Abstract
Background
We retrospectively reviewed the neuroimaging findings of patients with Cowden syndrome and determined their frequency in a single cohort.
Methods
Electronic medical records were queried from January 1999 to January 2017 to identify patients who fit the clinical criteria for diagnosis of Cowden syndrome with or without a documented PTEN mutation. Patients with brain MRI examinations were then identified.
Results
We retrospectively identified 44 patients with Cowden syndrome, 22 of whom had neuroimaging for review. Eleven (50%) had Lhermitte-Duclos disease, 4 (18.1%) had meningiomas, 13 (59.1%) had at least one developmental venous anomaly, 3 had cavernous malformations, 2 had evidence of dural arteriovenous fistula, 7 had increased white matter signal abnormalities relative to age (31.8%), 4 had prominent perivascular spaces, cerebellar tonsillar ectopia was present in 7 of 21 (33.3%), and 1 had cortical malformation.
Conclusions
It is important to recognize that in addition to Lhermitte-Duclos disease, other intracranial findings such as multiple venous anomalies, meningiomas, greater than expected white matter signal abnormality, prominent perivascular spaces, and cortical malformations may warrant a thorough evaluation for Cowden syndrome in the appropriate clinical setting. We further recommend that this broader spectrum of intracranial abnormalities be considered for addition to the Cowden syndrome diagnostic criteria at the time of next revision.
Cowden syndrome (CS) (also known as Cowden disease or multiple hamartoma syndrome, OMIM 158350) is a rare autosomal dominant genodermatosis characterized by multiple hamartomas of ectodermal, mesodermal, and endodermal origin. It is caused by loss of function mutations in the PTEN gene located on chromosome 10q23.1 Loss of function of the PTEN gene contributes to overgrowth and risk for a variety of cancers including breast, thyroid, endometrium, skin, kidneys, and colon. Other phenotypes associated with PTEN mutations include Bannayan-Riley-Ruvalcaba syndrome (BRRS) and PTEN-related Proteus syndrome.1 Revised diagnostic criteria for CS were proposed in 2013, which also recognized that CS can be more broadly considered as part of a spectrum of disorders termed PTEN hamartoma tumor syndrome.2 Lhermitte-Duclos disease (LDD) is a major criterion for the diagnosis of CS.2 It is a slow-growing tumor, containing dysplastic rather than neoplastic cells in the cerebellum, and is also referred to as a dysplastic gangliocytoma. Abel et al.3 suggest that LDD may be best considered as a hypertrophic cellular growth phenomenon superimposed upon a developmental malformation. MRI is the modality of choice for noninvasive diagnosis of LDD, as this reveals a characteristic striated appearance of the mass.4,5
In addition to LDD, other intracranial abnormalities are relatively common in patients with CS. These include meningiomas, vascular malformations including developmental venous anomalies (DVAs), cortical malformations, white matter signal abnormalities, and prominent perivascular spaces. However, a similar spectrum of intracranial findings has also been identified more broadly in patients without CS who have PTEN mutations, including those with BRRS.5–8
In this study, we retrospectively reviewed the neuroimaging findings of patients with CS (operational diagnosis based on 2013 criteria with or without PTEN mutation) to determine their frequency in a single cohort.2 Our primary aim was to synthesize these observations with previously published data to determine if additional intracranial abnormalities merit inclusion as diagnostic criteria for CS.
Methods
Standard protocol approvals, registrations, and patient consents
Our institutional review board with waiver of informed consent approved this retrospective study.
Electronic medical records were queried to identify patients with confirmed or possible diagnosis of CS, LDD, or dysplastic gangliocytoma of the cerebellum from January 1999 to January 2017.
These records were then manually reviewed by a physician who is dual board-certified in neurology and medical genetics to select patients who fit the clinical criteria for diagnosis of CS with or without a documented PTEN mutation.2 Patients with brain MRI examinations were then identified. For study inclusion, the MRI at a minimum had to include sagittal T1-weighted, axial T1-weighted, axial T2-weighted, axial T2-weighted fluid-attenuated inversion recovery (FLAIR), and axial diffusion-weighted (with apparent diffusion coefficient map) pulse sequences. MRI examinations that additionally included contrast-enhanced T1-weighted imaging, a T2* gradient echo sequence, or susceptibility-weighted imaging were also documented. Using a picture archiving and communication system, 2 board-certified neuroradiologists with 11 and 26 years of experience performed a joint consensus review of the MRI studies to evaluate for the following:
LDD: Presence of a cerebellar lesion with imaging features diagnostic of LDD by previously published criteria4,5
White matter lesions: A qualitative assessment was made as to whether the amount of cerebral white matter intraparenchymal T2 hyperintensity was greater than expected for patient age
Prominent perivascular spaces: Sharply defined foci that followed CSF signal on all pulse sequences without evidence of surrounding gliosis (i.e., FLAIR hyperintensity)
Cerebellar tonsillar ectopia: The presence of cerebellar tonsillar ectopia (>5 mm inferior to the plane of a line connecting the basion and opisthion) was determined, and the lowest-lying tonsil was measured
DVA: Based primarily upon postcontrast T1-weighted imaging, the number of DVAs was documented for each case; draining veins in the immediate vicinity of LDD were not included in this count, in spite of their resemblance to DVAs
Meningiomas: The presence and location of presumed meningiomas was recorded for each case, as defined by a dural-based, extra-axial mass with typical features of a meningioma
Magnetic susceptibility: Using T2* gradient echo sequences or susceptibility-weighted imaging, foci of abnormal intraparenchymal magnetic susceptibility suggestive of hemosiderin deposition within the brain were sought; foci of magnetic susceptibility morphologically consistent with a cavernous malformation on other MRI pulse sequences were specifically documented.
Brain malformations: The presence of any developmental brain malformation was noted
Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Study cohorts
A flow chart illustrating the assembly of the clinical cohorts used for neuroimaging review is shown in figure 1. We retrospectively identified a total of 44 patients with CS, 23 of whom were tested for a PTEN mutation and were positive. Twenty-two of the 44 patients with CS had an MRI that was digitally archived to allow for a detailed review. All of the MRI studies included T1-weighted postcontrast sequences except for one patient who only had a noncontrast MRI due to renal insufficiency. Sixteen of 22 patients with CS had either T2* gradient echo9 or susceptibility-weighted7 imaging for review. In 1 patient with CS who was status-post subtotal resection of LDD, only postoperative MRI was available for review, so the remainder of the brain was assessed as detailed in the Methods while the posterior fossa was excluded from assessment. Headache was the most common indication for neuroimaging.
Figure 1. Flow chart illustrating the assembly of the clinical cohorts used for brain MRI review.
Patients with Cowden syndrome (CS) all met criteria for operational diagnosis, either without or with a documented PTEN mutation.2 LDD = Lhermitte-Duclos disease.
Intracranial imaging features of CS
Fifteen of the 22 patients were women. Mean age (SD) at imaging was 46.1 years (18.7 years) with a median of 51.7 years. Neuroimaging was found to be abnormal in 21 of the 22 patients upon joint neuroradiologist review.
Eleven of 22 patients (50%), 7 of whom were PTEN-positive, had LDD, though preoperative imaging was only available for 10 of 11. Six of the 11 patients (54.5%) with LDD were women.
Four of 22 (18.1%) patients had meningiomas that were singular in 2 patients (left inferior parietal [4.0 cm × 5.1 cm × 5.2 cm], left superior frontal [1.6 cm × 1.0 cm × 1.5 cm]) and multiple in the remaining cases (2 right cavernous sinus [3.9 cm × 2.9 cm × 2.2 cm] and left parietal [1.0 cm × 0.5 cm × 1 cm] and 3 [2 anterior right frontal (1.0 cm × 1.1 cm × 1.2 cm; 0.5 cm × 0.6 cm × 0.6 cm) and 1 right prepontine (1.1 cm × 0.9 cm × 1.0 cm)] meningiomas, respectively). Only 1 patient with a single meningioma was symptomatic from it and underwent resection. Thirteen patients (59.1%) had at least 1 DVA while 8 (36.4%) had multiple DVAs. Three patients had definite cavernous malformations (right parietal [0.8 cm × 0.9 cm × 0.9 cm], left frontal [0.5 cm × 0.6 cm × 0.6 cm], right cerebral [1.2 cm × 1.3 cm × 1.1 cm]) based on the MRI appearance (2 of these had associated DVAs), and 3 additional patients had multiple small foci of magnetic susceptibility within the brain consistent with nonspecific chronic microhemorrhages. Two patients had evidence of dural arteriovenous fistulas on MRI that had been previously confirmed angiographically. Excluding the 1 case for which preoperative imaging was unavailable, cerebellar tonsillar ectopia was present in 7 of 21 patients (33.3%, range 5–14 mm). One patient exhibited malformations of cortical development that included right perisylvian polymicrogyria and left inferior frontal cortical dysplasia. Seven of the 22 patients had increased white matter signal abnormalities relative to age (31.8%), while 4 patients had prominent perivascular spaces within the cerebral white matter.
The table summarizes the intracranial findings. The 6 images in figure 2 illustrate some of the coexisting MRI abnormalities identified in this study.
Table.
Intracranial imaging abnormalities
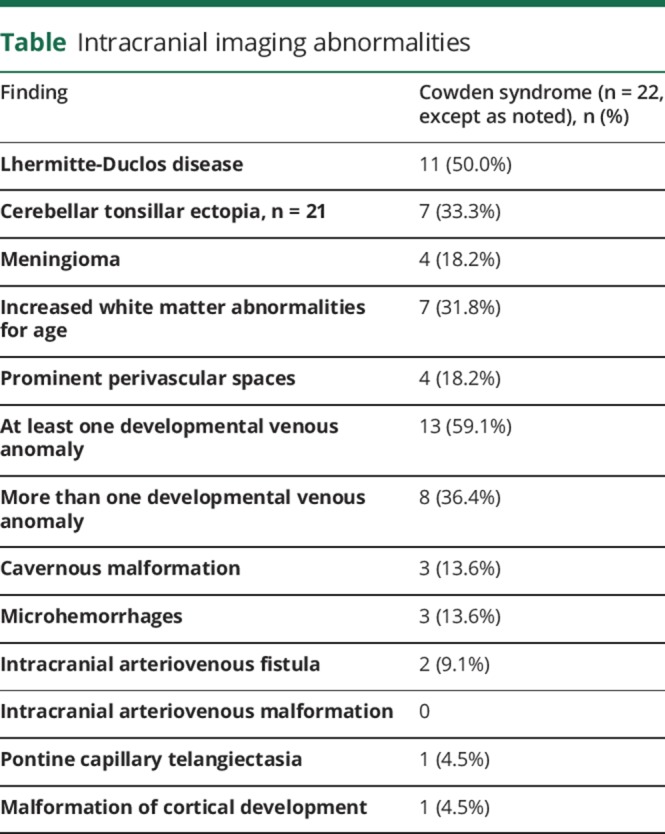
Figure 2. Associated MRI findings in patients with Cowden syndrome (CS).
(A) The classic striated appearance of Lhermitte-Duclos disease (LDD) (arrows) is well-demonstrated with T2-weighted imaging in a 67-year-old woman. (B) Axial T1 postcontrast MRI illustrates a large left parietal dural-based extra-axial mass (arrowheads), which was pathologically confirmed to represent a meningioma, in a 54-year-old woman. (C) Axial T1 postcontrast MRI demonstrates bilateral frontal developmental venous anomalies (arrows) in a 50-year-old woman. (D) Numerous foci of white matter hyperintensity (arrows), which are greater than expected for age, are identified on an axial T2-weighted fluid-attenuated inversion recovery sequence in a 39-year-old man. (E) As depicted on an axial T2-weighted sequence, multiple abnormally prominent perivascular spaces (arrows) are present in the cerebral white matter of a 61-year-old man. (F) In a 62-year-old woman, susceptibility-weighted imaging reveals multiple foci of magnetic susceptibility (arrows) in the cerebral white matter, which are presumably related to hemosiderin deposition from chronic microhemorrhages or possibly tiny cavernous malformations. (G) As seen on sagittal T1-weighted MRI, low-lying cerebellar tonsils (arrow) reach the upper aspect of the C1 arch in a 61-year-old woman who had CS without LDD. (H) In a 22-year-old woman with CS and LDD, sagittal T1-weighted image demonstrates cerebellar tonsillar ectopia (arrow) and typical striated appearance of the cerebellar gangliocytoma (asterisk). (I) Sagittal T1-weighted sequence depicts thick and irregularly bumpy cortex (arrows) consistent with a coarse pattern of right perisylvian polymicrogyria in an 8-year-old boy. A separate abnormality in the left frontal lobe had imaging features suspicious for cortical dysplasia (not shown).
Cerebellar tonsillar herniation from LDD vs Chiari I malformation
Excluding the patient with CS who only had a postoperative MRI, cerebellar tonsillar ectopia was present in 7 of 21 (33.3%) patients with CS. However, to account for the potential confounding effect of a space-occupying lesion in the posterior fossa (i.e., developmental vs acquired tonsillar herniation), it was believed to be more useful to assess tonsillar ectopia based on the presence or absence of LDD. Tonsillar ectopia was present in 5 of 10 patients with LDD (5, 6, 6, 10, 14 mm) and 2 of 11 patients without LDD (5, 8 mm). The relative proportions (p = 0.183, Fisher exact test) and mean tonsillar descent (p = 0.587, t test for independent samples, 2-tailed assuming equal variance) were not significantly different between the 2 groups based on the presence or absence of LDD.
Discussion
Our study adds to the existing literature through a comprehensive review of intracranial imaging abnormalities in a relatively large cohort comprising patients with CS. In doing so, we have further detailed the presence of a multitude of neuroimaging abnormalities that are increasingly recognized as common, but often asymptomatic, in patients with PTEN mutations.
Lhermitte-Duclos disease
LDD is a major criterion for the diagnosis of CS.2 Immunohistochemical analysis of LDD indicates activation of the PTEN/AKT/mTOR pathway, suggesting a central role for mTOR in the pathogenesis of LDD.3 LDD is histologically characterized by thickening and abnormal myelination of the molecular layer in the cerebellum, attenuated or absent Purkinje cells, infiltration of the granular cell layer by abnormal dysplastic ganglion cells, and variable vacuolization of white matter.3,9 LDD, the prototypical brain lesion in CS, was identified in 50% of our patients. This is a higher proportion than previously reported. For example, LDD was previously described in 3 of 20 patients (15%) with CS,6 and in 17 of 180 (9.4%) patients with a proven germline PTEN mutation.10 This may represent an overestimation due to referral bias since our institution is a tertiary neurosurgical center. As has been previously described,10 our patients with LDD were female predominant (54.5% CS).
Intracranial vascular malformations
In light of the increasingly understood PTEN-mediated control of angiogenesis,11 it is logical that patients with CS and other PTEN hamartoma tumor syndromes are systemically predisposed to vascular malformations.12–15 In our retrospective study, DVAs were actually more frequently seen in patients with CS than LDD (59.1% vs 50%), and we also observed a high frequency of patients with multiple DVAs. In a case series of 20 patients with CS, Lok et al.6 described single DVAs in 3 patients and multiple DVAs in 2, while an additional case report documents a 55-year-old woman with CS, LDD, and multiple DVAs.16 DVAs were also identified in 8 of 9 pediatric patients with PTEN mutations who underwent brain MRI, likewise with a tendency toward multiplicity.14 To put these observations in context, the incidence of DVAs noted in patients with CS is higher than in the general population, as noted in several studies, such as in the study of Gokce et al.,17 who noted 75 patients had DVAs (6.4%) in a series of 1,165 consecutive cranial MRIs, and most had only 1 (65/75). This apparent overrepresentation lends further support to the specific inclusion of multiple intracranial DVAs as a minor criterion for the diagnosis of CS.2
We identified intracranial cavernous malformations in 3 patients with CS (13.6%). An additional 3 patients with CS had evidence of previous microhemorrhages, which were believed to be nonspecific but one or more of them could be related to tiny cavernous malformations. Previous cohorts comprising patients with CS and PTEN mutations have likewise documented cavernous malformations.6,10,14 This is not surprising since DVAs have a high rate of associated cavernous malformations, which was estimated in one systematic review as 2%–40%.18
Meningiomas
In 1984, Starink7 summarized 83 patients with CS and identified 3 patients with meningiomas. However, given that some of these cases predated modern cross-sectional imaging of the head, small asymptomatic meningiomas would not have been reported. As part of a case report and literature review in 1993, Lyons et al.19 reported 160 cases of CS (all but 13 were after the advent of CT), and they concluded that a patient with CS is approximately 1,000 times more likely to develop a symptomatic intracranial meningioma than an unaffected person. Additional case series have subsequently documented meningiomas in 1 of 8 patients with LDD5 and 1 of 20 patients with CS.6 In the most recent case report and systematic review based upon 109 individuals with CS and confirmed PTEN mutations, the prevalence of meningiomas (8.25%) was similar to LDD (9.17%).8 The 18.2% frequency of meningiomas in patients with CS in our study was less than that of LDD (50%), though still much higher than expected, particularly given that the mean age was only 46.1 years. For comparison, an imaging-based screening study of middle-aged and older adults from the general population identified meningiomas in 0.9%.20 In a large epidemiologic study with over 13,000 newly diagnosed meningiomas, the mean age at presentation was 57.7 years.21 Thus, we propose that CS should be considered in the differential diagnosis of a young patient (<50 years) with asymptomatic or incidentally noted meningiomas, especially when associated with other supporting clinical and neuroimaging abnormalities. Although inclusion of meningioma as a major criterion for the diagnosis of CS has been suggested,8 it would be more appropriate as a minor criterion since it lacks the specificity of LDD.
Cerebellar tonsillar herniation from LDD vs Chiari I malformation
Cerebellar tonsillar herniation has been described in association with numerous cases of LDD.9,22–27 Other cases of patients with PTEN mutations and Chiari I malformation have been reported in the absence of LDD.24 However, the presence of a space-occupying lesion in the posterior fossa is confounding, so it remains unknown as to whether the tonsillar descent is entirely acquired (secondary) or partially developmental. We found that 5 of 10 patients with CS with LDD and 2 of 11 patients with CS but no LDD had tonsillar ectopia. While the relative proportions and severity were not significantly different, this assessment is limited by our small sample size. With the understanding that tonsil position varies with age and sex, the imaging prevalence of Chiari I malformation (defined by tonsillar descent greater than 5 mm) in the general population has been estimated as 0.24%–3.6%,28 suggesting an overrepresentation in our cohort. Our series and previous publications suggest that the association between CS and tonsillar herniation may not be coincidental or purely secondary to mass effect from LDD. It is worth noting that tonsillar herniation has been seen in other tumor suppressor syndromes like neurofibromatosis29 and is also a feature of overgrowth syndromes due to mutations in the PI3-AKT pathway.30
Malformations of cortical development
In our study, a single patient with CS (4.5%) was found to have malformations of cortical development that included perisylvian polymicrogyria and contralateral inferior frontal cortical dysplasia. Previous reports of similar malformations exist.31 The PI3K/AKT/mTOR signaling pathway has been shown to be important in brain development and loss of function PTEN mutations result in dysregulated AKT activity, which is one proposed pathway that could lead to these malformations.30,32
White matter abnormalities and prominent perivascular spaces
Although the white matter can be abnormal in the setting of developmental malformations, multifocal white matter signal abnormalities have been reported more broadly in the setting of PTEN hamartoma tumor syndrome, including foci that follow CSF signal on MRI, thereby making them compatible with dilated perivascular spaces.33–35 In our series, we found white matter signal abnormality that was greater than expected for age in 31.8% and prominent perivascular spaces in 18.2% of our patients with CS. The mechanism behind enlarged perivascular spaces in CS is not clear, though they are seen in other genetic syndromes such as storage disorders (mucopolysaccharidosis).36 Not only should PTEN mutation be added to the differential diagnosis for an MRI with enlarged perivascular spaces, but static multifocal white matter abnormalities and dilated perivascular spaces in a patient with an established PTEN mutation or clinical signs of a PTEN hamartoma tumor syndrome should not prompt additional testing for a separate white matter disorder.35
Limitations
Our study is limited given its retrospective nature that includes a cohort derived from single institution. Population frequency data cannot be defined due to the nature of this study. In particular, the high percentage of LDD and meningiomas in our cohort of patients with CS compared to a previous review may reflect a referral bias to a tertiary neurosurgical center.
Conclusions and recommendations
It is important to recognize that additional seemingly unrelated intracranial findings such as multiple DVAs, meningiomas, greater than expected white matter signal abnormality, prominent perivascular spaces, and cortical malformations may warrant a thorough evaluation for CS and other forms of PTEN hamartoma tumor syndrome in the appropriate clinical setting. We further recommend that this broader spectrum of intracranial abnormalities be considered for addition to the CS diagnostic criteria at the time of next revision. The relationship between LDD and cerebellar tonsillar herniation needs further investigation to better delineate the contribution of mass effect in the posterior fossa from a potential predisposition related to PTEN mutation.
Author contributions
Dr. Dhamija: drafting the original manuscript, analysis and interpretation of the data, revising the manuscript for intellectual content. Dr. Hoxworth: drafting the original manuscript, analysis and interpretation of the data, revising the manuscript for intellectual content, study supervision. Dr. Weindling, Dr. Porter, Dr. Hu, Dr. Wood: analysis and interpretation of the data, revising the manuscript for intellectual content.
Study funding
No targeted funding reported.
Disclosure
R. Dhamija and S.M. Weindling report no disclosures. A.B. Porter serves on the editorial board of Clinical Neurology News. L.S. Hu is author on patents re: Quantifying multiscale competitive landscapes of clonal diversity in glioblastoma, Patient-specific models of glioblastoma (GBM) heterogeneity and extent using transfer learning, Radiogenomics to characterize regional genetic heterogeneity in glioblastoma, and Multi-parametric MRI and texture analysis to visualize spatial histologic heterogeneity and tumor extent in glioblastoma; and receives research support from NIH/NCI, Arizona Department of Health Services, and the Ben and Catherine Ivy Foundation. C.P. Wood and J.M. Hoxworth report no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Eng C. PTEN: one gene, many syndromes. Hum Mutat 2003;22:183–198. [DOI] [PubMed] [Google Scholar]
- 2.Pilarski R, Burt R, Kohlman W, Pho L, Shannon KM, Swisher E. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst 2013;105:1607–1616. [DOI] [PubMed] [Google Scholar]
- 3.Abel TW, Baker SJ, Fraser MM, et al. Lhermitte-Duclos disease: a report of 31 cases with immunohistochemical analysis of the PTEN/AKT/mTOR pathway. J Neuropathol Exp Neurol 2005;64:341–349. [DOI] [PubMed] [Google Scholar]
- 4.Kulkantrakorn K, Awwad EE, Levy B, et al. MRI in Lhermitte-Duclos disease. Neurology 1997;48:725–731. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer CC, Smirniotopoulos JG, Jones RV. The striated cerebellum: an MR imaging sign in Lhermitte-Duclos disease (dysplastic gangliocytoma). Radiology 1995;194:699–703. [DOI] [PubMed] [Google Scholar]
- 6.Lok C, Viseux V, Avril MF, et al. Brain magnetic resonance imaging in patients with Cowden syndrome. Medicine 2005;84:129–136. [DOI] [PubMed] [Google Scholar]
- 7.Starink TM. Cowden's disease: analysis of fourteen new cases. J Am Acad Dermatol 1984;11:1127–1141. [DOI] [PubMed] [Google Scholar]
- 8.Yakubov E, Ghoochani A, Buslei R, Buchfelder M, Eyupoglu IY, Savaskan N. Hidden association of Cowden syndrome, PTEN mutation and meningioma frequency. Oncoscience 2016;3:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei G, Zhang W, Li Q, et al. Magnetic resonance characteristics of adult-onset Lhermitte-Duclos disease: an indicator for active cancer surveillance? Mol Clin Oncol 2014;2:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieuwenhuis MH, Kets CM, Murphy-Ryan M, et al. Cancer risk and genotype-phenotype correlations in PTEN hamartoma tumor syndrome. Fam Cancer 2014;13:57–63. [DOI] [PubMed] [Google Scholar]
- 11.Hamada K, Sasaki T, Koni PA, et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev 2005;19:2054–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenny B, Radovanovic I, Haenggeli CA, et al. Association of multiple vertebral hemangiomas and severe paraparesis in a patient with a PTEN hamartoma tumor syndrome: case report. J Neurosurg 2007;107:307–313. [DOI] [PubMed] [Google Scholar]
- 13.Prats-Sanchez LA, Hervas-Garcia JV, Becerra JL, et al. Multiple intracranial arteriovenous fistulas in Cowden syndrome. J Stroke Cerebrovasc Dis 2016;25:e93–e94. [DOI] [PubMed] [Google Scholar]
- 14.Tan WH, Baris HN, Burrows PE, et al. The spectrum of vascular anomalies in patients with PTEN mutations: implications for diagnosis and management. J Med Genet 2007;44:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turnbull MM, Humeniuk V, Stein B, Suthers GK. Arteriovenous malformations in Cowden syndrome. J Med Genet 2005;42:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams DW III, Elster AD, Ginsberg LE, Stanton C. Recurrent Lhermitte-Duclos disease: report of two cases and association with Cowden's disease. AJNR Am J Neuroradiol 1992;13:287–290. [PMC free article] [PubMed] [Google Scholar]
- 17.Gokce E, Acu B, Beyhan M, Celikyay F, Celikyay R. Magnetic resonance imaging findings of developmental venous anomalies. Clin Neuroradiol 2014;24:135–143. [DOI] [PubMed] [Google Scholar]
- 18.Hon JM, Bhattacharya JJ, Counsell CE, et al. The presentation and clinical course of intracranial developmental venous anomalies in adults: a systematic review and prospective, population-based study. Stroke 2009;40:1980–1985. [DOI] [PubMed] [Google Scholar]
- 19.Lyons CJ, Wilson CB, Horton JC. Association between meningioma and Cowden's disease. Neurology 1993;43:1436–1437. [DOI] [PubMed] [Google Scholar]
- 20.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med 2007;357:1821–1828. [DOI] [PubMed] [Google Scholar]
- 21.Zouaoui S, Darlix A, Rigau V, et al. Descriptive epidemiology of 13,038 newly diagnosed and histologically confirmed meningiomas in France: 2006-2010. Neurochirurgie 2018;64:15–21. [DOI] [PubMed] [Google Scholar]
- 22.Elia M, Amato C, Bottitta M, et al. An atypical patient with Cowden syndrome and PTEN gene mutation presenting with cortical malformation and focal epilepsy. Brain Dev 2012;34:873–876. [DOI] [PubMed] [Google Scholar]
- 23.Marcus CD, Galeon M, Peruzzi P, et al. Lhermitte-Duclos disease associated with syringomyelia. Neuroradiology 1996;38:529–531. [DOI] [PubMed] [Google Scholar]
- 24.Saletti V, Esposito S, Maccaro A, Giglio S, Valentini LG, Chiapparini L. Chiari I malformation in a child with PTEN hamartoma tumor syndrome: association or coincidence? Eur J Med Genet 2017;60:261–264. [DOI] [PubMed] [Google Scholar]
- 25.Spaargaren L, Cras P, Bomhof MA, et al. Contrast enhancement in Lhermitte-Duclos disease of the cerebellum: correlation of imaging with neuropathology in two cases. Neuroradiology 2003;45:381–385. [DOI] [PubMed] [Google Scholar]
- 26.Thomas B, Krishnamoorthy T, Radhakrishnan VV, Kesavadas C. Advanced MR imaging in Lhermitte-Duclos disease: moving closer to pathology and pathophysiology. Neuroradiology 2007;49:733–738. [DOI] [PubMed] [Google Scholar]
- 27.Wolansky LJ, Malantic GP, Heary R, et al. Preoperative MRI diagnosis of Lhermitte-Duclos disease: case report with associated enlarged vessel and syrinx. Surg Neurol 1996;45:470–475; discussion 475–476. [DOI] [PubMed] [Google Scholar]
- 28.Kahn EN, Muraszko KM, Maher CO. Prevalence of Chiari I malformation and syringomyelia. Neurosurg Clin N Am 2015;26:501–507. [DOI] [PubMed] [Google Scholar]
- 29.Miraglia E, Fabbrini G, Di Biasi C, et al. Chiari type 1 malformation in neurofibromatosis type 1: experience of a center and review of the literature. Clin Ter 2016;167:e6–10. [DOI] [PubMed] [Google Scholar]
- 30.Mirzaa GM, Riviere JB, Dobyns WB. Megalencephaly syndromes and activating mutations in the PI3K-AKT pathway: MPPH and MCAP. Am J Med Genet C Semin Med Genet 2013;163C:122–130. [DOI] [PubMed] [Google Scholar]
- 31.Klisch J, Juengling F, Spreer J, et al. Lhermitte-Duclos disease: assessment with MR imaging, positron emission tomography, single-photon emission CT, and MR spectroscopy. AJNR Am J Neuroradiol 2001;22:824–830. [PMC free article] [PubMed] [Google Scholar]
- 32.Jansen LA, Mirzaa GM, Ishak GE, et al. PI3K/AKT pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain 2015;138:1613–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busa T, Milh M, Degardin N, et al. Clinical presentation of PTEN mutations in childhood in the absence of family history of Cowden syndrome. Eur J Paediatr Neurol 2015;19:188–192. [DOI] [PubMed] [Google Scholar]
- 34.Merks JH, de Vries LS, Zhou XP, et al. PTEN hamartoma tumour syndrome: variability of an entity. J Med Genet 2003;40:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderver A, Tonduti D, Kahn I, et al. Characteristic brain magnetic resonance imaging pattern in patients with macrocephaly and PTEN mutations. Am J Med Genet A 2014;164A:627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reichert R, Campos LG, Vairo F, et al. Neuroimaging findings in patients with mucopolysaccharidosis: what you really need to know. Radiographics 2016;36:1448–1462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.




