Abstract
Purpose
Vertebral compression fracture (VCF) is increasingly recognized as an adverse event after spine stereotactic body radiotherapy (SBRT). We report a multi-institutional study aimed at clarifying the risk and predictive factors associated with VCF.
Patients and Methods
A total of 252 patients with 410 spinal segments treated with SBRT were included. The primary outcome was the development of VCF (a new VCF or progression of a baseline VCF). In addition to various patient-, treatment-, and tumor-specific factors, the Spinal Instability Neoplastic Scoring (SINS) system was applied to determine predictive value.
Results
The median follow-up was 11.5 months (range, 0.03 to 113 months). The median and mean overall survival rates were 16 and 26 months, respectively. We observed 57 fractures (57 of 410, 14%), with 47% (27 of 57) new fractures and 53% (30 of 57) fracture progression. The median time to VCF was 2.46 months (range, 0.03 to 43.01 months), and 65% occurred within the first 4 months. The 1- and 2-year cumulative incidences of fracture were 12.35% and 13.49%, respectively. Multivariable analysis identified dose per fraction (greatest risk for ≥ 24 Gy v 20 to 23 Gy v ≤ 19 Gy), in addition to three of the six original SINS criteria: baseline VCF, lytic tumor, and spinal deformity, as significant predictors of VCF.
Conclusion
Caution must be observed when treating with ≥ 20 Gy/fraction, in particular, for patients with lytic tumor, spinal misalignment, and a baseline VCF. Frequent short-term follow-up is required, as nearly two thirds of all VCF occurred within the first 4 months. We also conclude that SINS may have utility in predicting patients at high risk of SBRT-induced VCF.
INTRODUCTION
Spine stereotactic body radiotherapy (SBRT), also known as spine stereotactic radiosurgery, is an emerging treatment for patients with spinal metastases and is rapidly being adopted in the clinic without high-quality evidence and a firm understanding of the adverse events.1,2 The major complications are radiation myelopathy3,4 and vertebral compression fracture (VCF),5–7 and the latter is increasingly being recognized as a significant and common adverse event.8
The first major report on SBRT-induced VCF was by the Memorial Sloan-Kettering Cancer Center (MSKCC), who reported VCF in 27 (39%) of 71 sites treated with SBRT.5 This risk of VCF was alarming, and the median time to VCF was 25 months. Subsequently, it has been suggested by the MD Anderson Cancer Center (MDACC) and University of Toronto (UofT) that the risk may be closer to 11% to 20%, with a median time to VCF of 2 to 3 months.6,7
With respect to risk factors for VCF, there has also been variability among the reported series. The only consistent predictor on multivariable proportional hazards analysis, among the three major investigations,5–8 was that lytic tumors were at greater risk of SBRT-induced VCF, and the hazard ratios (HR) ranged from 3.9 to 12.2. Other significant factors identified have included vertebral body tumor involvement by at least 41% to 60%,5 age more than 55 years,7 pre-SBRT VCF,7 spinal deformity,6 histology,6 and a SBRT dose per fraction of ≥ 20 Gy.6 In particular, the dose per fraction finding led investigators to postulate that beyond anatomic and tumor-related risk factors, the radiation itself contributed to the mechanism of action.9
To gain robust data regarding the risk of VCF, time to developing VCF after SBRT, and to identify predictive factors, the UofT,6 MDACC,7 and Cleveland Clinic10 pooled their clinical data specific to SBRT-induced VCF for this first multi-institutional report. We also specifically investigated whether the components of the recently developed and reported reliable Spinal Instability Neoplastic Scoring (SINS) system11,12 can predict for this adverse event.
PATIENTS AND METHODS
The MDACC,7 Cleveland Clinic,10 and UofT6 pooled their reported clinical data of patients treated with spine SBRT for this specific VCF analysis. Institutional research board approval was obtained from each institution. The primary end point was the development of fracture (fracture progression), which could either be a new fracture (de novo) or progression of an existing fracture. This end point is consistent with prior reports.5–7 Exclusion criteria included patients who experienced local progression before or at the time of developing a VCF (by imaging or pathologically confirmed), any surgical or radiotherapy salvage therapy to the treated vertebral segment after spine SBRT and before development of VCF, and those who had insufficient information to provide a baseline SINS score. We specifically excluded patients with local progression to avoid any potential confounding effects from tumor growth on the vertebrae, as one of the major aims was to determine the risk of VCF and the dose prescribed. This methodology is consistent with prior reports.5,6
The final cohort consisted of 68 patients with 89 spinal segments treated at MDACC, 85 patients with 132 spinal segments treated at the Cleveland Clinic, and 99 patients with 189 spinal segments treated at UofT. Therefore, the total cohort for analysis consisted of 252 patients with 410 spinal segments treated. Fifty percent (205 spinal segments) were single targets treated, and the remaining had multiple spinal segments treated within a single target volume.
Each treated vertebral segment was scored according to the SINS criteria as described by Fisher et al.11 In brief, the individual SINS criteria consist of location (junctional, mobile, semi-rigid, and rigid spine), type of pain (mechanical, nonmechanical) versus pain-free, spinal misalignment (kyphosis/scoliosis, translation/subluxation) versus normal alignment, presence of baseline VCF (≥ 50% collapse, < 50% collapse) versus no collapse but ≥ 50% of the body involved by tumor versus neither, type of lesion (lytic, mixed, sclerotic), and whether tumor involves the posterolateral elements (bilateral, unilateral) or not. The SINS scoring system classifies patients as stable, potentially unstable (indeterminate), and unstable based on the overall score. Additional factors analyzed included histologic type, paraspinal/epidural disease extension, whether any targeted therapy or bisphosphonates had been given within 2 months before SBRT, prior radiation to the treated segment, total dose prescribed, number of fractions, and whether the treated segment included other adjacent segments in the target volume (single versus multiple). With respect to the dosimetric analysis, we did not equate the doses prescribed using the biologically equivalent dose model because the validity of the model given high-dose (> 15 Gy/fraction) per fraction radiation and inhomogeneous dose distributions has been questioned,13,14 and at this time there is no consensus regarding how to accurately model SBRT dose fractionations. All patients had clinical and radiographic follow-up at regular 2- to 4-month intervals; dates of fracture (based on the last imaging follow-up) and death were recorded. The spine SBRT technique at each institution has been previously reported.6,7,10,15
Statistics
Descriptive statistics were used to assess patient demographics, disease characteristics, and related covariates of interest. Categorical variables were expressed as count and proportions, whereas continuous variables, such as age and follow-up, were expressed as mean ± standard deviation or median and range. The primary outcome variable of interest was the time to fracture progression, and each treated vertebral segment was considered independent. In cases in which multiple vertebrae were treated in a single target volume, each vertebral segment was still considered independent and analyzed as such. At the same time, we considered the details of this factor (treated vertebral segment as a single target volume or within a multiple target volume) for analysis as a potential predictor, and assessed the impact to our outcome measure, time to fracture progression, in both univariate and multivariate models.
The time to event data was calculated in months from the start date of SBRT up to the event date for the treated vertebral segment, or last follow-up imaging study if fracture-free. Death of a patient before fracture was considered as competing risk to fracture. Cumulative incidence of vertebral compression fracture (CIF) rates were obtained using competing risk analysis using a method suggested by Pepe and Mori.16 Overall survival was calculated using the Kaplan-Meier product-limit method. The Fine and Gray method for competing risk was used for CIF to determine the impact of each variable of interest as a univariate analysis. We also incorporated those in to the multivariable Fine and Gray model to determine the joint effect of these factors that were found potential at the univariate level. All P values were two-sided. Results were considered significant if P < .05. Statistical analyses were performed using version 9.2 of the SAS system (SAS Institute, Cary, NC) and R version 2.15.2 (the R foundation for statistical computing).
RESULTS
We observed 57 fractures (57 of 410, 13.9%), with 47% (27 of 57) de novo and 53% (30 of 57) progression of an existing fracture. The median follow-up for the entire cohort was 11.53 months (range, 0.03 to 113.02 months). In total, 183 of 252 patients (72.6%) had died. The median and mean survival rates were 16 and 26 months, respectively. The 1- and 2-year overall survival rates were 59% and 41%, respectively. Baseline characteristics of the spinal segments treated are summarized in Table 1 and based on each of the six SINS criteria in Table 2, according to those who experienced fracture and those who did not.
Table 1.
Baseline Patient, Tumor, and Dosimetric Factors
| Factor | Fracture (n = 57) Vertebral Segments | No Fracture (n = 353) Vertebral Segments | Fractures |
||
|---|---|---|---|---|---|
| No. | Total No. | % | |||
| Histology | |||||
| Breast | 3 | 50 | 3 | 53 | 5.66 |
| GI | 3 | 9 | 3 | 12 | 25 |
| Gynecologic | 1 | 3 | 1 | 4 | 25 |
| Kidney | 36 | 191 | 36 | 227 | 15.86 |
| Lung | 6 | 38 | 6 | 44 | 13.64 |
| Melanoma | 2 | 9 | 2 | 11 | 18.18 |
| Myeloma | 2 | 3 | 2 | 5 | 40 |
| Prostate | 0 | 15 | 0 | 15 | 0 |
| Sarcoma | 2 | 5 | 2 | 7 | 28.57 |
| Thyroid | 0 | 17 | 0 | 17 | 0 |
| Other | 2 | 13 | 2 | 15 | 13.33 |
| Spine level | |||||
| Cervical | 5 | 42 | 5 | 47 | 10.47 |
| Thoracic | 26 | 173 | 26 | 199 | 13.07 |
| Lumbar | 26 | 112 | 26 | 138 | 18.84 |
| Sacrum | 0 | 26 | 0 | 26 | 0 |
| Paraspinal/epidural disease | |||||
| Present | 38 | 159 | 38 | 197 | 19.29 |
| Absent | 19 | 194 | 19 | 213 | 8.92 |
| Receiving targeted systemic therapy | 31 | 193 | 31 | 224 | 13.84 |
| Receiving bisphosphonate therapy | 14 | 122 | 14 | 136 | 10.29 |
| Prior radiation | |||||
| Present | 7 | 87 | 7 | 94 | 7.45 |
| Absent | 50 | 266 | 50 | 316 | 15.82 |
| Age, years | |||||
| Mean | 57.48 | 57.56 | 57.55 | ||
| Range | 28-87.67 | 18-90 | 18-90 | ||
| Single segment target | 43 | 161 | 43 | 204 | 20.08 |
| Single versus multiple segments within single target volume | 14 | 191 | 14 | 205 | 6.83 |
| Total dose/fraction | |||||
| 8-17 Gy/1 fraction | 16 | 121 | 16 | 137 | 11.68 |
| 18-26 Gy/1 fraction | 20 | 52 | 20 | 72 | 27.78 |
| 18-26 Gy/2 fractions | 6 | 41 | 6 | 47 | 12.77 |
| 18-35 Gy/3 fractions | 11 | 92 | 11 | 103 | 10.68 |
| 25-35 Gy/4 fractions | 2 | 4 | 2 | 6 | 33.33 |
| 25-35 Gy/5 fractions | 2 | 43 | 2 | 45 | 4.44 |
| Dose/fraction | |||||
| < 8 | 4 | 51 | 4 | 55 | 7.27 |
| 8-11 | 13 | 101 | 13 | 114 | 11.40 |
| 12-19 | 22 | 162 | 22 | 184 | 11.96 |
| 20-23 | 6 | 20 | 6 | 26 | 23.08 |
| ≥ 24 | 12 | 19 | 12 | 31 | 38.71 |
| Follow-up, months | |||||
| Median | 2.46 | 13.39 | 11.53 | ||
| Range | 0.03-43.01 | 0.53-113.02 | 0.03-113.02 | ||
Table 2.
Breakdown According to Each SINS Criterion
| Factor | Fracture (n = 57) Vertebral Segments | No Fracture (n = 353) Vertebral Segments | Fractures |
||
|---|---|---|---|---|---|
| No. | Total No. | % | |||
| Location | |||||
| Junctional (occiput-C2, C7-T2, T11-L1, L5-S1) | 25 | 139 | 25 | 164 | 15.24 |
| Mobile spine (C3-C6, L2-L4) | 20 | 91 | 20 | 111 | 18.02 |
| Semirigid (T3-T10) | 12 | 108 | 12 | 120 | 10 |
| Rigid (S2-S5) | 0 | 15 | 0 | 15 | 0 |
| Pain | |||||
| Mechanical | 23 | 92 | 23 | 115 | 20 |
| Occasional and nonmechanical | 18 | 157 | 18 | 175 | 10.29 |
| Pain free | 16 | 104 | 16 | 120 | 13.33 |
| Bone lesion type | |||||
| Lytic | 48 | 208 | 48 | 256 | 18.75 |
| Mixed | 6 | 83 | 6 | 89 | 6.74 |
| Blastic | 3 | 62 | 3 | 65 | 4.62 |
| Alignment | |||||
| Subluxation/translation | 1 | 0 | 1 | 1 | 100 |
| Kyphosis/scoliosis | 9 | 22 | 9 | 31 | 29.03 |
| Normal | 47 | 331 | 47 | 378 | 12.43 |
| Vertebral body collapse | |||||
| ≥ 50% | 3 | 9 | 3 | 12 | 25 |
| < 50% | 27 | 44 | 27 | 71 | 38.03 |
| No collapse but > 50% body involved by tumor | 13 | 65 | 13 | 78 | 16.67 |
| None of the above | 14 | 235 | 14 | 249 | 5.62 |
| Posterior element involvement | |||||
| Bilateral | 7 | 59 | 7 | 66 | 10.61 |
| Unilateral | 32 | 152 | 32 | 184 | 17.39 |
| Not involved | 18 | 142 | 18 | 160 | 11.25 |
| SINS classification | |||||
| Stable | 13 | 185 | 13 | 198 | 6.57 |
| Indeterminant | 42 | 167 | 42 | 209 | 20.1 |
| Unstable | 2 | 1 | 2 | 3 | 66.67 |
Abbreviation: SINS, Spinal Instability Neoplastic Scoring system.
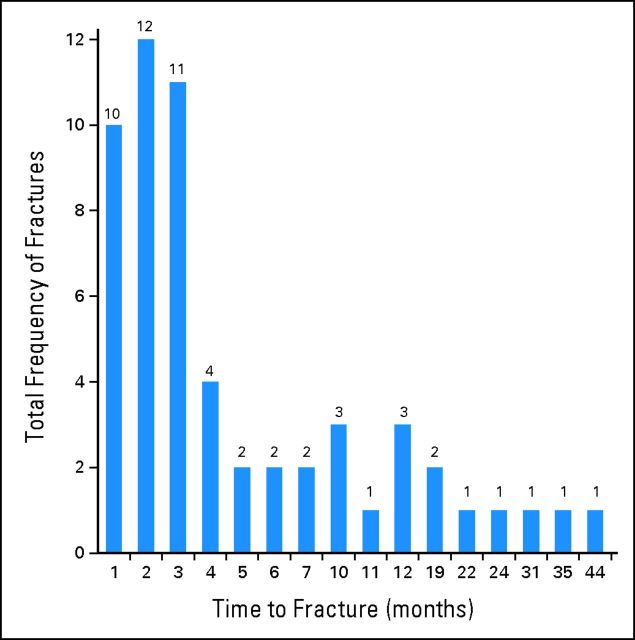
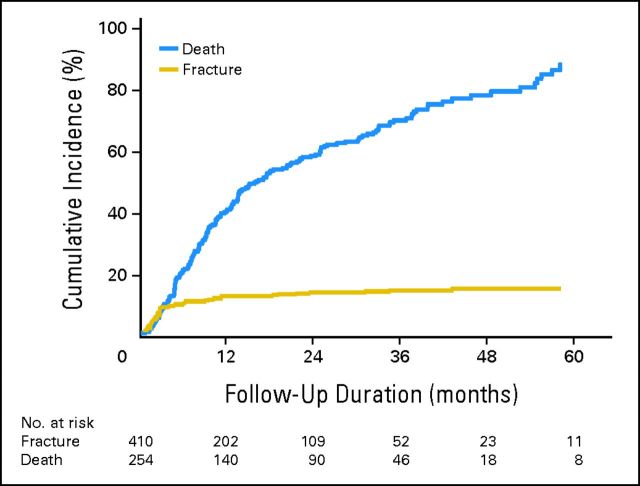
The median and mean times to fracture for the entire group who experienced fracture were 2.46 months and 6.33 months, respectively (range, 0.03 to 43.01 months). The distribution of fractures according to monthly intervals post-SBRT is described in Figure 1. Sixty-five percent of all VCFs occurred within the first 4 months post-SBRT. The 1- and 2-year CIF rates were 12.35% (95% CI, 7.59% to 17.11%) and 13.49% (95% CI, 6.84% to 20.14%), respectively (Fig 2).
Fig 1.
Distribution of the events of vertebral compression fracture over time in 1-month time intervals after spine stereotactic body radiotherapy.
Fig 2.
Cumulative incidence of vertebral compression fracture and death for the entire cohort.
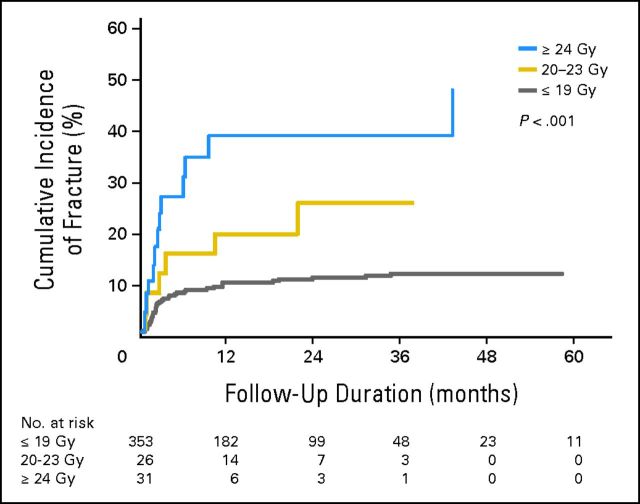
According to univariate analysis, only those factors found to be significant (P < .05) are described in Table 3. More specifically, we observed a relationship between dose per fraction and risk of VCF (P < .001), described in Figure 3. The multivariable Fine and Gray model confirmed significance for dose per fraction (global P < .001), and on pairwise comparison referenced to the ≤ 19 Gy/fraction cohort, treatment with 20 to 23 Gy/fraction and ≥ 24 Gy/fraction were significant predictors, with P < .001/HR = 4.91 and P < .001/HR = 5. 25, respectively (Table 3). The multivariate analysis also confirmed that baseline VCF, lytic tumor, and spinal misalignment (kyphosis/scoliosis and subluxation/translation) were predictive (Table 3).
Table 3.
Significant Predictors of VCF on Univariate and Multivariate Analysis
| Factor | Univariate P | Multivariable Fine and Grey Model |
||
|---|---|---|---|---|
| P | HR | 95% CI | ||
| Vertebral body collapse | < .001 | Global, < .001 | ||
| ≥ 50% VCF | .0189 | 6.92 | 1.38 to 34.77 | |
| < 50% VCF | < .001 | 8.98 | 4.48 to 18.00 | |
| No VCF but > 50% of vertebral body involved | < .001 | 4.46 | 2.08 to 9.57 | |
| Dose/fraction, Gy | < .001 | Global, < .001 | ||
| ≥ 24 | < .001 | 5.25 | 2.29 to 12.01 | |
| 20-23 | < .001 | 4.91 | 1.96 to 12.28 | |
| Alignment | .0027 | < .001 | 2.99 | 1.57 to 5.70 |
| Bone lesion type | < .001 | .0022 | 3.53 | 1.58 to 7.93 |
| Paraspinal/epidural extension | .0036 | NS | ||
NOTE. For vertebral body collapse, the reference is no VCF and less than 50% vertebral body involvement; for dose/fraction, the reference is ≤ 19 Gy/fraction; the reference for alignment was normal, and yphosis/scoliosis and subluxation/translation were grouped as only one patient had subluxation; and the reference for bone lesion was grouped according to mixed and osteoblastic tumor versus osteolytic, given that the majority of VCFs occurred in lytic tumors.
Abbreviations: HR, hazard ratio; NS, not significant; VCF, vertebral compression fracture.
Fig 3.
Cumulative incidence of vertebral compression fracture stratified according to the dose per fraction delivered.
Salvage interventions were performed for 43% of the VCFs, and included 17 cement augmentation procedures, one percutaneous instrumentation procedure, and six invasive instrumented stabilization surgeries.
DISCUSSION
We report the largest experience with spine SBRT evaluating the adverse event of VCF and evaluation of SINS in predicting for this potentially destabilizing complication. The crude risk was 14%, and the 1-year cumulative incidence of fracture was 12.35% (Fig 2), for the entire cohort. The median and mean times to VCF were 2.46 months and 6.33 months, respectively (range, 0.03 to 43.01 months), and the majority occurred within the first 4 months after SBRT (65%, Fig 1). These data are robust as they represent multi-institutional practice at major established centers performing spine SBRT, and there were no significant differences according to the treating institution (data not shown).
With respect to predictive factors, of the original six SINS criteria,11 we confirm that baseline VCF, lytic tumor, and misalignment were predictive, whereas presence of mechanical pain, location, and posterolateral involvement of spinal elements were not. We suspect that pain was not significant because of the subjectivity in the assessment and likelihood that patients describing frank mechanical pain would have been surgically stabilized and not included in this analysis. Similarly, if posterolateral elements were significantly compromised, then again these patients would likely be selected out of the analysis, as they would have been operated on. With respect to location, we had a nearly equal proportion of tumors in the junctional, semi-rigid, and the mobile spine (minority were in the rigid spine, S2-S5). The lack of significance may reflect the independent effect of the radiation itself on the VCF risk,6,9 and therefore, location is not specific to the outcome measure of SBRT-induced VCF. It is important to note that we investigated the role of SINS in predicting for this specific end point, and the criteria were developed to identify and communicate on in general a potentially mechanically unstable patient.17 Therefore, these findings do not reflect the utility of SINS as a tool to communicate spinal instability, but clarify the ability of this tool to predict for those at higher risk of VCF post-SBRT.
With respect to baseline VCF, we observed a significant relationship with increasing HRs, as each of the factors within the SINS vertebral body collapse criteria increased in severity (Table 3). The highest risk was observed for those with ≥ 50% baseline collapse, with an HR of 9.158. This risk factor had only been previously identified by the MDACC experience7 (univariate analysis only in the prior UofT report6), is biomechanically sound,8 and we now confirm its importance in risk stratification. Lytic tumor was also observed to be a significant risk factor for VCF (Table 3), and this is consistent among each of the prior major reports.5–7 We also confirm that baseline spinal misalignment is a significant risk factor (Table 3), and this had been previously reported only by the UofT group.6 Therefore, this multi-institutional study serves to clarify the significance of each of the SINS criteria, as the sample size is major with 410 tumors analyzed and 57 VCFs. Finding that three of the six original SINS criteria11 were indeed predictive is a critical finding, and although the overall score was not predictive (data not shown), we can conclude that SINS is an important tool to identify the patient at greater risk for SBRT-induced VCF. However, on its own, our findings suggest that SINS is only one component in the overall risk stratification, given that we also observed a significant predictive role for dose per fraction (Fig 3).
The impact of dose per fraction and risk of subsequent VCF is a major finding from this study. On multivariate analysis (Table 3), it is clear that as the dose per fraction increases beyond 19 Gy, the risk of fracture significantly increases, with significantly higher HRs for the 24 Gy/fraction group and 20 to 23 Gy/fraction group (which represents single-fraction SBRT). Although the HRs are significantly different for these two subgroups at 5.25 and 4.91, respectively, we acknowledge that the HRs are still similar, which indicates the significance of treating with high-dose single-fraction SBRT ≥ 20 Gy and risk of VCF. This threshold of ≥ 20 Gy is supported by the work by Cunha et al.6 From Figure 3, we observe 1-year VCF cumulative incidences of 39% with ≥ 24 Gy/fraction, 19% with 20 to 23 Gy/fraction, and 10% with ≤ 19 Gy/fraction.
These data serve to confirm and expand on results from two previously reported studies. First, we confirm the prior observation of an increased VCF risk when treating with ≥ 20 Gy/fraction versus less than 20 Gy/fraction by the UofT group,6 and we expand on this result by further stratifying the risk within three subgroups (≤ 19, 20 to 23, and ≥ 24 Gy/fraction). Second, our result of a 39% CIF at 1 year in those treated with 24 Gy in a single fraction both confirm and explain the previously reported 39% risk of VCF by the MSKCC5 given that they exclusively treated patients with high-dose single-fraction SBRT (median dose was 24 Gy in one fraction). However, unlike the MSKCC series,5 we report that fractures are occurring early after SBRT as opposed to what their findings suggest is a delayed event with a median time to VCF of 25 months. Therefore, our results suggest that if you reduce the dose per fraction, then you can improve the safety profile of spine SBRT with respect to VCF. Furthermore, that frequent follow-up in the short term is required given that nearly two thirds of all VCFs occurred within the first 4 months (Fig 1) after SBRT, and approximately half of these patients underwent salvage treatment with some form of surgical stabilization.
A recent clinicopathologic correlation analysis clarified the potential mechanism underlying SBRT-induced VCF. The work from Al-Omair et al9 suggests that radiation-induced osteoradionecrosis is the likely cause on the basis of biopsy results in two cases of SBRT-induced VCF. The authors postulate that the necrotic friable tissue compromises the ability of the vertebrae to withstand the axial loading forces, increasing the risk of VCF. Given that our aim was to determine the predictors of VCF secondary to the treatment itself, this is why we excluded those patients with tumor progression before or at the time of developing a VCF. We postulated that the destabilizing effects of tumor destroying existing bone as it grows would confound the analysis. Therefore, our results can confidently conclude that radiation itself is an independent factor with respect to the risk of VCF, and the dose-fractionation prescribed should be considered in treatment decisions.
Whether the practice of high-dose single-fraction SBRT (≥ 20 Gy/fraction) is justified outside of a clinical trial is a major clinical question that needs to be answered, given that patients are being exposed to a prohibitive risk of VCF and radionecrosis of the bone.9 Although there is evidence supporting high-dose single-fraction SBRT,18 there is also evidence to support more fractionated SBRT,19 with respect to local control. What is required is a randomized controlled trial focused on comparing various SBRT dose-fractionation schemes (much like what has been done with conventional radiation for bone metastases20) and specific to spinal metastases to clarify/justify current practice trends.
Our study supports caution with respect to spine SBRT dose-fractionation selection, in particular, for patients with lytic tumor, spinal misalignment, and a baseline fracture. We also conclude that SINS may have utility in predicting SBRT-induced VCF, and is a useful tool in the communication of high-risk features in a patient at risk of subsequent VCF and instability.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Arjun Sahgal, Medtronic Kyphoplasty; Lilyana Angelov, Brainlab Research Funding: None Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Arjun Sahgal, Eshetu G. Atenafu, Ameen Al-Omair, Michael G. Fehlings, Eric Chang
Provision of study materials or patients: Arjun Sahgal, Sam Chao, Lilyana Angelov, John Suh, Laurence D. Rhines, Michael G. Fehlings, Eric Chang
Collection and assembly of data: Arjun Sahgal, Eshetu G. Atenafu, Sam Chao, Ameen Al-Omair, Nicholas Boehling, Ehsan H. Balagamwala, Marcelo Cunha, Isabelle Thibault, Lilyana Angelov, Paul Brown, John Suh, Laurence D. Rhines, Eric Chang
Data analysis and interpretation: Arjun Sahgal, Eshetu G. Atenafu, Michael G. Fehlings, Eric Chang
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1. Sahgal A Roberge D Schellenberg D , etal: The Canadian Association of Radiation Oncology Scope of Practice Guidelines for Lung, Liver and Spine Stereotactic Body Radiotherapy Clin Oncol (R Coll Radiol) 24: 629– 639,2012. [DOI] [PubMed] [Google Scholar]
- 2. Pan H Simpson DR Mell LK , etal: A survey of stereotactic body radiotherapy use in the United States Cancer 117: 4566– 4572,2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sahgal A Ma L Weinberg V , etal: Reirradiation human spinal cord tolerance for stereotactic body radiotherapy Int J Radiat Oncol Biol Phys 82: 107– 116,2012. [DOI] [PubMed] [Google Scholar]
- 4. Sahgal A Weinberg V Ma L , etal: Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice Int J Radiat Oncol Biol Phys 85: 341– 347,2013. [DOI] [PubMed] [Google Scholar]
- 5. Rose PS Laufer I Boland PJ , etal: Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases J Clin Oncol 27: 5075– 5079,2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cunha MV Al-Omair A Atenafu EG , etal: Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): Analysis of predictive factors Int J Radiat Oncol Biol Phys 84: e343– e349,2012. [DOI] [PubMed] [Google Scholar]
- 7. Boehling NS Grosshans DR Allen PK , etal: Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases J Neurosurg Spine 16: 379– 386,2012. [DOI] [PubMed] [Google Scholar]
- 8. Sahgal A Whyne CM Ma L , etal: Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases Lancet Oncol 14: e310– e320,2013. [DOI] [PubMed] [Google Scholar]
- 9. Al-Omair A Smith R Kiehl TR , etal: Radiation-induced vertebral compression fracture following spine stereotactic radiosurgery: Clinicopathological correlation J Neurosurg Spine 18: 430– 435,2013. [DOI] [PubMed] [Google Scholar]
- 10. Balagamwala EH Angelov L Koyfman SA , etal: Single-fraction stereotactic body radiotherapy for spinal metastases from renal cell carcinoma J Neurosurg Spine 17: 556– 564,2012. [DOI] [PubMed] [Google Scholar]
- 11. Fisher CG DiPaola CP Ryken TC , etal: A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group Spine (Phila Pa 1976) 35: E1221– E1229,2010. [DOI] [PubMed] [Google Scholar]
- 12. Fourney DR Frangou EM Ryken TC , etal: Spinal instability neoplastic score: An analysis of reliability and validity from the spine oncology study group J Clin Oncol 29: 3072– 3077,2011. [DOI] [PubMed] [Google Scholar]
- 13. Ma L Sahgal A Larson D , etal: A generalized biologically effective dose model and it's application for radiosurgery and hypofractionated body radiotherapy Med Phys 34: 2403,2007. [Google Scholar]
- 14. Park C Papiez L Zhang S , etal: Universal survival curve and single fraction equivalent dose: Useful tools in understanding potency of ablative radiotherapy Int J Radiat Oncol Biol Phys 70: 847– 852,2008. [DOI] [PubMed] [Google Scholar]
- 15. Hyde D Lochray F Korol R , etal: Spine stereotactic body radiotherapy utilizing cone-beam CT image-guidance with a robotic couch: Intrafraction motion analysis accounting for all six degrees of freedom Int J Radiat Oncol Biol Phys 82: e555– e562,2012. [DOI] [PubMed] [Google Scholar]
- 16. Pepe MS, Mori M: Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med 12: 737– 751,1993. [DOI] [PubMed] [Google Scholar]
- 17. Sahgal A, Fehlings MG: In reply to Fourney Int J Radiat Oncol Biol Phys 85: 894– 895,2013. [DOI] [PubMed] [Google Scholar]
- 18. Yamada Y Bilsky MH Lovelock DM , etal: High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions Int J Radiat Oncol Biol Phys 71: 484– 490,2008. [DOI] [PubMed] [Google Scholar]
- 19. Heron DE Rajagopalan MS Stone B , etal: Single-session and multisession CyberKnife radiosurgery for spine metastases: University of Pittsburgh and Georgetown University experience J Neurosurg Spine 17: 11– 18,2012. [DOI] [PubMed] [Google Scholar]
- 20. Chow E Harris K Fan G , etal: Palliative radiotherapy trials for bone metastases: A systematic review J Clin Oncol 25: 1423– 1436,2007. [DOI] [PubMed] [Google Scholar]