Abstract
To determine the diagnostic value of computed tomography (CT) for prediction of microvascular invasion (MVI) in hepatocellular carcinoma (HCC). Preoperative CTs for 160 patients with 57 MVI-positive and 103 MVI-negative HCCs diagnosed by surgical pathology were reviewed retrospectively. CT parameters and serum α-fetoprotein (AFP) level were analyzed in SPSS 16.0. Although univariate analysis showed that tumor size (P = .012), grade (Z = −2.114, P = .034), and peritumoral enhancement (χ2 = 4.464, P = .035) were associated with MVI, multiple logistic regression analysis showed that capsular invasion (odds ratio [OR] = 23.469, P < .001), margins (OR = 6.751, P < .001), and serum AFP level (OR = 1.001, P = .038) were associated with MVI in HCC (P < .05). Radiographic hepatic capsular invasion and nonsmooth tumor margins identified by preoperative CT images, along with AFP levels greater than 232.2 ng/mL, are important predictors of MVI.
Keywords: α-fetoprotein, computed tomography, hepatocellular carcinoma, microvascular invasion
1. Introduction
Hepatocellular carcinoma (HCC), a very common cancer in Guangxi, China, may be cured through surgical resection or liver transplantation (LT).[1] However, the 5-year recurrence rates following curative resection and LT are only 70% and 35%, respectively.[2] Microvascular invasion (MVI) in early-stage HCC is a powerful independent prognostic factor for tumor recurrence and metastasis following surgery.[2–4] Preoperative MVI prediction is difficult because some researchers think that limited preoperative biopsy specimens are insufficient for accurate diagnosis;[2,5,6] thus, diagnosis is made mainly based upon histologic examination of resected specimens. Noninvasive, preoperative MVI assessment would assist providers in optimizing treatment. Wide surgical resection margins are needed to improve overall survival and reduce tumor recurrence in patients with MVI.[7]
Recent studies have suggested that computed tomography (CT),[8] CT perfusion,[9] PET-CT,[10] gadoxetic acid-enhanced magnetic resonance imaging (MRI),[11,12] and apparent diffusion coefficient (ADC) measurement[13–15] could be used to predict MVI. However, there have been no studies of capsular invasion on CT (a standard HCC imaging modality) to predict MVI. Furthermore, CT perfusion delivers relatively high radiation doses, and ADC and hepatobiliary contrast agent imaging are not widely accepted because of the loud noise, long scan time, and expense, despite the additional clinical information they may provide.
In this study, we assessed retrospectively whether preoperative CT parameters of tumor size, grade, margins, capsule, necrosis, peritumoral enhancement, tumor-enhanced ratio, capsular invasion, and serum α-fetoprotein (AFP) level could be used to predict MVI in HCC.[16–20] Histopathology findings were compared with radiography results to confirm CT accuracy.
2. Materials and methods
2.1. Patients
This retrospective study included 160 patients (129 males and 31 females) aged 24 to 79 years (mean, 48.4 years) with solitary HCC who underwent preoperative CT and AFP measurement at our hospital from July 1, 2014 to June 22, 2016. All patients provided written informed consent, and the institutional review board of Affiliated Tumor Hospital of Guangxi Medical University approved this retrospective study. All HCC cases met 3 criteria: no cancer-related treatment or biopsy before CT for solitary hepatic tumors; hepatic tumor diagnoses were confirmed by postoperative histopathological examination; no CT evidence of macrovascular invasion or metastasis. CT findings were compared with pathology findings. With respect to operative treatment, 127 patients were elected to have partial hepatectomy, and 33 patients were elected to have anatomic hemihepatectomy. Of the 160 HCC cases, 151 were Child-Pugh class A and 9 were Child-Pugh class B.
2.2. Computed tomography imaging protocol
Hepatic CT images were obtained using a 64-MDCT scanner (SOMATOM Sensation 64, Siemens Healthcare, Erlangen, Germany) equipped with z-axis modulation, a spiral pitch of 1, a 5-mm section thickness, a 2-mm reconstruction gap, a field of view of 311 mm, 120 kVp, 230 mA, and a standard reconstruction algorithm. Nonionic contrast medium (iopromide, 300 mgI/mL) was administered at an injection rate of 3 mL/s for a total dose of 100 mL. The hepatic arterial phase images were started approximately 25 seconds after contrast medium injection, with portovenous phase images taken 60 seconds later, and equilibrium phase images taken 180 seconds later. This protocol was conducted 3 times. The scanning range included the whole-liver zone while patients held their breath. Coronary and sagittal images were reconstructed using a 5-mm section thickness.
2.3. Image evaluation
Tumor size was divided into 4 grades[21]: Grade 1 was 3 cm or less; Grade 2 was 3.1 to 5 cm; Grade 3 was 5.1 to 6.5 cm; and Grade 4 was greater than 6.5 cm. Tumor margins on the liver map (location on transverse, coronary, and sagittal planes)[3,8,22,23] were categorized using 3 subtypes (Fig. 1): smooth margins (simple nodular, frequently with a complete capsule); nonsmooth margins including focal extranodular type (single nodule outside the tumor capsule); and multinodular type (confluence of several small nodules). Tumor capsules were defined by their linear and enhanced structure that surrounded the tumor on equilibrium phase imaging. Capsules were categorized into 3 groups: complete capsule encasing the tumor, incomplete tumor capsule, or absent tumor capsule. Negative or positive peritumoral enhancement was observed using multiphase CT. Positive peritumoral enhancement was defined in relationship to the liver parenchyma as hyperdensity proximal the tumor border on arterial phase imaging that changed to isodensity on equilibrium phase imaging. Radiographic evidence of hepatic capsular invasion was defined by the presence of any of several characteristics: incomplete or absent tumor capsule; tumor growth beyond the liver contour or a lack of liver parenchyma between the tumor and liver capsule; liver capsule that was fuzzy, interrupted, or thickened; or detectable subcapsular effusion accumulation (Fig. 2). In the absence of these CT findings, arch distance to maximum tumor diameter ratio was used to identify hepatic capsular invasion (Fig. 2). The length of the interface between the tumor and liver capsule (arch distance) was drawn freehand and the maximum tumor diameter was measured. The arch distance to maximum tumor diameter ratio was then calculated, and a ratio greater than 0.9 was considered evidence of hepatic capsular invasion.[24] The tumor enhanced ratio was calculated as
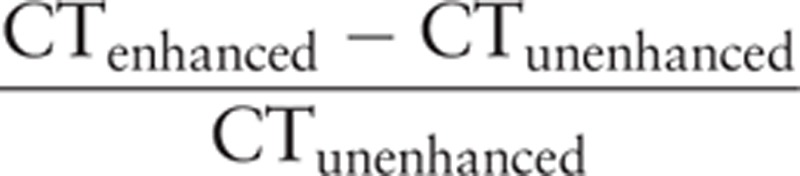 |
Figure 1.
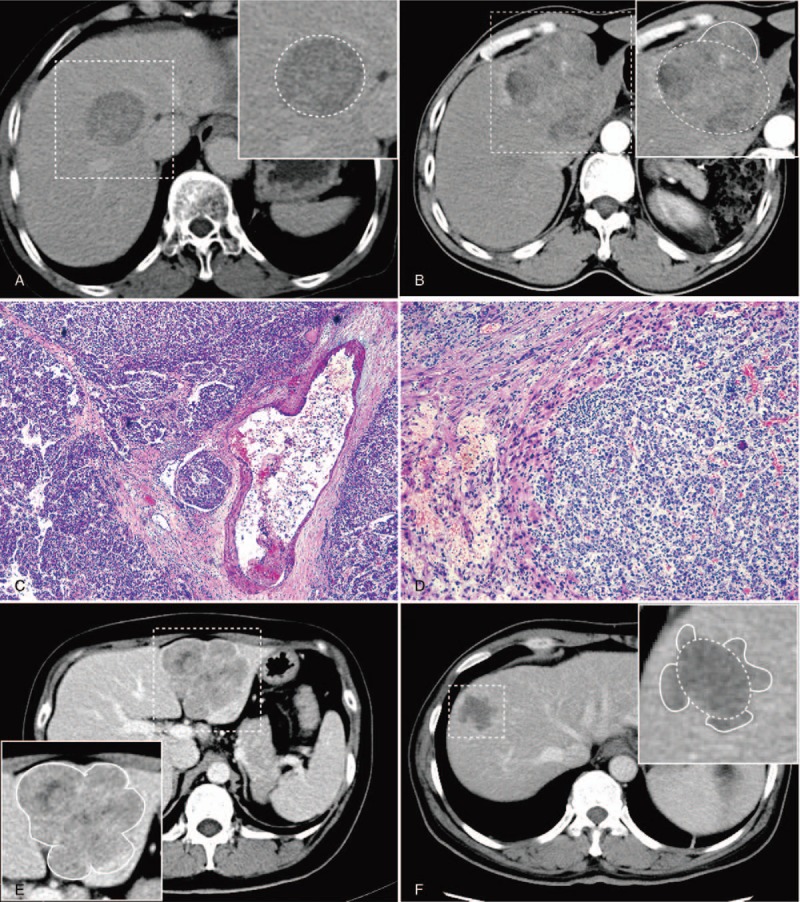
(A) 42-year-old male patient with solitary hepatocellular carcinoma at segments V and VIII; Portovenous phase computed tomography (CT) showing smooth margin (dashed box). (B) 55-year-old male patient with solitary hepatocellular carcinoma at left lobe; arterial phase CT showing focal extranodular type (dashed box). (C) Photomicrograph (hematoxylin-eosin staining, ×50) of nodular showing microvascular invasion. (D) Photomicrograph (hematoxylin-eosin staining, ×100) of nodular, nonsmooth tumor margins rich with tumor angiogenesis. (E) 37-year-old female patient; portovenous phase CT showing contiguous multinodular type at segments II and III (dashed box). (F) 51-year-old male patient; portovenous phase CT showing multinodular type at segments V and VIII (dashed box).
Figure 2.
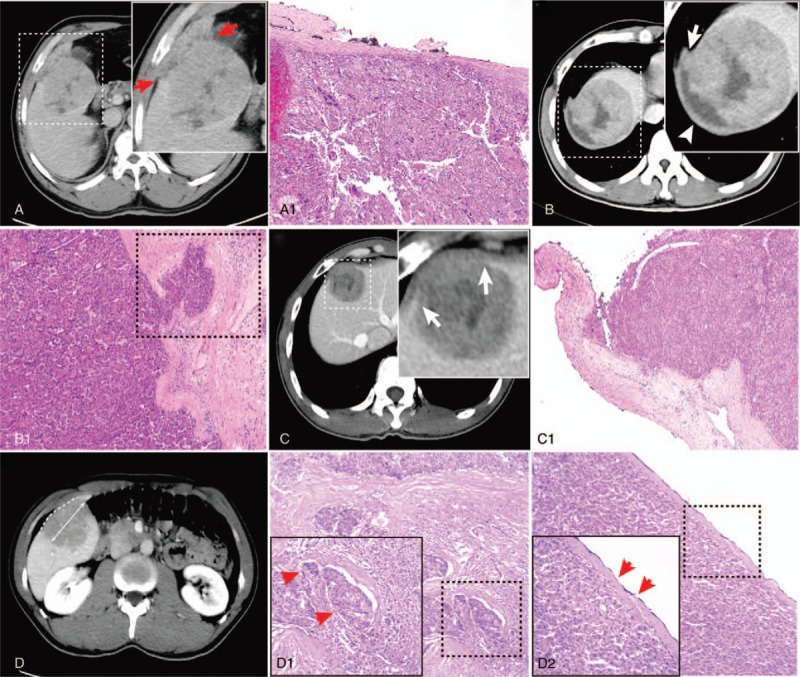
(A) 50-year-old male patient; portovenous phase-computed tomography (CT) showing tumor growth beyond the liver contour at segment V (arrows); (A-1) Photomicrograph showing liver capsule interruption. (B) 37-year-old male patient; portovenous phase CT showing liver capsular thickening (arrowhead) and subcapsular effusion; (B-1) photomicrograph showing liver capsule thickening and hepatic capsular invasion (dotted line). (C) 38-year-old male patient; portovenous phase CT showing smooth margins and liver capsule interruption (arrows); (C-1) Photomicrograph showing liver capsule interruption. (D) 35-year-old male patient; measurement of arch distance to maximum tumor diameter ratio on equilibrium phase CT. The single image that provided the maximum tumor diameter was used for analysis (actual line). The arch distance was drawn freehand and measured on the same image (dotted line). The arch distance to maximum tumor diameter ratio was then calculated; (D-1) photomicrograph showing microvascular invasion (dotted line; arrows); (D-2) photomicrograph showing hepatic capsular invasion (dotted line; arrows).
All imaging features were examined in consensus by 2 radiologists with 21 and 23 years of experience in reading liver CTs. A third radiologist joined the consensus conference when needed to resolve disagreements in assessments.
2.4. Histopathology
A pathologist with 8 years of experience interpreted and confirmed the pathology diagnoses using hematoxylin-eosin staining and immunohistochemistry. MVI was defined as tumor cell invasion into the portal vein, hepatic vein, or a large capsular vessel of the surrounding hepatic tissue that was partially or totally lined by endothelial cells visible only by microscopy.[25]
2.5. Data analysis
Statistical analysis was performed using SPSS 16.0 statistical software (SPSS, Chicago, IL). An independent t-test was used to compare tumor size, arterial phase-enhanced ratio, portovenous phase-enhanced ratio, and serum AFP level between MVI-positive and MVI-negative groups. Using a P value of less than 0.05, receiver operator characteristic (ROC) curve analyses were used to determine the optimal cutoff value and diagnostic performance. The χ2 test was used to compare tumor margins, necrosis, peritumoral enhancement, and radiographic hepatic capsular invasion between MVI-positive and MVI-negative groups. Tumor size and capsule were analyzed by the Mann–Whitney U test. The Hosmer–Lemeshow test was performed to assess the goodness of fit of the logistic regression model. A P value less than 0.05 was considered statistically significant.
3. Results
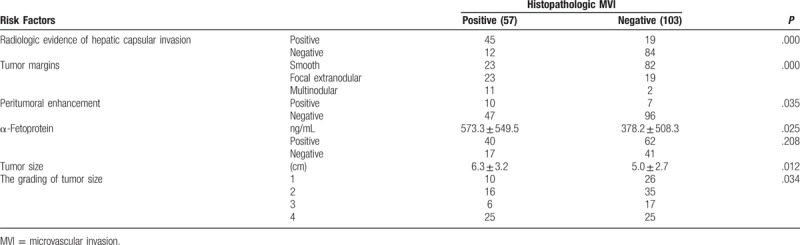
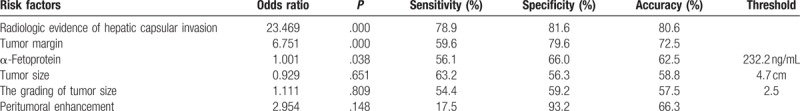
Quantitative and qualitative findings are summarized in Table 1. Of 160 specimens (57 MVI-positive and 103 MVI-negative), there were no differences between the MVI-positive and MVI-negative groups on univariate analysis with respect to arterial phase-enhanced ratio (P = 0.358), portovenous phase-enhanced ratio (P = 0.745), or necrois (χ2 = 3.298, P = .069). However, the MVI-positive and MVI-negative groups did differ with respect to tumor grade (Z = −2.114, P = .034), size (P = .012), serum AFP level (P = .025), peritumoral enhancement (χ2 = 4.464, P = .035), tumor margins (χ2 = 25.073, P < .001), and hepatic capsular invasion (χ2 = 55.963, P < .001). The tumor capsule did not differ between the 2 groups when analyzed with the Mann–Whitney U test (Z = −1.793, P = .073). Multiple logistic regression analysis showed that capsular invasion (odds ratio [OR] = 23.469, P < .001), margins (OR = 6.751, P < .001), and serum AFP level were associated with MVI (OR = 1.001, P = .038). The Hosmer–Lemeshow goodness-of-fit χ2 value was 10.914 (P = 0.207).
Table 1.
Quantitative measurements and qualitative findings identify hepatocellular carcinoma with MVI.
In retrospective cohort, 30 cases were located in the intrahepatic liver parenchyma. The 93 cases are applicable to identify hepatic capsular invasion by arch distance to maximum tumor diameter ratio. The presence of arch distance to maximum tumor diameter ratio on CT had a sensitivity of 71.1%, a specificity of 90.9%, and an accuracy rate of 82.8% in predicting histopathological evidence of hepatic capsular invasion.
Of 64 specimens with radiographic signs of hepatic capsular invasion, 5 were observed to have no histopathological evidence of hepatic capsular invasion. Conversely, of 96 specimens with no radiographic evidence of hepatic capsular invasion, 11 did present histopathologically observed hepatic capsular invasion. Of 57 HCC specimens with histopathological evidence of MVI, 45 also had radiographic evidence of hepatic capsular invasion. The presence of hepatic capsular invasion on CT had a sensitivity of 78.9%, a specificity of 81.6%, and an accuracy rate of 80.6% in predicting MVI.
Of the 57 HCCs with MVI, 23 were focal extranodular type, 11 were multinodular type, and 23 had smooth margins. Of the 103 HCCs without MVI, 19 were of the focal extranodular type, 2 were of the multinodular type, and 82 had smooth margins. The optimal sensitivity, specificity, and accuracy rate of tumor margins to predict MVI were 59.6%, 79.6%, and 72.5%, respectively. AFP level was higher in patients with MVI-positive than MVI-negative tumors (573.3 ± 549.5 vs 378.2 ± 508.3 ng/mL; P = .025).
Based on ROC analysis, the area under the ROC curve for using AFP to predict MVI was 0.58. Using the Youden index maximum value, the optimum AFP threshold was calculated as 232.2 ng/mL. Sensitivity, specificity, and accuracy rate values for AFP to predict MVI were 56.1%, 66.0%, and 62.5%, respectively (Table 2).
Table 2.
Multivariate logistic regression analysis and diagnostic performance of risk factors in predicting microvascular invasion.
4. Discussion
In this study, we conducted a retrospective evaluation of the association between radiographic hepatic capsular invasion and histopathological evidence of MVI. Multiphase CT was useful for identifying hepatic capsular invasion and predicting MVI in HCC. Multiple logistic regression analysis also showed that radiographic hepatic capsular invasion was an independent predictor of MVI, with a high associated OR value of 23.469 (P < .001). Because we did not exclude any tumors based on location, the actual accuracy of our new method to predict MVI in HCC may be higher than what was found in the present study. To our knowledge, the radiographic hepatic capsule invasion was defined and verified for the first time, and there are no other reports of using radiographic hepatic capsular invasion to identify MVI. In a follow-up study, we will aim to identify the predictive efficiency of MVI in HCC using multiphasic CT. Specifically, hepatic capsular invasion and arch distance to maximum tumor diameter ratio will be studied.
Nodular HCC, which can be subclassified into single nodular type, single nodular type with extranodular growth, or contiguous multinodular type,[23] is highly correlated with microscopic clinical features and prognosis. Nonsmooth tumor margins are associated with increased MVI risk.[8,17,26–28] Our retrospective study provided a detailed evaluation of tumor margin pathology to show that nodular borders of nonsmooth tumors are rich in pathological vessels, which suggests the presence of MVI. Nonsmooth tumor margins identified by CT were found to be directly associated with MVI, as has been shown previously.[8,17,26–28] However, the 59.6% sensitivity of tumor margins for predicting MVI in our study was much lower than expected.
The univariate and multivariate analysis findings showing that serum AFP level was a significant risk factor for MVI were consistent with previous reports.[2,18,19,29] However, we found a threshold value of 232.3 ng/mL in our study. Although serum AFP level and MVI were associated, the sensitivity (56.1%) and specificity (66.0%) for predicting MVI were low. Therefore, one should note that serum AFP level had low power to predict MVI.
Interestingly, univariate analysis showed that peritumoral enhancement, tumor size, and grading of tumor size helped predict MVI, but multivariate analysis did not confirm this association. Although peritumoral enhancement appeared to suggest increased MVI risk,[30,31] the sensitivity was only 17.5%. Chou et al[8] previously reported that peritumoral enhancement was not associated with MVI. Like other researchers,[2,8,21,30–34] we found that tumor size and grade may predict poor HCC prognosis for tumors that are 5 cm or larger. Zheng et al[33] reported that quantitative image analysis can be a preoperative predictor of MVI among HCC tumors both <5 and >5 cm, with angle co-occurrence matrix and local binary patterns, respectively. In stratified analyses, they found that higher serum AFP level, larger tumor size, and viral hepatitis history were associated with MVI among tumors >5 cm. The serum AFP level and tumor size were not associated with MVI among tumors <5 cm. They thought that the rate of MVI was relatively low among tumors <5 cm.[33] Larger tumor size appears more heterogeneity and poor cell differentiation.[34]
This study has several limitations. First, we focused on radiographic hepatic capsular invasion rather than pathological hepatic capsular invasion to determine MVI in HCC because pathology diagnoses cannot be made preoperatively. A future study will be conducted to further investigate the relationship between hepatic capsular invasion detected by multiphasic CT and pathology. Second, the arch distance-to-maximum tumor diameter ratio calculation was based on a small sample size in this study. A follow-up study will be performed to confirm the correlation between hepatic capsular invasion and arch distance-to-maximum tumor diameter ratio. A third potential limitation is the use of CT, which is commonly used for HCC evaluation, but may be inferior to MRI.
In conclusion, the presence of radiographic hepatic capsular invasion and nonsmooth tumor margins on preoperative multiphasic CT, along with serum AFP level greater than 232.2 ng/mL, were found to be important predictors of MVI. Radiographic evidence of hepatic capsular invasion appears to be the most important predictor. The treatment plan for patients with evidence of radiographic hepatic capsular invasion on preoperative CT should be made in consideration of the need for LT or extensive surgery.
Author contributions
Conceptualization: Danke Su.
Data curation: Wei Zhang, Lijuan Liu, Peng Wang, Lili Wang, Lidong Liu.
Formal analysis: Lijuan Liu, Peng Wang, Lili Wang, Jie Chen.
Funding acquisition: Wei Zhang, Danke Su.
Investigation: Lijuan Liu, Peng Wang, Lili Wang.
Methodology: Wei Zhang, Jie Chen.
Project administration: Danke Su.
Software: Lijuan Liu, Peng Wang.
Supervision: Jie Chen, Danke Su.
Validation: Wei Zhang, Jie Chen, Danke Su.
Visualization: Jie Chen.
Writing – original draft: Wei Zhang.
Writing – review & editing: Wei Zhang, Danke Su.
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, AFP = α-fetoprotein, CT = computed tomography, HCC = hepatocellular carcinoma, LT = liver transplantation, MVI = microvascular invasion, OR = odds ratio, ROC = receiver operator characteristic.
WZ, LJL, PW, and LLW have contributed equally to this article.
Funding/support: This work was supported by Innovation Project of Guangxi Graduate Education (grant no. YCBZ2017042) and International Communication of Guangxi Medical University Graduate Education (2017), for which the authors are grateful. We also are very grateful to the editor and reviewers.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. [DOI] [PubMed] [Google Scholar]
- [2].Banerjee S, Wang DS, Kim HJ, et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology 2015;62:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang W, Lai SL, Chen J, et al. Validated preoperative computed tomography risk estimation for postoperative hepatocellular carcinoma recurrence. World J Gastroenterol 2017;23:6467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim BK, Han KH, Park YN, et al. Prediction of microvascular invasion before curative resection of hepatocellular carcinoma. J Surg Oncol 2008;97:246–52. [DOI] [PubMed] [Google Scholar]
- [5].Torbenson M, Schirmacher P. Liver cancer biopsy—back to the future?!. Hepatology 2015;61:431–3. [DOI] [PubMed] [Google Scholar]
- [6].Sherman M, Bruix J. Biopsy for liver cancer: how to balance research needs with evidence-based clinical practice. Hepatology 2015;61:433–6. [DOI] [PubMed] [Google Scholar]
- [7].Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg 2016;151:356–63. [DOI] [PubMed] [Google Scholar]
- [8].Chou CT, Chen RC, Lin WC, et al. Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol 2014;203:W253–9. [DOI] [PubMed] [Google Scholar]
- [9].Wu D, Tan M, Zhou M, et al. Liver computed tomographic perfusion in the assessment of microvascular invasion in patients with small hepatocellular carcinoma. Invest Radiol 2015;50:188–94. [DOI] [PubMed] [Google Scholar]
- [10].Kobayashi T, Aikata H, Honda F, et al. Preoperative fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography for prediction of microvascular invasion in small hepatocellular carcinoma. J Comput Assist Tomogr 2016;40:524–30. [DOI] [PubMed] [Google Scholar]
- [11].Kim KA, Kim MJ, Jeon HM, et al. Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodium-enhanced hepatobiliary phase images. J Magn Reson Imaging 2012;35:629–34. [DOI] [PubMed] [Google Scholar]
- [12].Ariizumi S, Kitagawa K, Kotera Y, et al. A non-smooth tumor margin in the hepatobiliary phase of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatectomy in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2011;18:575–85. [DOI] [PubMed] [Google Scholar]
- [13].Huang YQ, Liang HY, Yang ZX, et al. Value of MR histogram analyses for prediction of microvascular invasion of hepatocellular carcinoma. Medicine (Baltimore) 2016;95:e4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu P, Zeng M, Liu K, et al. Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion-weighted imaging? J Gastroenterol Hepatol 2014;29:330–6. [DOI] [PubMed] [Google Scholar]
- [15].Suh YJ, Kim MJ, Choi JY, et al. Preoperative prediction of the microvascular invasion of hepatocellular carcinoma with diffusion-weighted imaging. Liver Transpl 2012;18:1171–8. [DOI] [PubMed] [Google Scholar]
- [16].McHugh PP, Gilbert J, Vera S, et al. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB (Oxford) 2010;12:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eguchi S, Takatsuki M, Hidaka M, et al. Predictor for histological microvascular invasion of hepatocellular carcinoma: a lesson from 229 consecutive cases of curative liver resection. World J Surg 2010;34:1034–8. [DOI] [PubMed] [Google Scholar]
- [18].Duffy JP, Vardanian A, Benjamin E, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg 2007;246:502–9. Discussion 09–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Arrieta O, Cacho B, Morales-Espinosa D, et al. The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer 2007;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tsai TJ, Chau GY, Lui WY, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery 2000;127:603–8. [DOI] [PubMed] [Google Scholar]
- [21].Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005;11:1086–92. [DOI] [PubMed] [Google Scholar]
- [22].Nakashima Y, Nakashima O, Tanaka M, et al. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res 2003;26:142–7. [DOI] [PubMed] [Google Scholar]
- [23].Kanai T, Hirohashi S, Upton MP, et al. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer 1987;60:810–9. [DOI] [PubMed] [Google Scholar]
- [24].Imai K, Minamiya Y, Ishiyama K, et al. Use of CT to evaluate pleural invasion in non-small cell lung cancer: measurement of the ratio of the interface between tumor and neighboring structures to maximum tumor diameter. Radiology 2013;267:619–26. [DOI] [PubMed] [Google Scholar]
- [25].Rodriguez-Peralvarez M, Luong TV, Andreana L, et al. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol 2013;20:325–39. [DOI] [PubMed] [Google Scholar]
- [26].Sumie S, Kuromatsu R, Okuda K, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol 2008;15:1375–82. [DOI] [PubMed] [Google Scholar]
- [27].Nagano Y, Shimada H, Takeda K, et al. Predictive factors of microvascular invasion in patients with hepatocellular carcinoma larger than 5 cm. World J Surg 2008;32:2218–22. [DOI] [PubMed] [Google Scholar]
- [28].Hui AM, Takayama T, Sano K, et al. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol 2000;33:975–9. [DOI] [PubMed] [Google Scholar]
- [29].Dumitra TC, Dumitra S, Metrakos PP, et al. Pretransplantation alpha-fetoprotein slope and Milan criteria: strong predictors of hepatocellular carcinoma recurrence after transplantation. Transplantation 2013;95:228–33. [DOI] [PubMed] [Google Scholar]
- [30].Kim H, Park MS, Choi JY, et al. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol 2009;19:1744–51. [DOI] [PubMed] [Google Scholar]
- [31].Nishie A, Yoshimitsu K, Asayama Y, et al. Radiologic detectability of minute portal venous invasion in hepatocellular carcinoma. AJR Am J Roentgenol 2008;190:81–7. [DOI] [PubMed] [Google Scholar]
- [32].Kaibori M, Ishizaki M, Matsui K, et al. Predictors of microvascular invasion before hepatectomy for hepatocellular carcinoma. J Surg Oncol 2010;102:462–8. [DOI] [PubMed] [Google Scholar]
- [33].Zheng J, Chakraborty J, Chapman WC, et al. Preoperative prediction of microvascular invasion in hepatocellular carcinoma using quantitative image analysis. J Am Coll Surg 2017;225:778–88. e771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Davnall F, Yip CS, Ljungqvist G, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 2012;3:573–89. [DOI] [PMC free article] [PubMed] [Google Scholar]


