Abstract
Limited studies performed a comprehensive assessment of risk factors for internal mammary lymph nodes (IMLN) metastasis, and disease-free survival (DFS) difference between IMLN-positive and IMLN-negative breast cancer (BC) patients undergoing IMLN dissection and systemic therapies was not clear.
A retrospective study included 1977 BC patients from Western China Clinical Cooperation Group between January 2005 and December 2012. The impact of clinicopathological factors on the occurrence of IMLN metastasis was assessed in univariate and multivariate logistic regression analyses, and a nomogram (model) was constructed to predict the IMLN status. DFS difference was evaluated in univariate and multivariate Cox regression analyses between IMLN-negative and IMLN-positive patients, and univariate analysis was performed to compare DFS between individuals with high and low IMLN metastasis risk defined by proposed nomogram.
Of 1977 enrolled patients, 514 cases underwent IMLN dissection and 1463 cases did not undergo IMLN irradiation or dissection. We found that initial disease symptoms and signs, mammographic calcification, tumor site, number of positive axillary lymph nodes (ALNs), American Joint Committee on Cancer pT stage, and human epidermal growth factor receptor 2 status were associated with IMLN metastasis (all P < .05). Those variables were included in nomogram, whose predictive ability was better than that of ALN classification (area under the curve: 0.82 vs 0.76, P < .001). Univariate cox proportional hazards model indicated that better DFS was observed in IMLN-negative patients than IMLN-positive group (hazard ratio [HR] = 1.87, 95% confidence interval [CI] = 1.05–3.34; P = .04), whereas no significant differences in DFS (HR = 0.99, 95% CI = 0.49–2.00; P = .97) were found after adjusting patient-, disease-, and treatment-related factors.
Nipple inversion, mammographic calcification, larger tumor size, medial tumor site, negative HER-2 status, and more positive ALNs are independent risk factors for IMLN metastasis, and the individualized nomogram is a feasible tool to predict the status of IMLN. Equivalent DFS was found between positive and negative IMLN patients who all accepted IMLN dissection and systemic therapies.
Keywords: breast cancer, disease-free survival, internal mammary lymph node, nomogram
1. Introduction
Overwhelming evidence from recent years showed obvious survival advantages conferred by additionally internal mammary lymph nodes (IMLN) irradiation especially among axillary lymph nodes (ALNs) positive breast cancer (BC) patients.[1–3] Positron emission tomography/computed tomography, high-resolution ultrasound, magnetic resonance imaging, and IMLN-sentinel lymph nodes biopsy were introduced to directly detect IMLN involvement, still, all these techniques are not enough to guide individualized IMLN irradiation.[4,5]
Although the status of IMLN and its relevant concepts were incorporated into 6th edition of the American Joint Committee on Cancer (AJCC) staging system for more than a decade, the 2016 NCCN Breast Cancer Clinical Practice Guidelines just recommended that IMLN irradiation for patients with at least 4 positive ALNs, and strongly considers IMLN irradiation for patients with 1 to 3 positive ALNs.[6] Nevertheless, prior studies reported that 36.8% to 46.2% patients with more than 4 positive ALNs and 18.8% to 26.7% patients with 1 to 3 positive ALNs were identified as IMLN metastases, and negative IMLN was found in about 70% patients with more than 4 positive ALNs.[7–10] Besides, excess local irradiation therapy can also lead to radiation pneumonitis and myocardial damage.[11] Huang et al included 1679 Chinese BC patients who underwent extended radical mastectomy, and indicated that patients with medial tumor and positive ALNs had a considerable risk of IMLN metastasis.[9] This study did not include tumor biological characteristics in multivariate regression, whereas previous studies indicated that tumor with calcifications, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) are predictive of lymph nodes involvement.[12–14] Hence, applying status of ALN to select beneficiaries of IMLN irradiation was not feasible, and more comprehensive models/nomograms integrated with anatomical and biological features needed be proposed to predict the involvement of IMLN individually.
Previous studies documented that patients with IMLN metastasis had worse survival outcomes than those with IMLN-negative regardless of involvement of ALN,[15–18] but randomized controlled trails consistently indicated that additional IMLN dissection did not show survival benefits compared with radical or modified radical mastectomy.[19–22] Although Veronesi et al[22] suggested that IMLN-positive patients who underwent IMLN dissection had higher annual death rate than corresponding nodal-negative patients, all the subjects enrolled in this study did not received postoperative radiotherapy. Moreover, any anticancer therapy or hormonal manipulation were not considered as prognostic covariables due to absence of documented evidence of primary treatment in this study, and similar results were found by Donegan.[23] Thus, few studies examined the effect of IMLN dissection on disease-free survival (DFS) among patients with different IMLN statuses when system adjuvant therapies were guaranteed for postoperative treatments.
The aim of this study was to investigate the impact of tumor heterogeneity, status of ALN, and calcifications of primary tumor on IMLN status, and to develop a nomogram for clinicians in predicting the IMLN status based on variables available after surgery to decide the best regional nodal irradiation option. In addition, we assessed the DSF difference between IMLN-positive and IMLN-negative patients who all received IMLN dissection and system adjuvant treatments.
2. Methods
2.1. Study design
The data for this study were obtained from Western China Clinical Cooperation Group (WCCCG), which included 23 BC centers in 9 provinces of Western China (i.e., Chongqing, Sichuan, Yunnan, Guizhou, Shanxi, Gansu, Guangxi, Ningxia, and Xinjiang). The whole database included a total of 18,600 patients with BC, which was histologically confirmed. Details about WCCCG had been described previously.[24] Patients with primary BC had undergone breast surgery between January 2005 and December 2012 were potentially enrolled. We excluded patients with neoadjuvant chemotherapy, bilateral tumors, and insufficient data. Only patients with completed data on mammography, ER, PR, HER2 status based on immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) were included. ER and PR positivity were determined IHC when the staining of ≥1% of tumor cells appeared. Tumors were identified as HER2-negative if they received an IHC score of 1+ and as HER2-positive only if they received an IHC score of 3+ or exhibited a HER2 gene expression level that was at least 2-fold higher than normal, as determined FISH. Considering the patients in the previous period did not routinely receive IHC for some prognostic biomarkers like P53 and Ki67, we cannot extract these variables from medical records. Despite this, the tumors were still categorized into 4 BC subtypes according to 2013 St Gallen International Expert Consensus[25]: hormone receptor (HR)-positive/HER2-negative, HR-positive/HER2- positive, HR-negative/HER2-positive, and triple-negative BC. Patient medical records and WCCCG were reviewed for data regarding to age at diagnosis, mammography data, and histopathological information. The surgical field of IMLN dissection was from the first to the fourth intercostal space, involving involved lymph nodes or pleura. The tumor sites were defined according to the quadrant or angles with nipple of primary tumor, and the data were provided by mammography. If the left (right) tumor located in upper outer quadrant, lower outer quadrant or 3 o’clock (9 o’clock), we defined it as lateral site. If the tumor located in nipple–areola, 6 o’clock or 12 o’clock, we defined it as central site. If the left (right) tumor located in upper inner quadrant, lower inner quadrant or 9 o’clock (3 o’clock), we defined it as medial site. The number of metastatic axillary nodes were categorized into 3 groups (node-negative nodes, 1–3 positive nodes, and at least 4 positive nodes), which reflected the essential cut-offs for status of ALN based on the IMLN irradiation associated recommendations released by the 2016 NCCN Breast Cancer Clinical Practice Guidelines.[6]
We extracted the aforementioned patients with completed survival data including survival status and survival time, who were followed up from 2005 to 2015, and questionnaire results were obtained through phone and outpatient department follow-up ways. Patients in every registry would answered the questions through telephone follow-up or reexamining in outpatient department at least once every 3 months during the first 3 years and then every 6 months thereafter. Clinic doctors would take detailed history or have a completed physical examination at each follow-up visit. Residual breast ultrasound or mammogram, chest radiography, abdominal sonography, whole-body bone scan, or positron emission tomography–computed tomography was routinely performed annually or when tumor relapse was clinically suspected. DFS was defined as the date of the diagnosis to the locoregional or distant recurrence or death from BC, whichever came first, and DFS was considered as censored status if patients were alive until date of last contact.
At last, of 1977 enrolled patients, 514 cases underwent IMLN dissection, 1463 cases did not undergo IMLN irradiation or dissection. This observational study was entirely based on data extracted from patient medical records and was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
2.2. Statistical method
We evaluated the distribution of clinicopathological variables between patients with IMLN-positive and IMLN-negative groups using the Pearson chi-squared test or Fisher exact test for categorical variables, and Student t for continuous variables. Univariate logistic regression analysis was used to assess the strength of the association between each predictive variable and the status of IMLN, and multivariate logistic regression analysis was applied to identify independent effects of these univariate predictive variables (P < .05). To avoid the influence of multicollinearity between some highly correlated variables, we only included one of them into final model (e.g., N stage and ALN status). In addition of these, an individualized nomogram was constructed based on rms package in R software. To validate this model internally through 1000 bootstrap resamples, concordance index (C-index) was calculated for the evaluation of the performance of predicting and discrimination ability by test concordance between predicted probability and actual outcome. Given that the probability of IMLN status was predicted by points based on nomogram or ALN categories recommended by NCCN 2016, we also conducted receiver operating characteristic curve to compare the performance of predicting ability of 2 IMLN status prediction tools, which were measured by area under the curve (AUC) values.
We conducted log-rank tests and cox proportion hazard regressions to examine the difference between patients with IMLN-positive and IMLN-negative in DFS, and calculated hazard ratios (HRs) with 95% confidence interval (CI). We performed univariate analysis to determine potential prognostic variable on DFS, and multivariate analysis was conducted to acquire adjusted HRs. Similar analyses were performed between high and low IMLN metastasis risk groups, which were stratified by cut-off value of score based on developed nomogram.
All P values reported are 2-sided, which <.05 were considered statistically significant. All analyses were conducted using R software (version 3.4.1).
3. Results
3.1. Patient characteristics
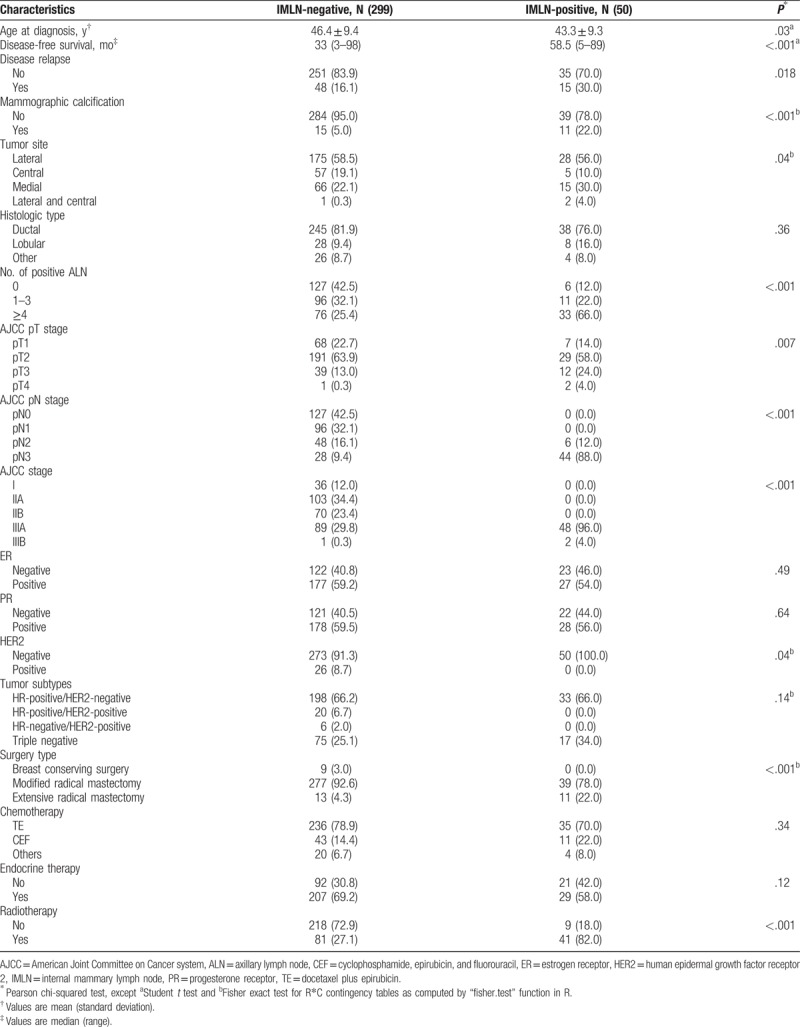
A total of 1977 eligible patients were enrolled in this study according to inclusion criteria, 514 individuals of them underwent IMLN dissection, 1463 patients of them with survival data did not undergo IMLN dissection or radiation. The flow chart was shown in Fig. 1, and Tables 1 to 3 illustrated clinicopathological characteristics of corresponding patients included. Of those patients who underwent IMLN dissection, 427 BC patients had IMLN-negative statuses and 87 patients had IMLN metastases. The mean age of the IMLN-negative group was similar with that of the patients who were IMLN involvement (46.8 ± 9.9 vs 44.9 ± 10.0, P = .10). Among patients with IMLN metastasis, 71.3% and 18.4% patients of them had at least 4 positive and 1 to 3 positive ALNs, respectively, and IMLN negative was found in 52.9% patients with ALN metastasis. In addition, mammographic calcification, AJCC pathological stage, HER2 status, and tumor subtype defined by IHC varied significantly across the 2 IMLN status groups (P < .05).
Figure 1.
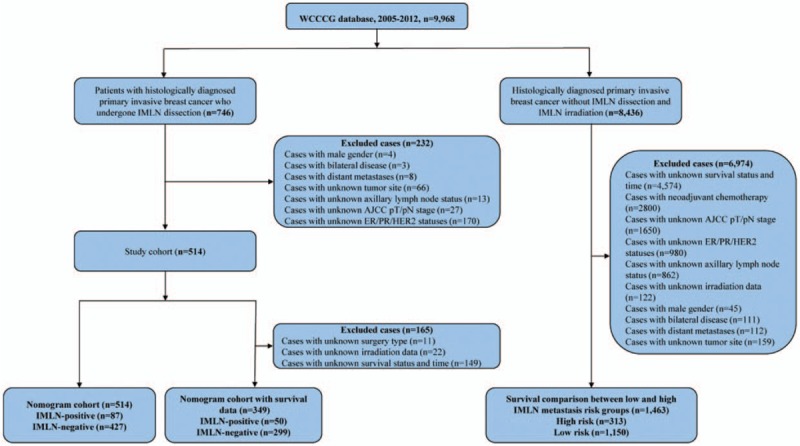
Flow chart for the data screening.
Table 1.
Demographics for eligible patients according to IMLN status (n = 514).

Table 3.
Demographics for patients with survival data according to IMLN risk stratified by nomogram (n = 1463).
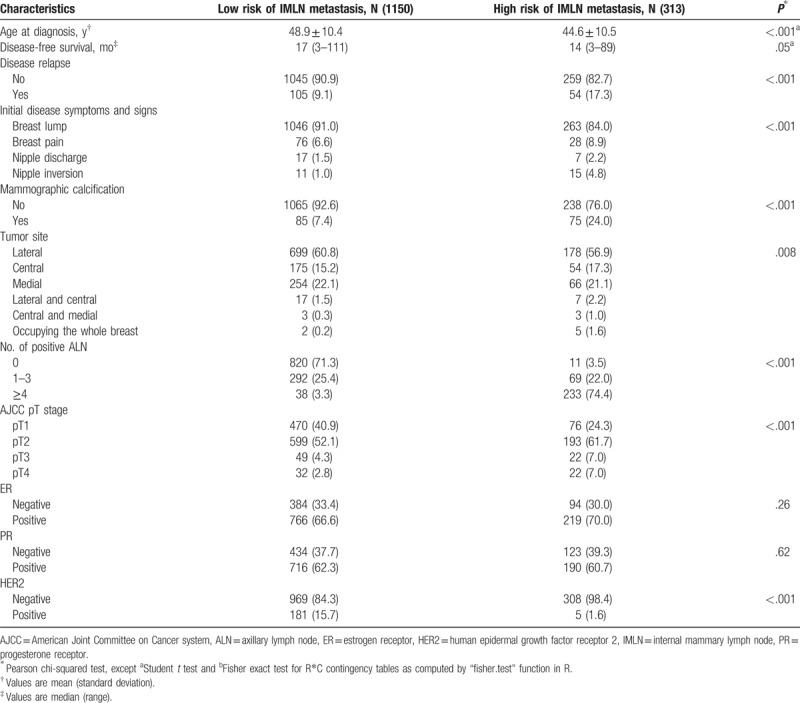
Table 2.
Demographics for patients with survival data according to IMLN status (n = 349).
3.2. Predictors of IMLN status
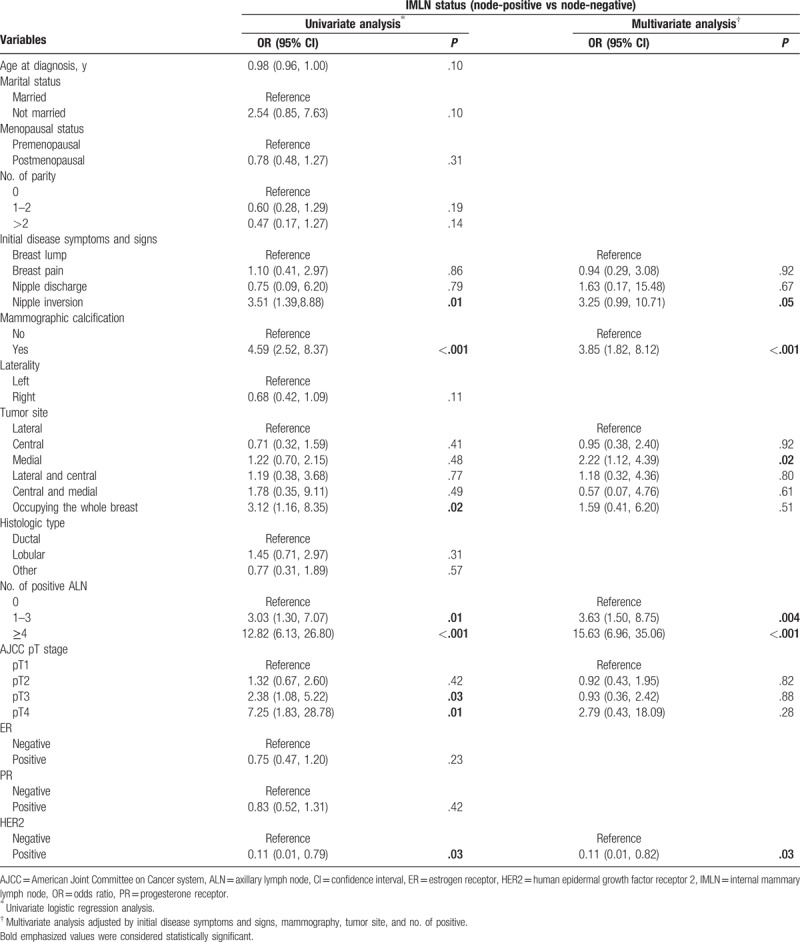
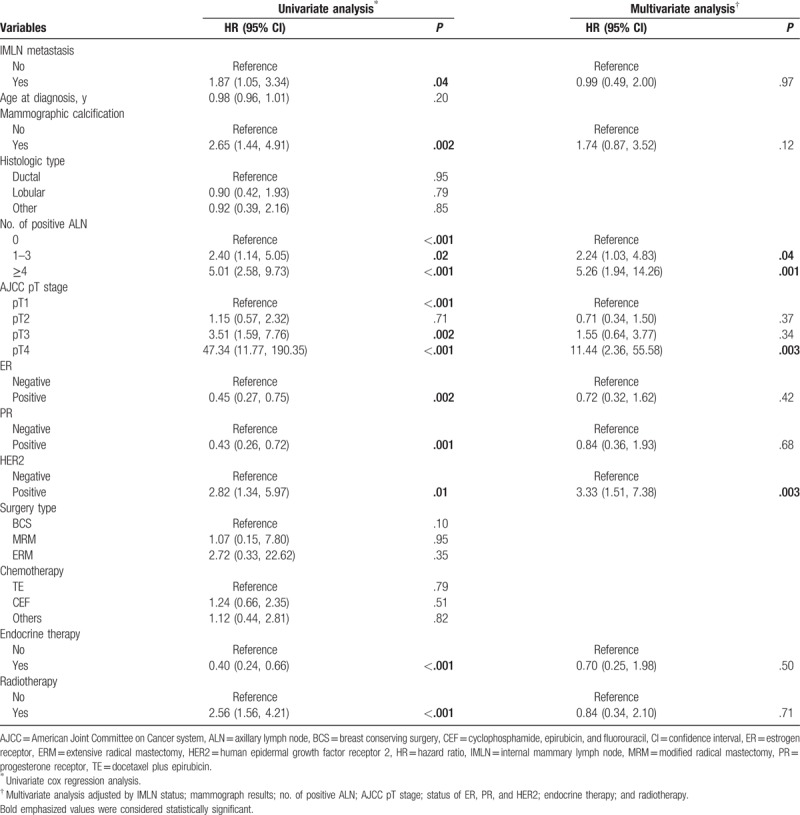
Associations of clinicopathological factors with metastatic IMLN incidence were studied by univariate and multivariate logistic regression analyses (Table 4). Univariate logistic regression analyses indicated that initial disease symptoms and signs, mammographic calcification, tumor site, number of positive ALN, AJCC pT stage, and HER2 status. After adjusting other predictive variables, exception for AJCC pT stage, residual variables remained to be independent risk factors (P < .05). Patients with nipple inversion breast, mass with calcification, foci located in medial site, 1 to 3, or at least 4 metastatic ALNs were all strongly associated with IMLN metastasis (Table 3).
Table 4.
Univariate and multivariate logistic regressions for prediction of IMLN status (n = 514).
3.3. Nomograms predicting IMLN status
The results from the multivariate regression analyses were used to construct the nomogram that predicted involvement of internal mammary nodes (Fig. 2). In internal validation, C-index for the nomograms to predict IMLN status was 0.82 (95% CI: 0.81–0.83). The AUC of nomogram (0.82, 95% CI: 0.71–0.81) is higher than that of ALN classification (0.76, 95% CI: 0.78–0.87) (Fig. 3), and the cut-off value of score based on nomogram is 192. These results consistently indicated that the predicting ability and discrimination of the models were generally good.
Figure 2.
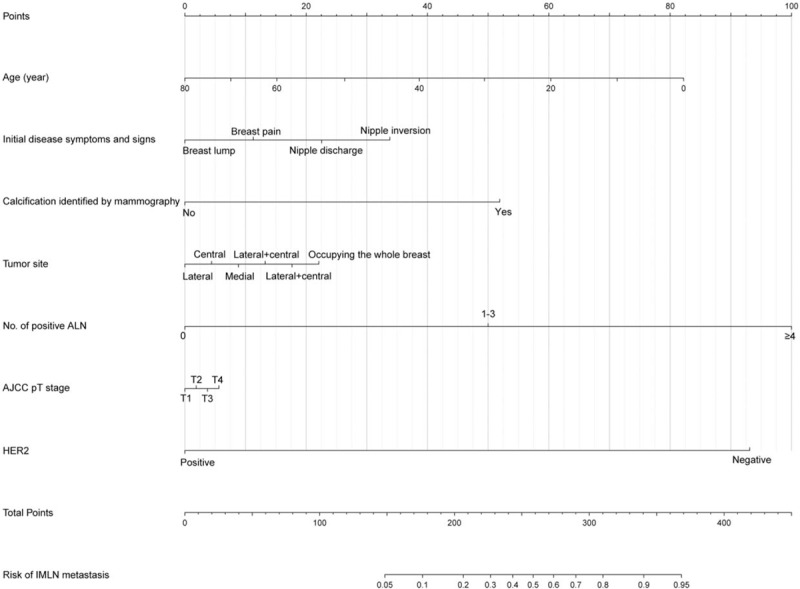
Nomogram predicting the status of IMLN. The total score for each patient is assigned by drawing a vertical line from the appropriate point for each predictor down to the score scale, and summing these scores. To obtain the predicted probability of IMLN metastasis, a vertical line is drawn from the total score scale up to the predicted probability scale in the lower part of the nomogram. AJCC = American Joint Committee on Cancer system, ALN = axillary lymph node, BCS = breast conserving surgery, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, IMLN = internal mammary lymph nodes, PR = progesterone receptor.
Figure 3.
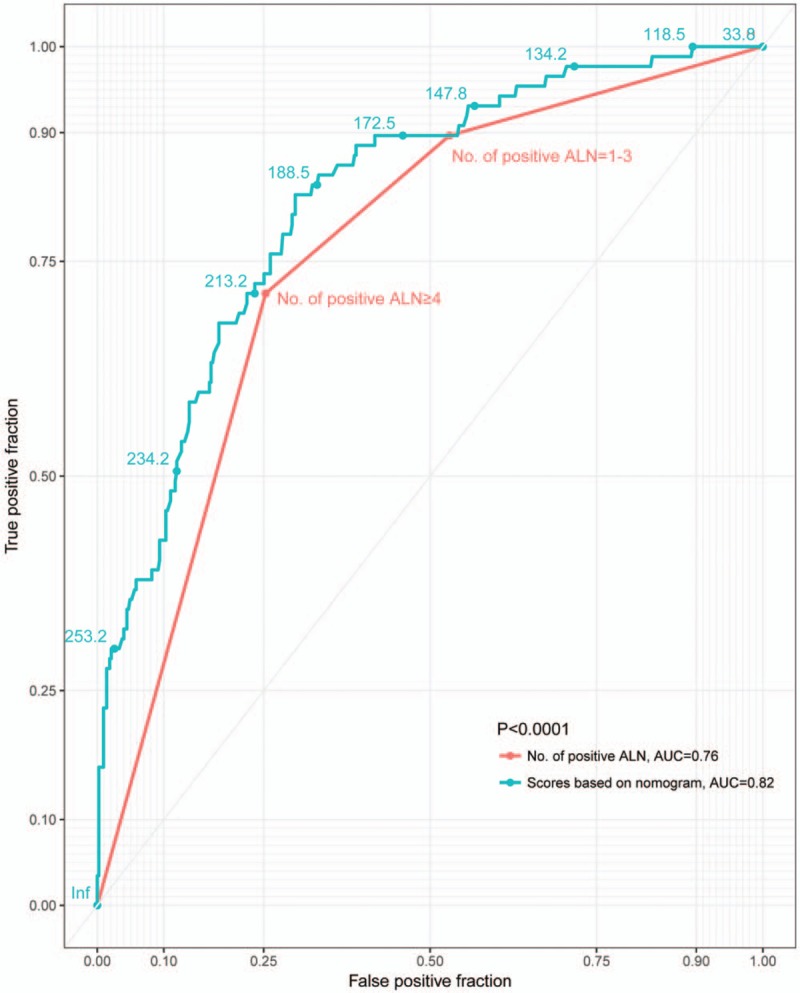
Receiver operating characteristic curves representing the discriminatory ability of the nomograms and ALN categories in predicting axillary nodal status. ALN = axillary lymph node.
3.4. Survival analysis
The median DFS for 349 enrolled patients who underwent IMLN dissection was 33 months (range: 3–98) in IMLN-negative group and 58.5 months (range: 5–89) in IMLN-positive group, respectively. The 3- and 5-DFS rates in the IMLN-negative group are 89.3% and 86.6%, respectively, which were significantly higher than those in the IMLN-positive group (78% and 72%, respectively, log-rank P value = 0.04; HR = 1.87, 95% CI: 1.05–3.34; Fig. 4A). To adjust potential modifier-effects, multivariate cox proportional hazards model including significant variables in univariate analysis was conducted, whereas no significant differences in DFS (HR = 0.99, 95% CI: 0.49–2.00; P = .97, Table 5) between IMLN-positive group and IMLN-negative group.
Figure 4.
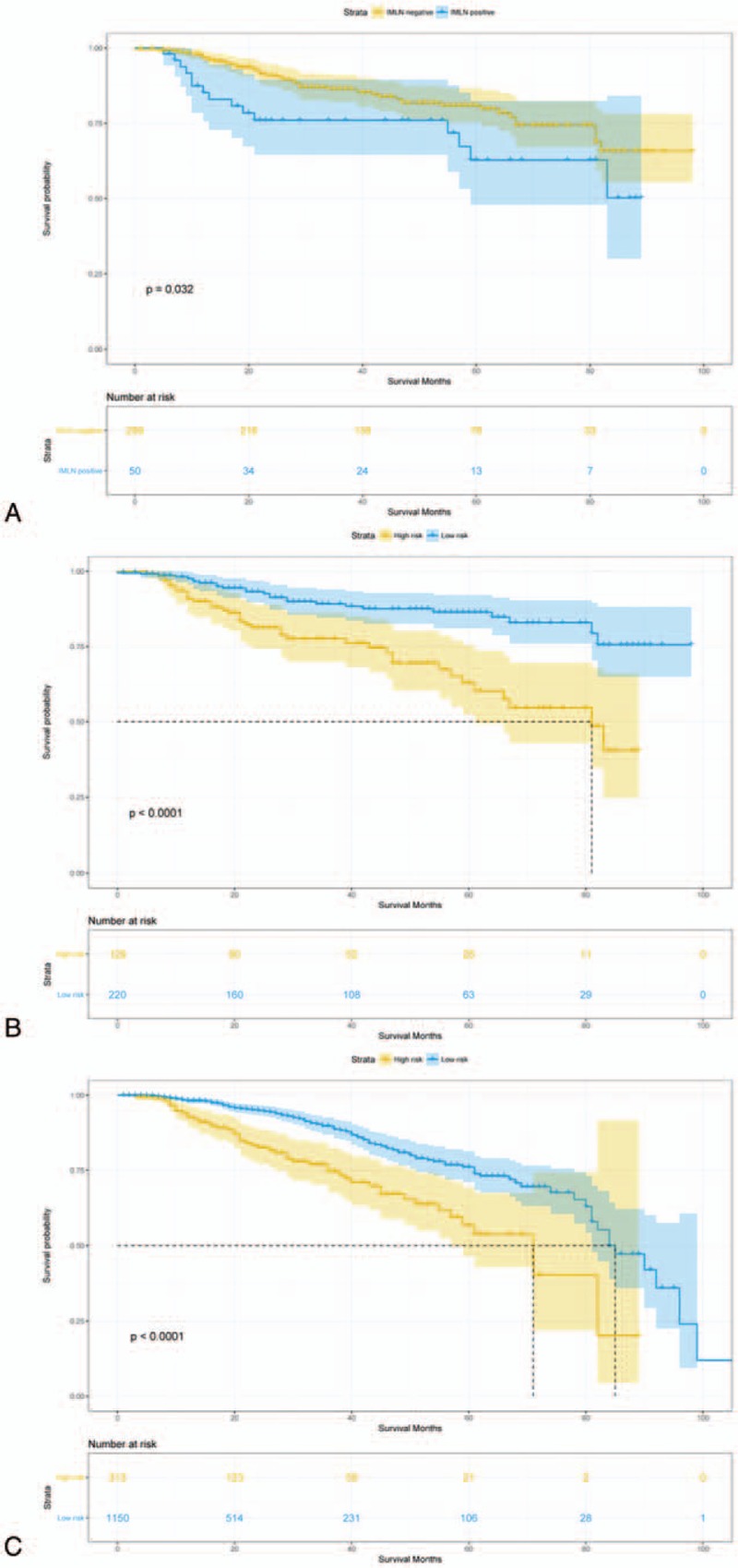
Disease-free survival comparison between (A) IMLN-positive and IMLN-negative patients who underwent IMLN dissection (N = 349). (B)∗ High and low risk of IMLN metastasis patients who underwent IMLN dissection (N = 349). (C)∗ High and low risk of IMLN metastasis patients who did not underwent IMLN dissection or IMLN irradiation (N = 1463). IMLN = internal mammary lymph nodes. ∗High- and low-risk IMLN metastasis group was stratified according to the IMLN nomogram.
Table 5.
Univariate and multivariate cox regressions for breast cancer patients with known IMLN status (n = 349).
IMLN metastasis risk stratification was carried out by stratifying patients into low-risk and high-risk groups based on cut-off value of scores calculated by our nomogram. As results, among 349 with known IMLN status, 220 patients were placed in the low-risk group with a score of <192, and 129 patients were placed in the high-risk group with a score of >192. Among 1463 patients without receiving IMLN dissection or irradiation, 313 of them were categorized into low-risk group, remaining 1150 patients in low-risk group. The DFS was significantly different between the low- and high-risk groups, regardless of whether comparisons were conducting among patients who underwent IMLN local-therapy or not (all P < .05) (Fig. 4B and C).
4. Discussion
This study provides contemporary information from a multicentral population-based data set on the association of tumor anatomical and biological characteristics with IMLN metastasis risk. This updates earlier reports[4,7,9,10,22] and indicates that ALN status, tumor with calcification, initial symptoms and signs, tumor site, and the status of HER2 are independent risk factors for IMLN metastasis, which is comparable with previous studies.[6,7,9,12,14] The developed nomogram is a valid tool that can help doctors in IMLN irradiation strategy making. Meanwhile, equivalent DFS is found between IMLN-positive and IMLN-negative groups after IMLN dissection, which may owe more to the influence of system adjuvant therapies.
In the final nomogram, ALN status remained a key factor to predict the IMLN metastasis. Of those positive IMLN patients, nearly 80% subjects were identified with ALN metastasis. Nevertheless, we found that 304 patients with ALN metastasis occurred IMLN metastasis (25%), which was similar with that reported by Huang et al (27%). Obviously, over-treatment like irradiation for internal mammary district would conducted according to the current NCCN Breast Cancer Clinical Practice Guidelines. A large amount of studies[9,18,19,23,26] also found medial tumor to be strongly associated with a higher rate of IMLN involvement. We defined the tumor site as more comprehensive model that considered those cross-sectional tumors and multiple lesions; unexpectedly, medial tumor was still an independent risk factor to impact IMLN metastasis. Subsequently, Chen et al[16] raised that ALN status combined with tumor site could be an effective way for IMLN prediction, and up to 65% patients with medial tumors and positive ALN had involvement of IMLN. Nevertheless, the effect of tumor site on prediction of IMLN status in the nomogram was limited. Accordingly, Wang et al[27] hypothesized that IMLN sentinel lymph nodes received the lymphatic drainage from not only the primary tumor area but also the entire breast parenchyma, which was also validated that different tracers injected into the different sites of the intraparenchymal reached the same IMLN sentinel lymph node. Furthermore, a prospective cohort study[1] based on Danish Breast Cancer Cooperative Group documented that IMLN irradiation increased overall survival in patients with early-stage node-positive BC, but no survival difference between irradiation and nonirradiation groups was found in medial/central subgroup regardless of number of positive ALNs. In addition, we seemed to ignore that tumor site included not only horizontal position but also vertical position, after all, the dermal and subdermal lymphatic flow is rarely directed to IMLN.[28–31]
A recent study[12] revealed that HER2-positive BC was associated with ALN metastasis, which was independent with HR status. Controversially, Crabb et al[13] suggested that HER2-positive tumors do not differ in risk of ALN involvement compared with the luminal subtype, and they yielded that basal BC molecular subtype predicted a lower incidence of axillary nodal metastasis. In our study, we are first to report that significantly a lower proportion of HER2-positive tumors were found in IMLN-positive group compared with those in IMLN-negative group. We had tried to use molecular subtypes as a covariable (HR-positive/HER2-negative, HR-positive/HER2-positive, HR-negative/HER2-positive, and triple-negative), and no difference in IMLN metastasis risk was observed between groups. Interestingly, Gingras et al[32] failed to demonstrate a DFS benefit of regional nodal irradiation in HER2-positive, node-positive patients treated with adjuvant HER2-targeted therapy, and 131 enrolled patients treated with IMLN irradiation. It was inferred that HER2-positive tumor tends to be nodal negative based on this indirect outcome. More evidence for associations of HR/HER2 status with IMLN metastasis risk should be provided to guide clinicians’ decision making like axillary surgery and locoregional radiation.
The associations of tumors with calcifications and nipple inversion with increased IMLN metastasis risk were first revealed in this study. Likewise, our previous study[14] had pointed out that patients with mammographic calcifications were also characterized by large tumor sizes, ALN positivity, and other unfavorable pathological features. Interestingly, although incidence of an underlying breast carcinoma in subjects with nipple inversion varied from 5% to >50%,[33] the relationships between BC with nipple inversion and clinicopathological features or prognostic outcomes had been little addressed.
A prior early BC trialists’ collaborative group meta-analysis[34] showed the favorable effect of regional radiotherapy including IMLN irradiation after mastectomy on survival outcomes especially in patients with positive nodes, which was comparable with results reported by recent large-scale studies.[1–3] Inversely, 2 cooperative randomized trials[19,22] revealed no benefit from surgical dissection of the IMLN. Unexpectedly, this study demonstrated that patients with positive IMLN had better median DFS compared with negative nodal patients (58.5 vs 33 months). On the one hand, almost all of patients with positive IMLN were 100% HER2-negative, which contributed to satisfying DFS. On the other hand, almost all of patients (82%) with positive IMLN received radiotherapy that was also a crucial factor for DFS. Naturally, no difference in DFS was further found after adjusting these prognostic variables. These findings simultaneously supported that the effect of surgical dissection of IMLN was limited from another angle, and randomized, well-designed clinical trials are urgently needed to compare the effectiveness of IMLN dissection and IMLN irradiation.
To our knowledge, this is the first clinical study including a large number of surgical series in western China to explore the associations of contemporarily clinicopathological characteristics with IMLN metastasis risk among BC patients, and to develop a nomogram to predict IMLN status individually. Some limitations of this study should be acknowledged, and our results ought to be interpreted with cautions. Some important confounding factors such as status of Ki67 and P53, nuclear grade, and anti-HER2 therapy were missing in most of enrolled patients, which may have influenced our results. IMLN irradiation had favorable effects on survival among early BC patients,[1–3] and question remains whether survival advantages would be changed when IMLN was involved or not. In addition, although the sample size in this study was only next to that in Huang et al's study, limited numbers of patients maybe lead to decline of statistical power especially in survival analyses (n = 349). Lastly, we cannot entirely control the quality of primary data, and pathological diagnosis from multiple hospital will lead to inevitable bias.
5. Conclusion
The independent risk factors of IMLN metastasis are nipple inversion, mammographic calcification, large tumor size, medial tumor site, negative HER2 status, and more positive ALNs. The developed nomogram is a valid predictive tool to facilitate postoperative decision making for additional irradiation therapy of internal mammary district. Among those patients who underwent IMLN dissection, DFS in positive IMLN patients was no different with negative nodal patients.
Author contributions
Hong-Yuan Li and Kang Wang conceived the study idea. Kang Wang and Xiang Zhang performed data mining and statistical analyses, interpreted results of statistical analyses. Kang Wang drafted the initial manuscript. Hong-Yuan Li made critical comment and revision for the initial manuscript, and had primary responsibility for the final content. All authors reviewed and approved the final manuscript.
Conceptualization: Kang Wang, Lei Xing, Fan Li.
Data curation: Xiang Zhang, Ai-Jie Zhang, Hui Li.
Formal analysis: Jin-Ping Liu.
Methodology: Xue-Dong Yin, Yang Shi, Bin-Lin Ma.
Project administration: Ling-Quan Kong, Jun Jiang, Guo-Sheng Ren.
Resources: Ke Zheng, Lei Xing, Fan Li.
Software: Kang Wang.
Supervision: Hong-Yuan Li.
Writing – original draft: Kang Wang.
Writing – review and editing: Hong-Yuan Li.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer system, ALN = axillary lymph node, AUC = area under the curve, BC = breast cancer, C-index = concordance index, CI = confidence interval, DFS = disease-free survival, ER = estrogen receptor, FISH = fluorescence in situ hybridization, HER2 = human epidermal growth factor receptor 2, HR = hazard ratio, IMLN = internal mammary lymph nodes, PR = progesterone receptor.
Kang Wang (kang_wang0822@hotmail.com) and Xiang Zhang have contributed equally to this work and should be considered as co-first authors.
This study was supported by grants from the National Key Clinical Specialty Construction Program of China, the Chinese Academy of Medical Sciences, and the Peking Union Medical College (2014BAI08B03), the Zhejiang Province Key Project of Science and Technology (2014BAI08B00). The funder of this study had no role in the decisions about the design and conduct of the study; collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The views expressed in this review are the opinions of the authors.
The authors have no conflicts of interest to disclose.
References
- [1].Thorsen LB, Offersen BV, Dano H, et al. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol 2016;34:314–20. [DOI] [PubMed] [Google Scholar]
- [2].Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med 2015;373:307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 2015;373:317–27. [DOI] [PubMed] [Google Scholar]
- [4].Noguchi M, Tsugawa K, Miwa K. Internal mammary chain sentinel lymph node identification in breast cancer. J Surg Oncol 2000;73:75–80. [DOI] [PubMed] [Google Scholar]
- [5].Sugg SL, Ferguson DJ, Posner MC, et al. Should internal mammary nodes be sampled in the sentinel lymph node era? Ann Surg Oncol 2000;7:188–92. [DOI] [PubMed] [Google Scholar]
- [6].Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive breast cancer Version 1.2016, NCCN Clinical Practice Guidelines in oncology. J Natl Compr Canc Netw 2016;14:324–54. [DOI] [PubMed] [Google Scholar]
- [7].Veronesi U, Cascinelli N, Bufalino R, et al. Risk of internal mammary lymph node metastases and its relevance on prognosis of breast cancer patients. Ann Surg 1983;198:681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Noguchi M, Yabushita K, Tajiri K, et al. Five year results of radical mastectomy for breast cancer, by a sternal splitting, intrapleural en bloc resection of the internal mammary lymph nodes. Jpn J Surg 1987;17:63–71. [DOI] [PubMed] [Google Scholar]
- [9].Huang O, Wang L, Shen K, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat 2008;107:379–87. [DOI] [PubMed] [Google Scholar]
- [10].Noguchi M, Ohta N, Thomas M, et al. Risk of internal mammary lymph node metastases and its prognostic value in breast cancer patients. J Surg Oncol 1993;52:26–30. [DOI] [PubMed] [Google Scholar]
- [11].Postma EL, van Wieringen S, Hobbelink MG, et al. Sentinel lymph node biopsy of the internal mammary chain in breast cancer. Breast Cancer Res Treat 2012;134:735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ugras S, Stempel M, Patil S, et al. Estrogen receptor, progesterone receptor, and HER2 status predict lymphovascular invasion and lymph node involvement. Ann Surg Oncol 2014;21:3780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Crabb SJ, Cheang MC, Leung S, et al. Basal breast cancer molecular subtype predicts for lower incidence of axillary lymph node metastases in primary breast cancer. Clin Breast Cancer 2008;8:249–56. [DOI] [PubMed] [Google Scholar]
- [14].Zheng K, Tan JX, Li F, et al. Relationship between mammographic calcifications and the clinicopathologic characteristics of breast cancer in Western China: a retrospective multi-center study of 7317 female patients. Breast Cancer Res Treat 2017;166:569–82. [DOI] [PubMed] [Google Scholar]
- [15].Veronesi U, Fau-Cascinelli N, Cascinelli N, et al. Prognosis of breast cancer patients after mastectomy and dissection of internal mammary nodes. Ann Surg 1985;202:702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen RC, Lin NU, Golshan M, et al. Internal mammary nodes in breast cancer: diagnosis and implications for patient management—a systematic review. J Clin Oncol 2008;26:4981–9. [DOI] [PubMed] [Google Scholar]
- [17].Dahl-Iversen E, Tobiassen T. Radical mastectomy with parasternal and supraclavicular dissection for mammary carcinoma. Ann Surg 1963;157:170–3. [PubMed] [Google Scholar]
- [18].Livingston SF, Arlen M. The extended extrapleural radical mastectomy: its role in the treatment of carcinoma of the breast. Ann Surg 1974;179:260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lacour J, Le M, Caceres E, et al. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Ten year results of an international cooperative trial in breast cancer. Cancer 1983;51:1941–3. [DOI] [PubMed] [Google Scholar]
- [20].Hennequin C, Bossard N, Servagi-Vernat S, et al. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat Oncol Biol Phys 2013;86:860–6. [DOI] [PubMed] [Google Scholar]
- [21].Meier P, Ferguson DJ, Karrison T. A controlled trial of extended radical mastectomy. Cancer 1985;55:880–91. [DOI] [PubMed] [Google Scholar]
- [22].Veronesi U, Marubini E, Mariani L, et al. The dissection of internal mammary nodes does not improve the survival of breast cancer patients. 30-year results of a randomised trial. Eur J Cancer (Oxf, Engl 1990) 1999;35:1320–5. [DOI] [PubMed] [Google Scholar]
- [23].Donegan WL. The influence of untreated internal mammary metastases upon the course of mammary cancer. Cancer 1977;39:533–8. [DOI] [PubMed] [Google Scholar]
- [24].Wang K, Ren Y, Li H, et al. Comparison of clinicopathological features and treatments between young (≤40 years) and older (>40 years) female breast cancer patients in West China: a retrospective, epidemiological, multicenter, case only study. PLoS ONE 2016;11:e0152312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Caceres E. Incidence of metastasis in the internal mammary chain in operable carcinoma of the breast and 5 year results. Acta Unio Int Contra Cancrum 1963;19:1566–9. [PubMed] [Google Scholar]
- [27].Cong BB, Qiu PF, Liu YB, et al. Validation study for the hypothesis of internal mammary sentinel lymph node lymphatic drainage in breast cancer. Oncotarget 2016;7:41996–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Park C, Seid P, Morita E, et al. Internal mammary sentinel lymph node mapping for invasive breast cancer: implications for staging and treatment. Breast J 2005;11:29–33. [DOI] [PubMed] [Google Scholar]
- [29].Shimazu K, Tamaki Y, Taguchi T, et al. Lymphoscintigraphic visualization of internal mammary nodes with subtumoral injection of radiocolloid in patients with breast cancer. Ann Surg 2003;237:390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang L, Yu JM, Wang YS, et al. Preoperative lymphoscintigraphy predicts the successful identification but is not necessary in sentinel lymph nodes biopsy in breast cancer. Ann Surg Oncol 2007;14:2215–20. [DOI] [PubMed] [Google Scholar]
- [31].Sun X, Liu JJ, Wang YS, et al. Roles of preoperative lymphoscintigraphy for sentinel lymph node biopsy in breast cancer patients. Jpn J Clin Oncol 2010;40:722–5. [DOI] [PubMed] [Google Scholar]
- [32].Gingras I, Holmes E, De Azambuja E, et al. Regional Nodal Irradiation After Breast Conserving Surgery for Early HER2-Positive Breast Cancer: Results of a Subanalysis From the ALTTO Trial. Journal of the National Cancer Institute 2017;109: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Neville EM, Freeman AH, Adiseshiah M. Clinical significance of recent inversion of the nipple: a reappraisal. J R Soc Med 1982;75:111–3. [PMC free article] [PubMed] [Google Scholar]
- [34].McGale P, Taylor C, et al. EBCTCG. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


