Abstract
Background
We sought to identify clinical associations and potential triggers of Guillain-Barré syndrome (GBS) within 6 weeks of surgery.
Methods
We retrospectively reviewed consecutive patients diagnosed with GBS within 6 weeks of a surgery between January 1995 and June 2014 at Mayo Clinic. Postsurgical GBS was defined as symptom onset within 6 weeks of surgery. Patients with postsurgical GBS were compared with patients who did not have a surgery prior to GBS onset to determine differences between groups.
Results
A total of 208 patients with GBS, median age 55 years (interquartile range [IQR] 41–68), were included. Nineteen patients (9.1%) developed postsurgical GBS. Median duration from the surgery to onset of first GBS symptom was 15 days (IQR 9–37). The main types of surgeries preceding GBS were gastrointestinal, orthopedic, and cardiac. General anesthesia was used in 18 (95%) and conscious sedation in 1 (5%) patient. Among the 19 patients with postsurgical GBS, 11 (57.9%) had a known diagnosis of malignancy. Autoimmune conditions were present in 5 (26.3%) patients. Postoperative infection was found in 4 (21%) patients. On univariate analysis, the factors that showed an association with postsurgical GBS were age (p = 0.02), malignancy (p ≤ 0.0004), active malignancy (p = 0.03), preexisting autoimmune disorder (p = 0.02), and infection (p = 0.0001). On multivariate analysis, only active malignancy (0.03) remained associated.
Conclusions
Surgery antedated GBS in 9.1% of patients. Postsurgical GBS was more common in patients with an active malignancy. A prospective study is needed to determine whether active malignancy represents an independent risk factor for the development of postsurgical GBS.
Patients with Guillain-Barré syndrome (GBS) may recall a flu-like syndrome weeks before the onset of symptoms. Other antecedent events have been reported including trauma, vaccination, blood transfusion, administration of chemotherapy, or surgery. Two publications from Mayo Clinic and Massachusetts General Hospital first reported surgical procedures as a trigger for GBS.1,2 Since these observations were published, 2 retrospective series noted an incidence of GBS after surgery of 5% and 9.5%, and multiple subsequent case reports have been published, most commonly in patients undergoing cardiovascular, gastrointestinal, or neurosurgical procedures.1–8 The first detailed study on postoperative polyneuritis by Arnason and Asbury1 reported that postsurgical GBS occurred as a result of the release of nerve antigen during the course of surgery. Further, they hypothesized an association with other host factors that caused a vigorous immunologic response, culminating in the development of polyneuritis. Others have postulated that surgical stress induced the activation of the neuroendocrine stress axis and cell-mediated immunosuppression, which in turn might promote infections resulting in the production of cross-reactive antibodies.9,10 We aimed to understand whether patients who presented with GBS within 6 weeks of undergoing a surgery are potentially different from other patients developing GBS.
Methods
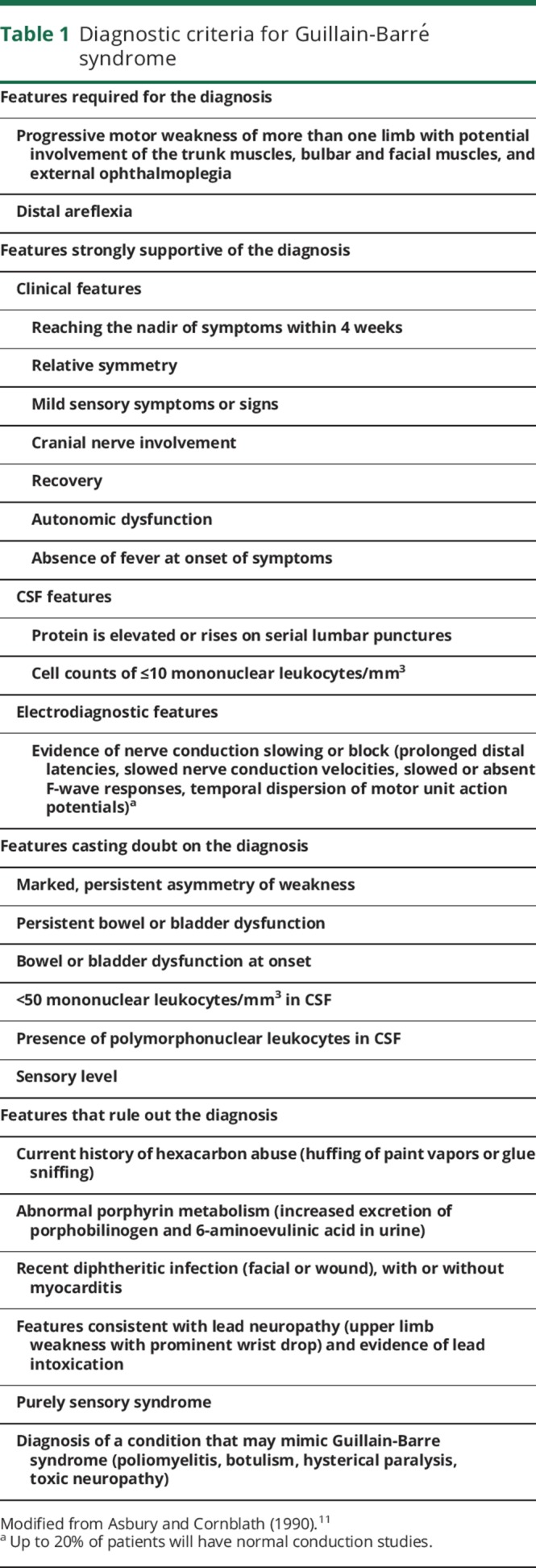
We retrospectively reviewed medical records of patients diagnosed with GBS between January 1995 and June 2014 at Mayo Clinic. We used the search terms acute demyelinating polyneuropathy, Guillain-Barré syndrome, and Miller Fisher syndrome in the reason for admission, discharge diagnosis, body of the clinical documents, and problem list to identify patients. All charts were reviewed and only patients aged 18–90 years with GBS who met diagnostic criteria proposed in 1990 (table 1) were included.11 We excluded patients who were diagnosed with GBS outside Mayo Clinic or were experiencing a recurrent episode of GBS.
Table 1.
Diagnostic criteria for Guillain-Barré syndrome
Variables collected included basic demographics, history of autoimmune disorders and malignancy, established GBS triggers, surgery type, type of anesthesia, number of days from the surgery to onset of GBS, details of treatment for the GBS episode, duration of hospital stay, and recurrence of GBS during follow-up. Active malignancy was defined as a diagnosis of cancer within 6 months of enrollment, any treatment for cancer within the previous 6 months, or documented recurrent or metastatic cancer.12 We defined postoperative infection based upon the Liu et al.13 study. This includes superficial wound infection, deep wound infection, organ space site infection, surgical wound distribution, pneumonia, urinary tract infection, sepsis and septic shock, and any other systemic infectious complications as the occurrence of any infection within 30 days from the surgery. The study group was defined as those patients who developed postsurgical GBS, defined as GBS symptom onset within 6 weeks of surgery.3 Patients with postsurgical GBS were compared with those with GBS and no prior surgery to determine the differences between groups.
Standard protocol approvals, registrations, and patient consents
This study was reviewed by and received approval from our institutional review board. Patients who had not given consent to use their medical records for research were excluded.
Statistical analysis
Descriptive summaries were reported using frequencies and percentages for categorical variables and as median and interquartile range (IQR) for continuous variables. Statistical significance was set at the conventional 2-tailed α level of 0.05. Associations between the categorical variables was assessed using the χ2 test or Fisher exact test as appropriate and Kruskal-Wallis test was used to compare the continuous variables as they were not normally distributed. Further analysis was performed using multivariate logistic regression. Variables with p values of <0.05 were considered as candidate variables for multivariate regression. Since only 3 variables could be analyzed simultaneously in the model (due to small size of the population and to avoid overfitting), multiple models with all possible combinations of important variables were analyzed, before arriving at the final set of included variables. Odds ratio and 95% confidence interval were used to quantify the strength of associations. All statistical analysis was performed using JMP 10.0.0 (SAS Institute Inc., Cary, NC).
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
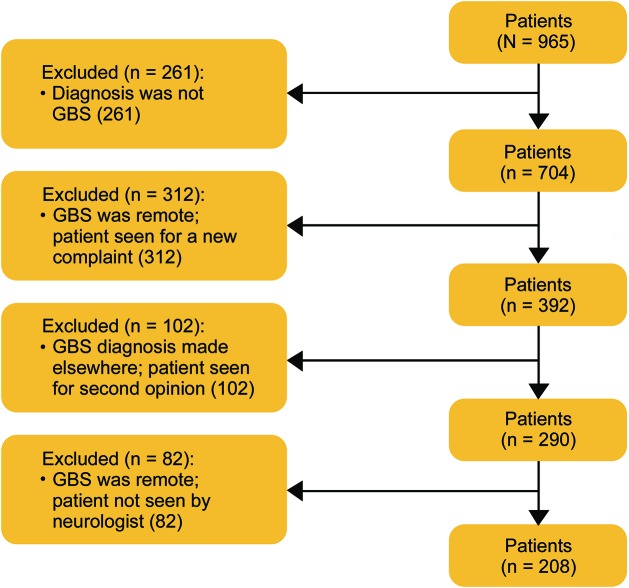
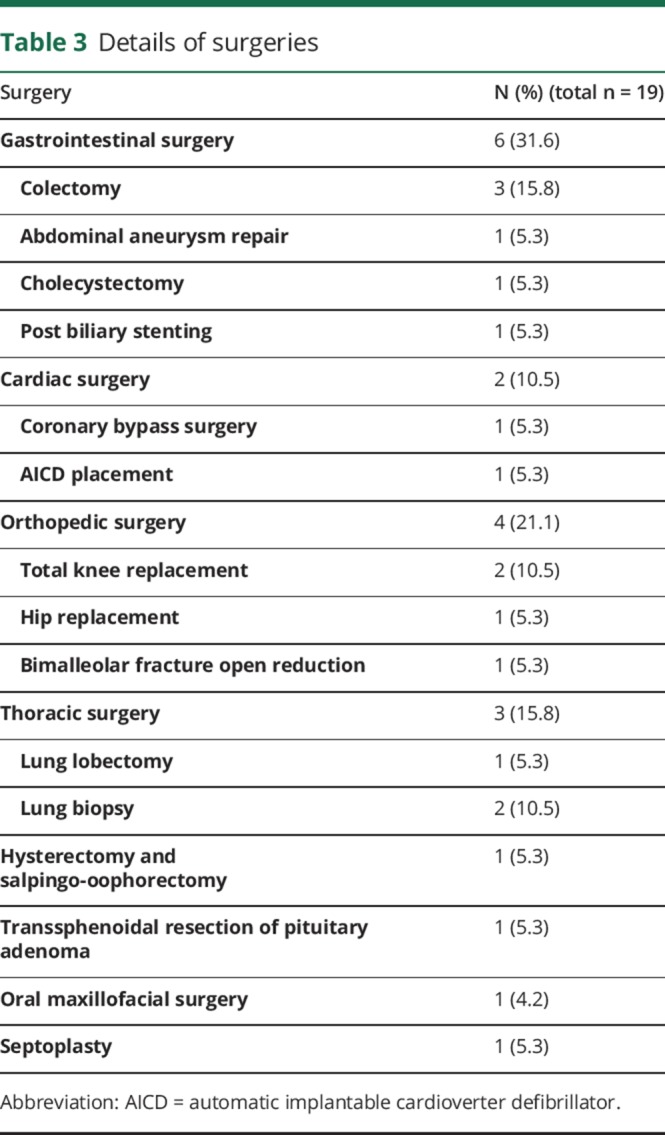
We identified 965 patients using our search terms, of whom 208 met inclusion criteria (figure). Median age was 55 years (IQR 41–68), and 120 (58%) were male. The demographic details are presented in table 2. Nineteen (9.1%) patients developed postsurgical GBS. The median age of patients with postsurgical GBS was 63 years (IQR 59–70) and 13 (68.4%) were male. Details of the surgeries are included in table 3. The most commonly used type of anesthesia was general in 18 (95%) patients, followed by conscious sedation in 1 (5%). No patient developed GBS after a procedure in which he or she was administered only spinal or local anesthesia. The median duration from the surgery to the onset of GBS symptoms was 15 days (IQR 9–37).
Figure. Patient flow diagram.
GBS = Guillain-Barré syndrome.
Table 2.
Demographic and clinical features of adults with Guillain-Barré syndrome
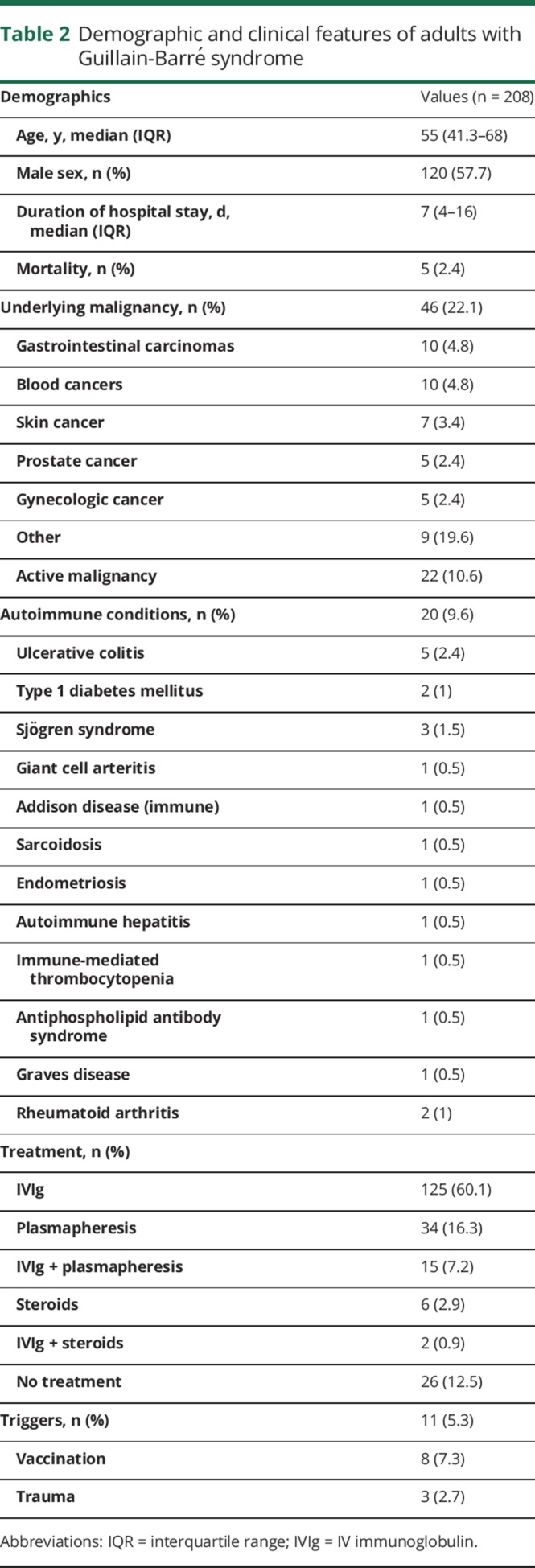
Table 3.
Details of surgeries
Eleven (57.9%) patients had an associated malignancy and 8 (42.1%) patients had active malignancies. Gastrointestinal (4 [21%]) malignancies were the most common type, followed by hematologic (2 [10.5%]) and gynecologic cancer (2 [10.5%]), followed by prostate (1 [5.2%]) and lung cancer (1 [5.2%]). Comorbid autoimmune conditions were present in 5 (26.3%) patients with postsurgical GBS. We found out that 4 (21%) of 19 patients had a postoperative infection. Infections included urinary tract infection in 2 (10.5%) patients, upper respiratory tract infection in 1 (5.2%) patient, and systemic infection in 1 (5.2%) patient. The median number of days to onset of infection was 9 (IQR 2–11). Fourteen (73.6%) patients were treated with IV immunoglobulin, 5 (26.4%) with plasmapheresis. The median duration of hospital stay was 14 days (IQR 5–23). Three (15.7%) patients died during the hospital stay. The cause of death is unknown in 1 patient and related to underlying malignancy in 2 patients. The median duration of follow-up after the diagnosis was 28.4 months (IQR 2.1–116.3). Eight patients underwent surgery after the diagnosis of GBS and the median number of surgeries was 0 (IQR 0–2). There were no recurrences of GBS in these patients.
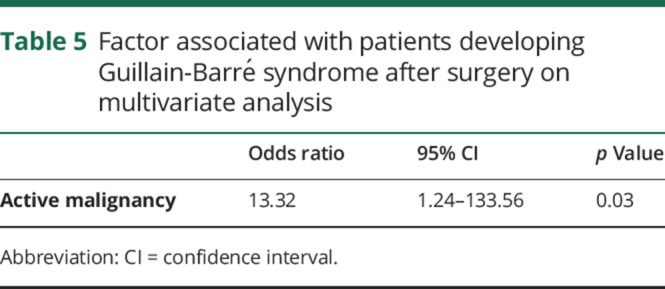
On univariate analysis, the factors associated with GBS after a surgery were age (p = 0.02), malignancy (p < 0.0004), active malignancy (p = 0.03), preexisting autoimmune disorder (p = 0.02), and infection (p = 0.0001) (table 4). On multivariate logistic regression analysis, only active malignancy (p = 0.03) remained significant (table 5).
Table 4.
Factors associated with patients developing Guillain-Barré syndrome (GBS) after surgery on univariate analysis
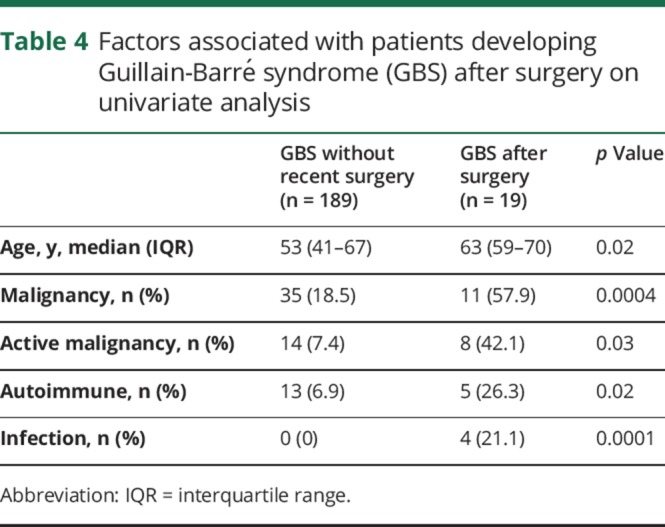
Table 5.
Factor associated with patients developing Guillain-Barré syndrome after surgery on multivariate analysis
Discussion
In this large consecutive series of patients with GBS, we found that malignancy and comorbid autoimmune disease were associated with the development of postsurgical GBS.
A positive history of autoimmune conditions was present in nearly 10% of the patients with nonsurgical GBS compared with nearly 30% of those with postsurgical GBS in our series. Existing literature with regard to adult populations reported autoimmune conditions to be more prevalent in patients with GBS, accounting for 10%–14%.14,15 This finding was consistent with the results of the present study and higher than the incidence of autoimmune disorders in the population at large. Multiple previously published case reports have documented the association between GBS and malignancy.16–22 Vigliani et al.19 carried out a population-based study in patients with GBS and concluded that cancer patients were more prone to develop GBS, with a reported prevalence of 2.4 times higher when compared to the general population. Interestingly, comorbid malignancy was an independent risk factor for the development of postsurgical GBS in the present study, when compared to the control group. Still, there is no consensus about the association between postsurgical GBS and malignancy or preexisting autoimmune disease. The cause of postsurgical GBS is not understood and explanations can only be speculative. In the Massachusetts General Hospital series of 169 patients with GBS in the 1960s and 1970s, 10 cases were found with “common wound infections” and half with blood transfusions, of which one had a subsequent cytomegalovirus infection.5 This may suggest a bacterial trigger or a transfusion-transmitted viral infection may play a role. Another (unproven) possibility is immunosuppression triggering, secondary to surgery, underlying malignancy, or subclinical infections.
Other factors known to be associated with GBS include transplantation, Hodgkin disease, leukemia, infection, and vaccination.16–23 Our study population was comparable to previously published series with respect to the distribution of these triggering factors.
During the study period, more than 50,000 surgeries were performed under general anesthesia and conscious sedation at the institution where the present study was undertaken. The occurrence of GBS after a surgery was therefore extremely rare and this was demonstrated earlier in large population studies.3,4,23 A further limitation of this analysis is the potential for referral bias because of the large number of patients referred to our institution for the diagnosis and management of their malignancy, including surgery.
A surgery preceded the onset of GBS symptoms in 9.1% of patients diagnosed with GBS over 18.5 years, which is slightly higher than previous published series.3–5,23 The findings of the present study were higher than what was previously reported with smaller study populations.3–5,23 One study reported an incidence of postsurgical GBS of 9.5%. Within a relatively small cohort of 63 patients, 6 patients developed GBS within 6 weeks of a surgery. The affected group included patients with exposure to general anesthesia during the surgical procedure only.4 We included a large series of 208 patients who underwent both general and conscious sedation. Traditionally, most surgeries were done under general anesthesia. However, over the last 2 decades, due to recent advances in surgical techniques, good anesthetic medications, and higher prevalence of side effects associated with general anesthesia and endotracheal intubation, some surgeries such as endotracheal biopsy and laparoscopic surgeries were performed under conscious sedation.24–27 We presumed that the risk of nerve damage associated with surgery and transient immunosuppression remained the same, regardless of the duration of the procedure and type of anesthetic medications used.
In another study, inclusive of pediatric and adult patients, 1,034 patients developed GBS during the study period, among whom 52 (5%) experienced symptom onset within 8 weeks of a surgical procedure.3 In that study, as in ours, patients with other potential triggering factors for GBS occurring within 8 weeks of symptom onset were not excluded.
Mortality rate was 16% among patients with postsurgical GBS. Deaths were predominantly noticed during the acute and early plateau phase. Mortality has been previously shown to be higher in cancer patients who developed GBS when compared to the general population.19 In an earlier study involving a large series of patients with GBS, the mortality rate was 2.8%–3.9% at 1-year follow-up. It was also observed that death occurred predominantly during the recovery phase.28 We therefore attributed the higher mortality in our postsurgical GBS patients to the preexisting comorbid conditions and complications of the surgery in these patients, since the mortality rate for the entire GBS series, including patients without an antecedent surgery, was 2.4%.
The duration of hospital stay in patients with GBS after surgery was found to be longer when compared to that in patients who did not undergo surgery. This might be due to the time required for postoperative recovery. However, future studies should examine if the course of GBS would be more severe and prolonged in patients with antecedent surgery than without.
Surgery antedated GBS in 9.1% of patients. We were not able to document the incidence and attributable risk in the study population without an exact total number of surgical procedures performed during the study period. Nonetheless, we found that patients developing GBS after surgery represent a rare, distinct cohort with more frequent history of malignancy and autoimmunity. Prospective multicenter confirmation is necessary to ensure the validity of this finding and eliminate the possibility that it can be explained by referral bias alone.
Acknowledgment
The authors thank Dr. Tarun Singh for assistance with creating the tables and statistical review.
Author contributions
S. Hocker was responsible for study design, study concept, data collection, data analysis, drafting the manuscript, and manuscript revision. E. Nagarajan was responsible for study design, study concept, data collection, data analysis, and drafting the manuscript. M. Rubin was responsible for data collection and revision of the manuscript. E.F.M. Wijdicks was responsible for study concept and manuscript revision.
Study funding
No targeted funding reported.
Disclosure
E. Nagarajan and M. Rubin report no disclosures. E.F.M. Wijdicks receives publishing royalties from books published by Oxford University Press. S. Hocker serves on a scientific advisory board for SAGE Therapeutics; has received speaker honoraria from the AAN and honoraria from Continuum; and serves on the editorial board of Journal of Stroke and Cerebrovascular Diseases and as Review Editor for Frontiers in Stroke and Frontiers in Neurocritical and Neurohospitalist Care. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Arnason BG, Asbury AK. Idiopathic polyneuritis after surgery. Arch Neurol 1968;18:500–507. [DOI] [PubMed] [Google Scholar]
- 2.Wiederholt WC, Mulder DW, Lambert EH. The Landry-Guillain-Barré Strohl syndrome or polyradiculoneuropathy: historical review, report on 97 patients, and present concepts. Mayo Clin Proc 1964;30:427. [PubMed] [Google Scholar]
- 3.Hurwitz ES, Holman RC, Nelson DB, Schonberger LB. National surveillance for Guillain-Barré syndrome: January 1978–March 1979. Neurology 1983;33:150–157. [DOI] [PubMed] [Google Scholar]
- 4.Gensicke H, Datta AN, Dill P, Schindler C, Fischer D. Increased incidence of Guillain-Barré syndrome after surgery. Eur J Neurol 2012;19:1239–1244. [DOI] [PubMed] [Google Scholar]
- 5.Ropper AH, Wijdicks EFM, Truax BT. Antecedent and associated illness, Guillain-Barré syndrome. In: Contemporary Neurology Series, vol. 34 Philadelphia: FA Davis; 1991:71. [Google Scholar]
- 6.Algahtani H, Moulin DE, Bolton CF, Abulaban AA. Guillain-Barre syndrome following cardiac surgery: difficult diagnosis in the intensive care unit. Neurosciences 2009;14:374–378. [PubMed] [Google Scholar]
- 7.Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg 2002;12:592–597. [DOI] [PubMed] [Google Scholar]
- 8.Papantoni E, Sakorafas GH, Zouros E, Peros G. Guillain-Barré syndrome following total gastrectomy/esophagectomy: a very rare and dramatic post-operative complication with a favourable outcome. ANZ J Surg 2010;80:858. [DOI] [PubMed] [Google Scholar]
- 9.Hogan BV, Peter MB, Shenoy HG, Horgan K, Hughes TA. Surgery induced immunosuppression. Surgeon 2011;9:38–43. [DOI] [PubMed] [Google Scholar]
- 10.Vucic S, Kiernan MC, Cornblath DR. Guillain-Barré syndrome: an update. J Clin Neurosci 2009;16:733–741. [DOI] [PubMed] [Google Scholar]
- 11.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol 1990;27(suppl):S21–S24. [DOI] [PubMed] [Google Scholar]
- 12.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumadin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–153. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Ma C, Elkassabany N, Fleisher LA, Neuman MD. Neuraxial anesthesia decreases postoperative systemic infection risk compared with general anesthesia in knee arthroplasty. Anesth Analg 2013;117:1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korn-Lubetzki I, Abramsky O. Acute and chronic demyelinating inflammatory polyradiculoneuropathy: association with autoimmune diseases and lymphocyte response to human neuritogenic protein. Arch Neurol 1986;43:604–608. [DOI] [PubMed] [Google Scholar]
- 15.Abramsky O, Korn-Lubetzky I, Teitelbaum D. Association with autoimmune diseases and cellular immune response to the neuritogenic protein in Guillain-Barré syndrome. Trans Am Neurol Assoc 1980;105:350–354. [PubMed] [Google Scholar]
- 16.Ozkan A, Taskapilioglu O, Bican A, et al. Hairy cell leukemia presenting with Guillain-Barre syndrome. Leuk Lymphoma 2007;48:1048–1049. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi AI, Cook AA, Mishu HP, Krendel DA. Guillain-Barré syndrome in immunocompromised patients: a report of three patients and review of the literature. Muscle Nerve 1997;20:1002–1007. [DOI] [PubMed] [Google Scholar]
- 18.Re D, Schwenk A, Hegener P, Bamborschke S, Diehl V, Tesch H. Guillain-Barré syndrome in a patient with non-Hodgkin's lymphoma. Ann Oncol 2000;11:217–220. [DOI] [PubMed] [Google Scholar]
- 19.Vigliani MC, Magistrello M, Polo P, Mutani R, Chiò A; Piemonte and Valled'Aosta Register for Guillain-Barré Syndrome. Risk of cancer in patients with Guillain-Barré syndrome (GBS): a population-based study. J Neurol 2004;251:321–326. [DOI] [PubMed] [Google Scholar]
- 20.Brannagan TH III, Zhou Y. HIV-associated Guillain-Barré syndrome. J Neurol Sci 2003;208:39–42. [DOI] [PubMed] [Google Scholar]
- 21.El-Sabrout RA, Radovancevic B, Ankoma-Sey V, Van Buren CT. Guillain-Barré syndrome after solid organ transplantation. Transplantation 2001;71:1311–1316. [DOI] [PubMed] [Google Scholar]
- 22.Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet 2005;366:1653–1666. [DOI] [PubMed] [Google Scholar]
- 23.Sipilä JO, Soilu-Hänninen M. The incidence and triggers of adult-onset Guillain-Barré syndrome in southwestern Finland 2004-2013. Eur J Neurol 2015;22:292–298. [DOI] [PubMed] [Google Scholar]
- 24.Lobo R, Kiernan T. The use of conscious sedation in elective external direct current cardioversion: a single centre experience. BMJ Qual Improv Rep 2015;4:eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosati M, Bramante S, Conti F, Rizzi M, Frattari A, Spina T. Laparoscopic salpingo-oophorectomy in conscious sedation. JSLS 2015;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grau-Talens EJ, Cattáneo JH, Giraldo R, Mangione-Castro PG, Giner M. Transcylindrical cholecystectomy under local anesthesia plus sedation: a pilot study. Endoscopy 2010;42:395–399. [DOI] [PubMed] [Google Scholar]
- 27.Sivalingam S, Tamm-Daniels I, Nakada SY. Office-based ureteral stent placement under local anesthesia for obstructing stones is safe and efficacious. Urology 2013;81:498–502. [DOI] [PubMed] [Google Scholar]
- 28.Van den Berg B, Bunschoten C, van Doorn PA, Jacobs BC. Mortality in Guillain-Barre syndrome. Neurology 2013;80:1650–1654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.