2–7. [DOI] [PubMed] [Google Scholar]
- 25.Hallett M. Physiology of human posthypoxic myoclonus. Mov Disord 2000;15(suppl 1):8–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.