Abstract
Purpose of review
We systematically appraised randomized controlled trials proposing exercise to influence cognition in older adults to (1) assess the methodologic quality using Cochrane criteria; (2) describe various exercise dose measures and assess their relationship with improved cognitive performance; and (3) identify consistent patterns of reported effects on cognition.
Recent findings
There was overall good methodologic quality in all 98 included studies. The assessment of the relationship between improved cognition and various measures of exercise dose (session duration, weekly minutes, frequency, total weeks, and total hours) revealed a significant correlation with total hours. Improvements in global cognition, processing speed/attention, and executive function were most stable and consistent.
Summary
We found that exercising for at least 52 hours is associated with improved cognitive performance in older adults with and without cognitive impairment. Exercise modes supported by evidence are aerobic, resistance (strength) training, mind–body exercises, or combinations of these interventions.
The population older than 60 years will reach 2 billion by 2050,1 and their highest health-related concern is being “mentally sharp.”2 Physiologic changes to the aging brain (i.e., cognitive aging) are complex and highly variable,3 resulting in difficulty projecting the trajectory of cognitive decline and identifying transition to pathologic states, such as mild cognitive impairment (MCI) and dementia. Given the limited effectiveness of available treatments for dementia, promotion of a healthy brain is relevant. Physical exercise can promote cognitive brain health (defined as the ability to remember, learn, plan, concentrate, and maintain a clear, active mind) and counteract many effects of cognitive aging.4-6
To date, there are over 1,000 clinical trials, 174 systematic reviews, and 50 meta-analyses examining effects of exercise on cognitive function in older adults. Effect sizes range from small to moderate, yet findings appear to be consistently positive.7-12 Despite the determined efficacy, the available literature offers no practical prescriptive guidance for physical exercise to promote cognitive brain health. The sufficient and optimal exercise dose and regimen to induce such effects have not been fully examined. We undertook an exhaustive systematic review of studies evaluating physical exercise to influence cognition in healthy older adults and those with MCI and dementia. We aimed our study to (1) assess methodologic quality using Cochrane criteria, (2) describe and assess the relationship between various exercise dose measures and improved cognitive performance in older adults, and (3) identify consistent patterns of reported effects on cognition.
Methods
The present systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement13 and Cochrane Handbook for Systematic Reviews and Interventions.14 The protocol is published (registration CRD42016049877).15 Randomized controlled trials (RCTs) with the following criteria were included: published in English; aimed at probing effects of regular physical exercise (>4 sessions) on cognition; included older adults (age ≥60 years) with or without cognitive impairment; and including at least one neuropsychological outcome measure. Exclusion criteria were studies with lower dosage than specified above or absence of a quantitative neuropsychological measure of cognitive performance.
Searches were conducted in December 2016, by 2 independent investigators, in the following indexed electronic medical databases: LILACS, MEDLINE/PubMed, SciELO, PEDro, the Cochrane Central Register of Controlled Trials, Scopus, and Clinicaltrials.gov utilizing the following keywords: exercise, cognitive function, older adults, elderly, cognitively impaired adults, and mild cognitive impairment (supplementary material e-1, links.lww.com/CPJ/A27). Data extraction was done according to the population intervention comparison outcome framework.16 Cochrane Review Manager 5.3 software (version 5.3, Cochrane Collaboration, Canada) was utilized to collect study information and to construct a risk of bias table14 that displayed the methodologic rating (low, high, or unclear risk) for each potential source of bias. The risk of bias analysis comprised (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; (7) other.
Exercise regimen information (session time, exercise intensity, frequency per week, and length of intervention and exercise intensity) was collected and averaged across studies. Exercise intensity was described using heart rate relative to age-predicted maximal heart rate (HRmax%) and conversions using rating of perceived exertion, percent of oxygen uptake reserve (VO2R%), absolute metabolic intensity, or percentage of maximum voluntary contraction were employed.17 We classified exercise intensity relative to HRmax as light (35%–54%), moderate (55%–69%), hard (70%–89%), and very hard (90%–100%),17 exercise modes into 6 categories (resistance training, aerobic training, combination, mind–body, stretching/balance/toning, or other), and calculated their relative frequency.
While interested in reporting on the patterns of exercise employed by all studies that met our inclusion criteria, we also aimed at assessing the relationship between each specific exercise measure (session duration, weekly minutes, frequency, total weeks, and total hours) and improved cognitive performance. For this analysis, we conducted a correlation utilizing Spearman rho statistic to examine relationships between an improvement in cognitive performance and each exercise measure. Intensity was not included in the analysis, as it was not reported in all studies. A p value <0.05 was considered statistically significant.
We grouped studies according to the cognitive status of the participants, creating 3 groups: older healthy adults (OHA), individuals with MCI, and individuals with dementia. Cognitive status was reported according to the mean score on the Mini-Mental State Examination (MMSE18), and scores were converted if the modified MMSE19 or Montreal Cognitive Assessment20 was used. For the analysis of cognitive domains most influenced by exercise, all neuropsychological tests employed by the included studies were classified into 5 possible domains: executive function, processing speed/attention, global cognition, visuospatial processes/memory, and working memory. A board-licensed PhD-trained clinical neuropsychologist (KM) oversaw this classification. Outcome measures pertaining to more than one domain were represented in both relevant cognitive domains (2 maximum). We calculated the frequency with which a neuropsychological test was associated with an improvement (statistically significant) or no improvement (nonsignificant) in cognitive performance, as reported in each study. For each domain, we carried out an analysis to test the hypothesis that the percentage of analyses with cognitive improvements was statistically different from 50% using a 2-tailed 1-proportion Z test. A Bonferroni correction was applied to correct for the inflation of type I error rate with statistical significance at p < 0.01. Domains that met this criterion (percentage successful outcomes statistically different from 50%) were classified as most consistently improved by exercise.
Data availability
All data reported are presented within the article and supplementary material. For data-related inquiries, contact the corresponding author.
Results
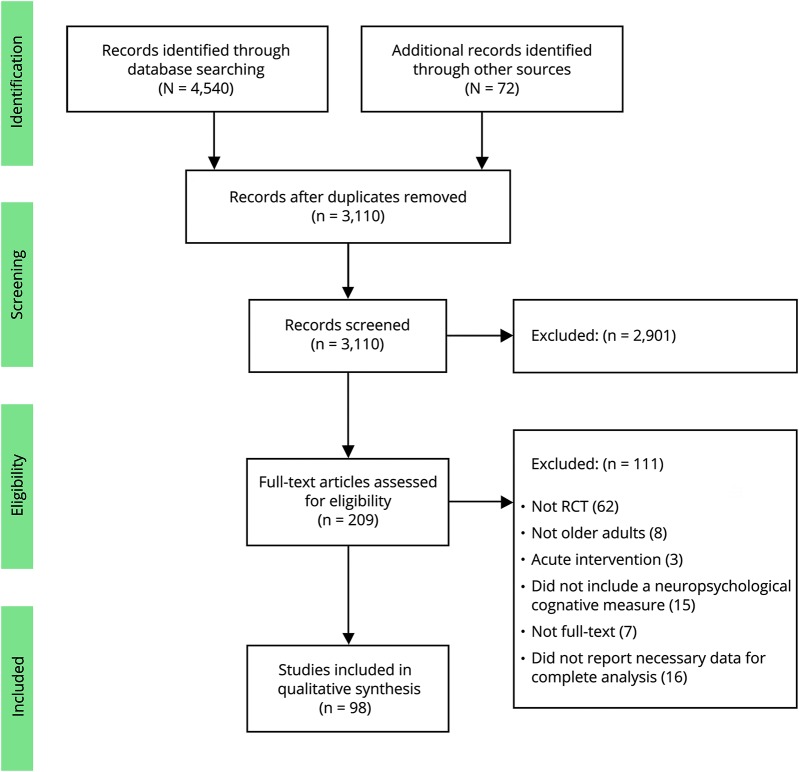
Of 4,612 studies, 98 met our inclusion criteria (figure 1).
Figure 1. Flow diagram of the study selection.
RCT = randomized controlled trial.
Participant characteristics
Data from a total of 11,061 participants (3,491 male [31.56%]; 7,475 female [67.58%]; 84 not reported) were included in the present study (tables e-1 through e-3 and e-references links.lww.com/CPJ/A27). The mean age of participants was 73 ± 2 years. According to standard exercise recommendations for physical health,21 most participants (58.2%) were sedentary prior to study enrollment, 11.25% were active, and 30.44% of studies did not report on prior activity levels.
The average baseline MMSE for all individuals was 25.03 ± 1.55. Utilizing reference values for the MMSE,18 59.41% of all participants were classified as OHA, 25.74% were classified as having MCI, and 14.85% had a score consistent with dementia. Within the dementia group, 73.3% of participants had moderate and 26.7% had severe dementia.
Risk of bias analysis of included studies
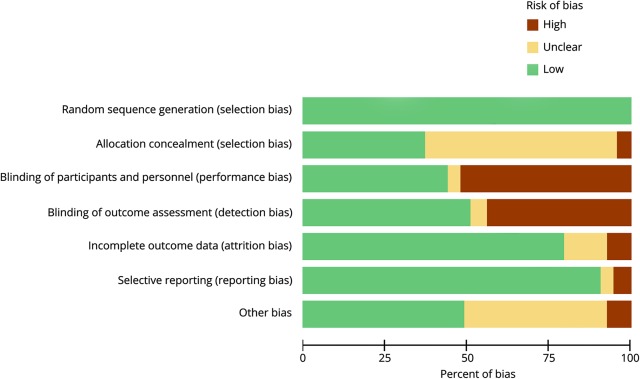
In the risk of bias analysis (figure 2), 100% of the RCTs included in the present study had low risk for selection bias due to random sequence generation. Concerning risk for selection bias due to allocation concealment, 60.2% of studies had unclear risk, 37.5% had low risk of bias, and the remaining 4% had high risk of bias. Over half of the studies (53.4%) had high risk of performance bias due to the lack of blinding procedures for participants and personnel, 43.8% had low risk, and the remaining 3% had unclear risk. Similarly, for detection bias nearly half (51%) of the studies had high risk, 43.8% had low risk, and 5.1% had unclear risk due to lack of blinding of outcomes assessment. Regarding attrition bias, the majority of studies (79.5%) had low risk, 13.2% had unclear risk, and 7.1% had high risk. Nearly all studies (90.8%) had low risk of bias for selective outcome reporting, 5.1% of studies had high risk, and 4% had unclear risk. Concerning “other” sources of bias, nearly half (48.9%) of studies had low risk, 43.8% had unclear risk, and 7.1% had high risk of bias. The most commonly observed “other” sources of bias were low reporting on activity/sedentary status and education level at baseline and lack of reporting on exercise intensity employed (individual scoring for each item and study can be seen in figure e-1, links.lww.com/CPJ/A27).
Figure 2. Risk of bias graph for all criteria in all included studies.
Exercise dosage analysis in included studies
Exercise modes
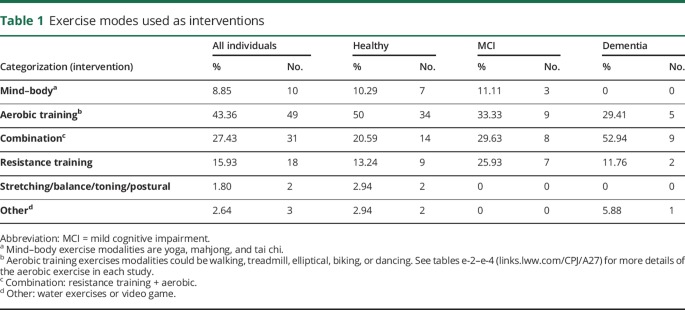
The most frequently reported exercise mode was aerobic (43.63%), followed by combined aerobic and resistance training (27.43%), resistance training in isolation (15.93%), and mind–body exercises (8.85%) (table 1). Stretching/balance/toning and water exercises/video games were utilized less often (1.8% and 2.64%, respectively). Due to studies with more than one intervention, the total number of interventions is greater than the number of studies included.
Table 1.
Exercise modes used as interventions
Walking was the most frequently used mode of aerobic exercise (51.7%), and self-selected exercise was the second (23.5%). The remainder of activities were cycling (10.59%), dancing (5.88%), and walking and biking (8.23%). No exercise was the most common control (58.4%), followed by stretching/toning/balance (25.7%), resistance training (4.95%), and cognitive training (3.96%). Other less frequent interventions were combined aerobic and resistance training (2.97%), aerobic exercise (1.98%), mind–body exercises (0.99%), and other (water exercises, Wii Fit [0.99%]).
Exercise measures per health status are seen in table 2.
Table 2.
Overview of exercise measures according to health status
Session time
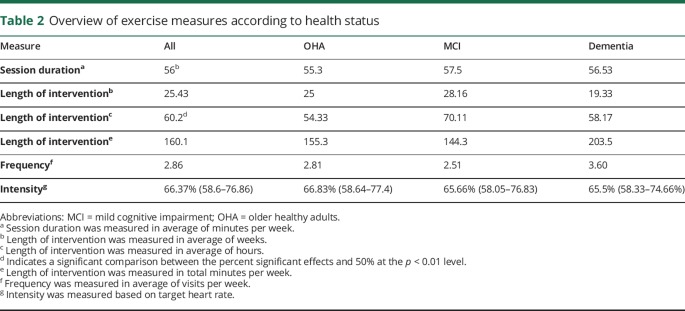
The mean session time across studies was 56 ± 19.11 minutes; 52.0 ± 18.2 minutes for the OHA group, 57.5 ± 20.57 minutes for the MCI group, and 56.53 ± 22.2 minutes for the dementia group.
Exercise frequency
The mean frequency of intervention (times per week) across all studies was 2.86 ± 1.19 times per week; 2.81 ± 0.98 for the OHA group, 2.51 ± 0.77 for the MCI group, and 3.6 ± 1.98 for the dementia group.
Length of intervention
The mean exercise duration across studies was 60.2 ± 49.4 hours distributed over an average of 25.43 ± 19.1 weeks (range 4–104). For the OHA group, the mean exercise duration was 54.33 ± 47 hours distributed over an average of 25.01 ± 20.3 weeks. The mean exercise duration for the MCI group was slightly higher, with 70.11 ± 58.4 hours, delivered over an average of 28.16 ± 17.96 weeks. For the dementia group, the mean exercise duration was 58.17 ± 38.6 hours, delivered over an average of 19.33 ± 14.61 weeks. The mean exercise time per week in minutes was 160.1 (all individuals), 155.3 (OHA), 144.3 (MCI), and 203.5 (dementia).
Exercise intensity
The majority of studies utilized either high (37.8%) or medium intensity (36.7%), and 6% of studies utilized light intensity. Nearly a fifth of studies (19.4%) did not report exercise intensity. The mean intensity across all studies was 66.36% ± 9.59% for the OHA group, 65.66% ± 10.01% for the MCI group, and 65.5% ± 9.6% for the dementia group.
Relationship between improvement in cognitive performance and exercise measures
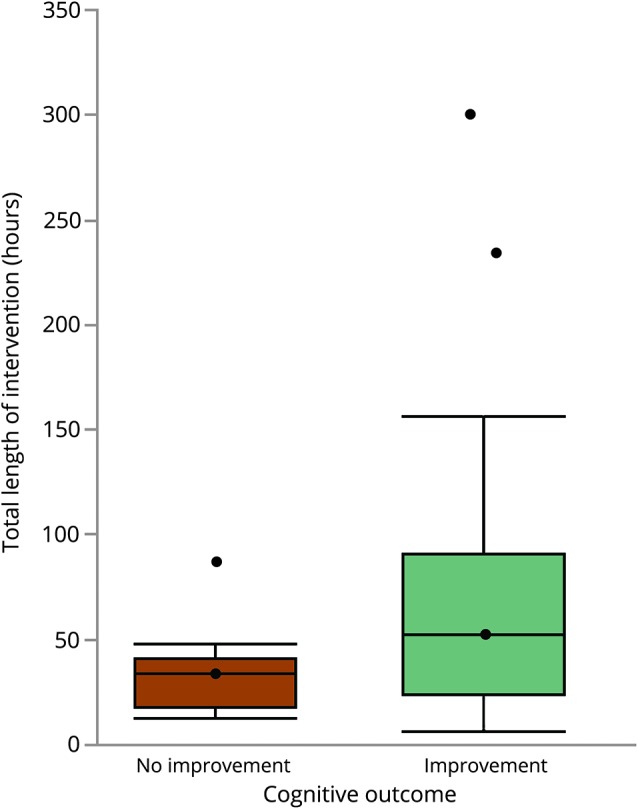
The bivariate correlation analysis revealed that the total length of intervention in hours was the only correlate of improved cognitive performance (r = 0.24, p = 0.01). The median length of intervention in hours (± lower and upper quartiles) for studies reporting improved cognitive outcomes was 52, compared to 33.8 in studies reporting no improvement in cognitive outcomes (figure 3). None of the remaining outcomes was correlated with improved cognitive performance (session time in minutes r = 0.20, p = 0.05; exercise frequency per week r = −0.04, p = 0.67; length of intervention in minutes r = 0.15, p = 0.15; total weeks r = 0.15, p = 0.12).
Figure 3. Boxplots indicating median intervention time in hours (± lower and upper quartiles) in studies reporting improvements (gray box) and no improvements (white box) on cognitive outcome.
Cognitive domains most consistently influenced by exercise
A total of 122 different neuropsychological outcome measures were utilized across all studies. The majority of tests (n = 34) primarily assessed executive function, followed by visuospatial/memory processes (n = 32), processing speed/attention (n = 27), global cognition (n = 18), and working memory (n = 18).
Consistency of reported effects
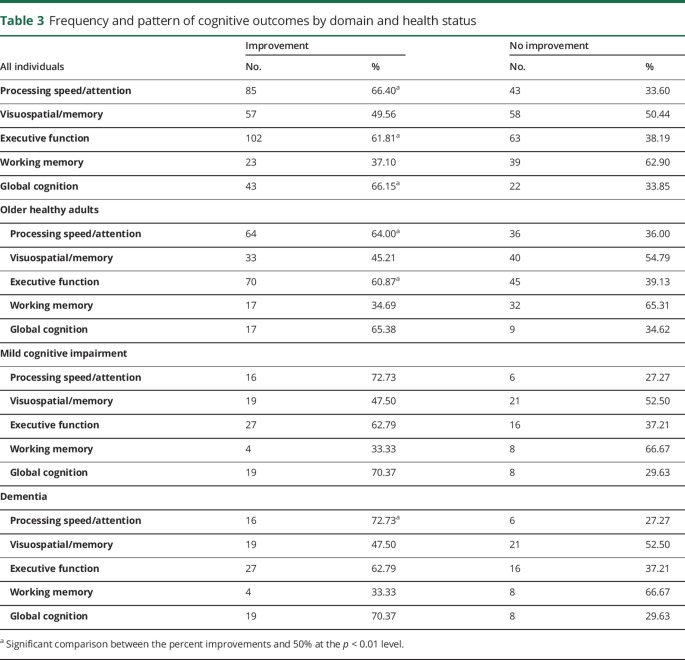
Of all 460 analyses, we found that 57.6% (265) were associated with improvements in cognitive function, while the remaining 42.4% (195) did not report improved cognitive outcomes. Table 3 presents the number of analyses and relative percentage per cognitive domain and health status. Data from all individuals revealed that improvements in processing speed/attention, executive function, and global cognition were most consistent (p = 0.0002, p = 0.002, p = 0.009, respectively) when compared to visuospatial/memory processes and working memory (p = 0.92, p = 0.04, respectively). Individual analyses for the OHA group revealed that improvements in processing speed/attention and executive function were the most consistent (p = 0.005 and p = 0.01, respectively) when compared to visuospatial/memory processes, working memory, and global cognition (p = 0.41, p = 0.03, and p = 0.11, respectively). In the MCI group, none of the domains showed consistent improvements (processing speed/attention p = 0.03, visuospatial/memory processes p = 0.75, executive function p = 0.09, working memory p = 0.24, global cognition p = 0.03). Finally, in the dementia group, improvements in processing speed/attention were the most consistent (p = 0.01) when compared to visuospatial/memory processes, executive function, working memory, and global cognition (p = 0.05, p = 0.40, p = 1.00, and p = 0.79, respectively).
Table 3.
Frequency and pattern of cognitive outcomes by domain and health status
Discussion
This exhaustive systematic appraisal of 98 RCTs evaluating physical exercise to influence cognitive performance in 11,061 older adults identified a high number of studies with good methodologic quality that assessed various exercise modes (comprising aerobic, resistance, combined, mind–body exercises), dosages, and regimens. At least 50% of articles had low risk of bias for all criteria assessed. The highest biases were due to the lack of blinding of participants and examiners and the low reporting on activity/sedentary status and education level at baseline and exercise intensity.
The average exercise dose from all included studies was 1 hour a day, 3 times per week, for 60 hours distributed over 25 weeks. However, the comparison between exercise dose measures in studies that reported positive and negative cognitive outcomes revealed that the most important correlate of improved cognition was the total intervention time. All studies associated with negative cognitive outcomes employed interventions lasting less than 52 hours, which was the median intervention time for studies associated with a positive cognitive outcome (figure 3). A cautious interpretation of this finding is that exercising for at least 52 total hours is likely to improve cognitive performance. While we found no relationships between cognitive improvements and session time, exercise frequency, intensity, and weekly minutes, we recommend that future studies examine these factors so that exercise guidelines for cognitive brain health can be developed.
A comparison between our results and other available exercise guidelines for physical health is difficult. The US Department of Health and Human Services,21 American Heart Association,22 and American College of Sports Medicine23 suggest that individuals engage in at least 150 minutes of moderate intensity exercise or 75 minutes of vigorous exercise per week to achieve benefits in physical health. However, we found no relationship between improved cognition and length of intervention in weekly minutes. This finding may suggest that when compared to physical health, a longer-term exposure to exercise may be necessary to achieve cognitive benefits. Nevertheless, a likely scenario pertains to the probability that gains in brain health are not limited to 52 total hours of exposure. Thus, akin to additional general health benefits derived from additional activity, it is likely that gains in cognitive brain health be seen from additional activity beyond this amount.
Approximately half of all studies used aerobic exercise alone, and another third combined aerobic and resistance training. The average intensity in aerobic interventions was moderate relative to the HRmax (60%–80%). We emphasize that this does not mean that these interventions are superior to the less often reported interventions such as mind–body exercises, but rather that presently the majority of available data is in support of these interventions. The finding that various modes are associated with beneficial effects on cognitive brain health is encouraging from a clinical perspective. Not all individuals will have sufficient cardiovascular endurance, functional capacity, or motivation to initiate moderate intensity aerobic exercise, but it appears that they can still benefit from lower intensity exercise.
In addition, it is conceivable that different types of exercise may affect cognition via different pathways. For example, a recent study in rodents24 showed that aerobic exercise and resistance training improved spatial working memory and hippocampal plasticity in aging rats, albeit by different molecular mechanisms. While both interventions increased neurotrophic signaling, aerobic exercise increased glutamatergic signaling and reduced DNA damage, and resistance training increased proinflammatory factors.24
Physical exercise has been suggested to be neuroprotective against the onset of neurodegenerative diseases, such as dementia.25-28 Nevertheless, a recent prospective study of 10,000 adults with a mean follow-up of 27 years found that midlife physical activity (10–28 years before diagnosis) did not prevent a dementia diagnosis later in life, suggesting a lack of neuroprotective effects.29 Our analysis does not approach the question of neuroprotection, yet it suggests that upon becoming symptomatic, exercise interventions can result in cognitive gains. It is worthwhile to note that nearly 60% of the total sample was sedentary, suggesting that combating sedentary behavior is a contributing factor to the results observed. It is unclear whether similar cognitive effects can be seen in response to exercise in physically active individuals. In addition, only 730-36 of the 98 studies assessed and demonstrated maintenance of effects at follow-up; future studies should assess short-term and long-term follow-up of effects.
In the overall analysis, processing speed/attention, executive function, and global cognition were most consistently associated with improvements. These findings suggest that cognitive-mediated improvements via exercise act on the same constructs most commonly affected by cognitive aging. In addition, the identification of the cognitive domains most susceptible to exercise-mediated improvements may enable identification of individuals who may be most responsive to exercise-mediated improvements in cognitive function. The diversity in outcomes utilized across studies (122 in total) makes comparisons difficult, and we recommend that a common core of neuropsychological outcome measures be delineated to facilitate further comparisons between studies.
We acknowledge that by limiting the analysis to the statistical significance, we could not capture important clinical information that is pertinent to cognition, such as the clinical significance. Our review synthesized dose of exercise in RCTs indirectly by averaging results across studies. While this allowed for comparison of a large number of studies, conclusions may be influenced by limitations in the design, power, and other logistical aspects of such studies, such as funding mechanisms and feasibility.
The present study advances the field by identifying practical information that can be used by individuals and practitioners for the implementation of exercise to promote cognitive brain health in older adults. We found that exercising for at least 52 hours in sessions lasting approximately an hour is associated with improved cognitive performance in older adults with and without cognitive impairment. Exercise improves processing speed in OHA and individuals with dementia, improves executive function in OHA, and appears to have little effect on memory (working memory and visuospatial memory). While promising, the effect on overall cognition is not clear, and suffers from a paucity of studies reporting the effect of exercise on overall cognition.
TAKE-HOME POINTS
→ Good quality evidence supports the use of exercise for the promotion of cognitive brain health in older adults.
→ Exercising for at least 52 hours in sessions lasting approximately an hour is associated with improved cognitive performance in older adults with and without cognitive impairment.
→ In order to achieve the exercise dose above, individuals can participate in aerobic, resistance (strength) training, mind–body exercises, or combinations of these interventions.
→ Improvements in processing speed/attention, executive function, and global cognition are most stable and consistently associated with exercise participation.
Author contributions
Joyce Gomes-Osman: study concept, design, acquisition, analysis and interpretation of data, manuscript preparation, critical revision of manuscript for intellectual content. Danylo F. Cabral: study design, acquisition of data, manuscript preparation. Timothy P. Morris: acquisition of data, manuscript preparation. Katalina McInerney: analysis and interpretation of data, manuscript preparation. Lawrence P. Cahalin: critical revision of manuscript for intellectual content and statistical analyses. Tatjana Rundek: critical revision of manuscript for intellectual content. Augusto Oliveira: manuscript preparation. Alvaro Pascual-Leone: study concept, analysis and interpretation of data, critical revision of manuscript for intellectual content.
Study funding
J.G-O. was supported by a grant from the Evelyn McKnight Institute at the University of Miami Miller School of Medicine. D.C. was supported by a studentship by the “Fundação de Apoio a Pesquisa do Estado De Alagoas (Brazil).”
Disclosure
J. Gomes-Osman, D.L. Ferreira-Cabral, T.P. Morris, K. McInerney, and L.P. Cahalin report no disclosures. T. Rundek serves as an Assistant Editor for stroke on the editorial boards of Neurology®, Cerebrovascular Disease, and Journal of Ultrasound in Medicine and receives research support from NIH/NINDS and Evelin F. McKnight Brain Institute. A. Oliveira reports no disclosures. A. Pascual-Leone serves on scientific advisory boards for Neosync, Starlab, Neuronix, Neuroelectrics, Constant Therapy, Cognito, and Novavision; serves as an Associate Editor for Annals of Neurology, European Journal of Neuroscience, and Frontiers in Neuroscience; is listed as co-inventor on several patents related to real-time integration of TMS with EEG and functional imaging; and receives research support from Neuronix, NIH (NEI, NINDS, NCRR, NIMH, NCATS), IARPA, DARPA, Michael J. Fox Foundation, Berenson-Allen Foundation, RJG Family Foundation, and Sidney Baer Jr. Foundation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP/248. 2017. Available at: esa.un.org/unpd/wpp/Publications/Files/WPP2017_KeyFindings.pdf. Accessed February 20, 2017. [Google Scholar]
- 2.National Council on Aging. The United States of Aging Survey: Full Research Findings. 2015;4–7. Available at: ncoa.org/wp-content/uploads/USA15-National-Fact-Sheet-Final.pdf. Accessed January 15, 2017. [Google Scholar]
- 3.Blazer DG, Yaffe K, Karlawish J. Cognitive aging. JAMA 2015;313:2121. [DOI] [PubMed] [Google Scholar]
- 4.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011;108:3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Best JR, Chiu BK, Liang Hsu C, Nagamatsu LS, Liu-Ambrose T. Long-term effects of resistance exercise training on cognition and brain volume in older women: results from a randomized controlled trial. J Int Neuropsychol Soc 2015;21:745–756. [DOI] [PubMed] [Google Scholar]
- 6.Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, Titterness AK. Exercising our brains: how physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecul Med 2008;10:47–58. [DOI] [PubMed] [Google Scholar]
- 7.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults. Psychol Sci 2003;14:125. [DOI] [PubMed] [Google Scholar]
- 8.Angevaren M, Aufdemkampe G, Verhaar HJJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment (review). Cochrane Database Syst Rev 1996;3. [DOI] [PubMed] [Google Scholar]
- 9.Ludyga S, Gerber M, Brand S, Holsboer-Trachsler E, Pühse U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: a meta-analysis. Psychophysiology 2016;53:1611–1626. [DOI] [PubMed] [Google Scholar]
- 10.Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev 2014;16:12–31. [DOI] [PubMed] [Google Scholar]
- 11.Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev 2015;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wayne PM, Walsh JN, Taylor-Piliae RE, et al. Effect of tai chi on cognitive performance in older adults: systematic review and meta-analysis. J Am Geriatr Soc 2014;62:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Telzlaff J, Altman DG; The PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 14.Shuster JJ, Higgins JPT, Green S. Review: Cochrane handbook for systematic reviews for interventions. Res Synth Methods 2011;2:126–130. [Google Scholar]
- 15.Cabral D, Oliveira CA, Gomes-Osman J. Physical exercise to promote brain health in older adults with or without cognitive impairment: a systematic review of randomized trials for dose-specific recommendations: PROSPERO International Prospective Register of Systematic Reviews. 2016. Available at: crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016049877. Accessed November 10, 2017.
- 16.Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club 1995;123:12–13. [PubMed] [Google Scholar]
- 17.American College of Sports Medicine. American College of Sports Medicine position stand. Med Science Sport Exerc 1998;30:975–991. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 19.Tombaugh TN, McDowell I, Kristjansson B, Hubley AM. Mini-Mental State Examination (MMSE) and the modified MMSE (3MS): a psychometric comparison and normative data. Psychol Assess 1996;8:48–59. [Google Scholar]
- 20.Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ. Alzheimer's Disease Neuroimaging Initiative for the ADN: Relationship between the Montreal Cognitive Assessment and Mini-Mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr 2015;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services. Physical activity guidelines for Americans. Pres Counc Phys Fit Sport Res Dig 2008;9:1–8. [Google Scholar]
- 22.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;129:1–46. [Google Scholar]
- 23.Garber CE, Blissmer B, Deschenes MR, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults. Med Sci Sport Exerc 2011;43:1334–1359. [DOI] [PubMed] [Google Scholar]
- 24.Vilela TC, Muller AP, Damiani AP, et al. Strength and aerobic exercises improve spatial memory in aging rats through stimulating distinct neuroplasticity mechanisms. Mol Neurobiol 2017;54:7928–7937. [DOI] [PubMed] [Google Scholar]
- 25.Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc 2003;51:459–465. [DOI] [PubMed] [Google Scholar]
- 26.Chieffi S, Messina G, Villano I, et al. Neuroprotective effects of physical activity: evidence from human and animal studies. Front Neurol 2017;8:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckett MW, Ardern CI, Rotondi MA. A meta-analysis of prospective studies on the role of physical activity and the prevention of Alzheimer's disease in older adults. BMC Geriatr 2015;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips C, Baktir MA, Srivatsan M, Salehi A. Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Front Cel Neurosci 2014;8:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabia S, Dugravot A, Dartigues JF, et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ 2017;357:j2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eggenberger P, Schumacher V, Angst M, Theill N, de Bruin ED. Does multicomponent physical exercise with simultaneous cognitive training boost cognitive performance in older adults? A 6-month randomized controlled trial with a 1-year follow-up. Clin Interv Aging 2015;17:1335–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Öhman H, Savikko N, Strandberg TE, et al. Effects of exercise on cognition: the Finnish Alzheimer disease exercise trial: a randomized, controlled trial. J Am Geriatr Soc 2016;64:731–738. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki T, Shimada H, Makizako H, et al. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: a randomized controlled trial. BMC Neurol 2012;12:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki T, Shimada H, Makizako H, et al. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS One 2013;8:e61483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smiley-Oyen AL, Lowry KA, Francois SJ, Kohut ML, Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: the “selective improvement” and “cardiovascular fitness” hypotheses. Ann Behav Med 2008;36:280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease. J Am Med Assoc 2008;300:1027–1037. [DOI] [PubMed] [Google Scholar]
- 36.Law LLF, Barnett F, Yau MK, Gray MA. Effects of functional tasks exercise on older adults with cognitive impairment at risk of Alzheimer's disease: a randomised controlled trial. Age Ageing 2014;43:813–820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data reported are presented within the article and supplementary material. For data-related inquiries, contact the corresponding author.