Abstract
An increased vascular risk is present in patients with ankylosing spondylitis (AS). In this report, we evaluate the presence and grade of atherosclerosis in patients with AS, uninterruptedly treated with tumor necrosis factor-α (TNF-α) antagonists for 2 years, in comparison to that in a nontreated group of healthy controls.
Fourteen patients with AS and 14 healthy controls underwent carotid sonography to measure intima-media thickness (IMT) and to evaluate the presence of plaque. Bath Ankylosing Spondylitis Disease Activity Index, Bath Ankylosing Spondylitis Metrology Index, Bath Ankylosing Spondylitis Functional Index scores, erythrocyte sedimentation rate, C-reactive protein, glycemia, total cholesterol, and triglyceride levels were also recorded.
Patients with AS showed significantly lower values of mean and maximum IMT at the level of the common carotid (P = .02 and .04, respectively) and the carotid bulb (P = .0006 and .0005, respectively) compared to those of healthy controls. They also had a number of carotid plaques significantly lower than that of healthy controls (P = .02). No differences were found in IMT values at the level of internal carotid between the 2 populations.
The significantly lower carotid atherosclerosis found in patients with AS treated with TNF antagonists than in healthy controls shows the important complementary role of this treatment in reducing vascular disease progression probably by decreasing inflammation.
Keywords: ankylosing spondylitis, carotid sonography, intima-media thickness, tumor necrosis factor-α antagonists
1. Introduction
The ankylosing spondylitis (AS) is a chronic arthritis that causes inflammation in several areas of the body. It has long been recognized that chronic inflammatory systemic diseases are associated with a major risk of cardiovascular diseases[1–4] but conflicting results are emerged in the literature about the actual role that AS plays. Indeed, atherosclerosis and endothelial dysfunction, identified in the form of increased carotid intima-media thickness (IMT), presence of carotid plaques, and altered values of flow-mediated vasodilation or pulse-wave velocity are increased in patients with AS according to some authors.[5–9] AS is also associated with an increased risk of atherosclerosis independent of traditional vascular risk factors[10] and of myocardial infarction and stroke[11]; furthermore, the presence of an increased inflammation and the impairment of endothelial function seem to play a crucial role in accelerating the development of atherosclerosis.[12] On the contrary, no difference in atherosclerosis has been found in patients with AS compared to general population, according to other authors.[13,14] The uncertainty about the association between AS and an increased subclinical atherosclerosis remains also in patients who underwent tumor necrosis factor-α (TNF-α) antagonists treatment. According to some authors, subclinical atherosclerosis in patients with AS treated with TNF-α antagonists is higher than in controls,[15] whereas others have observed a slowdown of the progression of atherosclerosis in patients with AS treated with TNF-α antagonists.[16,17] For several years, carotid IMT and endothelial dysfunction have been considered a proper marker of atherosclerotic progression[18–20] whose noninvasively measurement can be excellently made by means of B-mode ultrasonography.[21–23] Recently, it was emphasized the importance of the use of carotid sonography in the assessment of cardiovascular risk in patients with AS.[24]
Since poor information is present on carotid atherosclerosis in patients with AS treated with TNF-α antagonists, the aim of this study is to evaluate it in these patients comparing the results with those obtained in a nontreated group of healthy controls.
2. Materials and methods
2.1. Patients and healthy controls
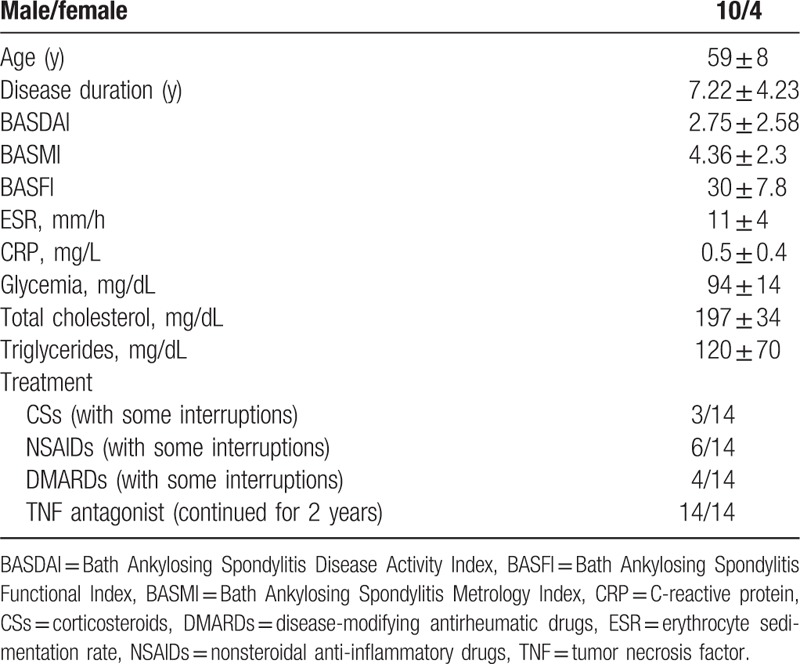
Fourteen patients with AS (10 males, 4 females; median age 59 ± 8 years) diagnosed, according to the Assessment of Spondylo Arthritis Society (ASAS) criteria[25] and already treated for 2 years with TNF-α antagonists according the ASAS guidelines,[26] were studied. Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), and Bath Ankylosing Spondylitis Metrology Index (BASMI) scores were also calculated through a questionnaire and the physical examination.
Table 1 shows the clinical data and activity indexes of these patients.
Table 1.
Main clinical and demographic features of 14 patients with ankylosing spondylitis.
Fourteen healthy individuals without any rheumatic diseases represented the nontreated control group (6 males, 8 females; median age 62 ± 7 years). Patients under 18 years of age and those with a known history of diabetes were excluded from the study.
Body mass index (BMI) was calculated as the ratio of weight (kg) to height (m) squared (kg/m2).
Standard commercial kits were employed by the analysis laboratory of our university to measure glycemia, total cholesterol and triglycerides, erythrocyte sedimentation rate, and C-reactive protein. All clinical and biochemical assessments were performed at the time of the recruitment.
2.2. Sonography
B-mode Doppler ultrasonography was performed by an expert investigator with more than 15 years of experience on this field, using a Toshiba Aplio 500 platinum sonographic machine (Toshiba Medical Systems Corporation, Otawara, Tochigi-ken, Japan), equipped with a high definition linear multifrequency probe (7–15 MHz) and with an automated edge-tracking package for IMT analysis. Each patient was examined in the morning while lying in the supine position with the outstretched neck resting on a soft cushion. The investigator was blinded to the clinical condition of the patients. Tissue harmonics imaging modality was adopted to improve visualization and details of the borders; the entire carotid artery was visualized from multiple angles in longitudinal and transverse scan before selecting the best view and obtain an image with the vessel in a longitudinal plane to measure the IMT from the lumen-intima border to the media-adventitia border. These measurements were made at the near and the far wall of the distal common carotid (1 cm proximal to the carotid bulb), of the carotid bulb, and of the internal carotid in both sides, specifically excluding plaques and selectively measuring IMT in a plaque-free region. To minimize measurement error and make a faster assessment of subclinical atherosclerosis, the automatic IMT measure modality of the echographic machine was used. Each image was immediately reviewed to ensure optimal visualization and exclude improper detection of the lumen-intima border and the media-adventitia borders. Both the mean IMT and the maximum IMT values, obtained in all locations from the right and the left side of the carotid, were recorded and separately averaged to obtain the respective mean values suitable for the statistical comparison between the 2 classes of patients following the European Stroke Conferences, Mannheim advices.[27]
We also quantified the presence or absence of plaque in one of the anterior or posterior segment of common, bulb or internal carotid, in both populations. The presence of atheromatous plaques was defined as an IMT of more than 1.5 mm or an increased thickness exceeding into the carotid lumen of the 50% of the surrounding IMT value.[27] Sonographic plaque characteristics (echogenicity, border, and morphology) were also recorded. Finally, the percentage of stenosis was quantified as the ratio between the narrowed diameter of the residual lumen and the luminal diameter.[28]
All the data were collected in an Excel Database.
2.3. Statistical analysis
Microsoft Excel 2013 for Windows was used to perform statistical examination. The intraclass correlation coefficient (ICC) was used for assessing intraoperator repeatability. The 2-tailed unpaired t test was used to compare laboratory results and the mean and highest IMT values of patients with AS and healthy controls. The Chi-squared test of homogeneity was used to evaluate whether the frequency count of carotid plaques was distributed identically across the 2 populations.
A p-value <.05 was considered statistically significant.
2.4. Ethical standard
The institutional ethic committee approved the study; the written informed consent was obtained by all participants in the study.
3. Results
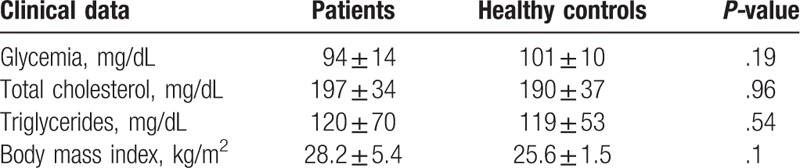
The clinical characteristics of the patients with AS are described in Table 1. There were no significant differences in the glycemia, total cholesterol, and triglyceride values between patients with AS and healthy nontreated controls (Table 2). Three healthy controls were normal weight, whereas the others were overweight; 4 patients with AS were normal weight, 2 were class II obese, and the others were overweight.
Table 2.
Biochemical characteristics of the study population.
Six of 14 healthy controls and 5 of 14 patients with AS were smoker. Five healthy controls and 5 patients with AS had a history of arterial hypertension and were treated with antihypertensive agents.
The sonographer performed a periodic control of the quality of the carotid IMT measurement to ensure repeatability and precision of the examination and the interobserver variability was good (ICC 0.9).
B-mode sonographic examination showed that patients with AS had significant lower mean and maximum IMT values, both at the level of the common carotid and of the bulb, in comparison with those of healthy controls (Table 3, Figs. 1 and 2). No significant differences were observed in mean and maximum IMT values on internal carotid between patients with AS and healthy controls (Table 3).
Table 3.
Differences in carotid IMT values among patients with AS treated with anti-tumor necrosis factor therapy and healthy controls.
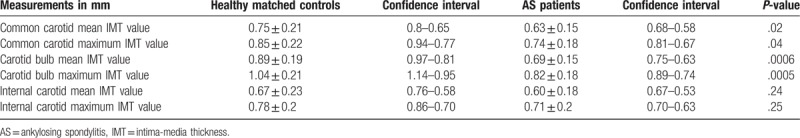
Figure 1.
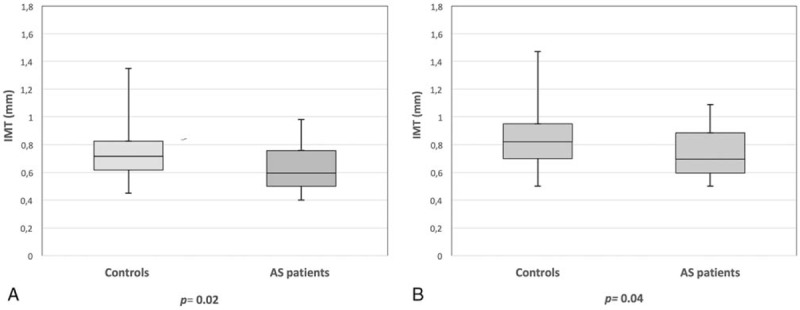
(A) Box plots of the mean intima-media thickness (IMT) of the common carotid artery in patients with ankylosing spondylitis (AS) treated with tumor necrosis factor-α (TNF-α) antagonists and in healthy controls. (B) Box plots of the maximum IMT of the common carotid artery in patients with AS treated with TNF-α antagonists and in healthy controls.
Figure 2.
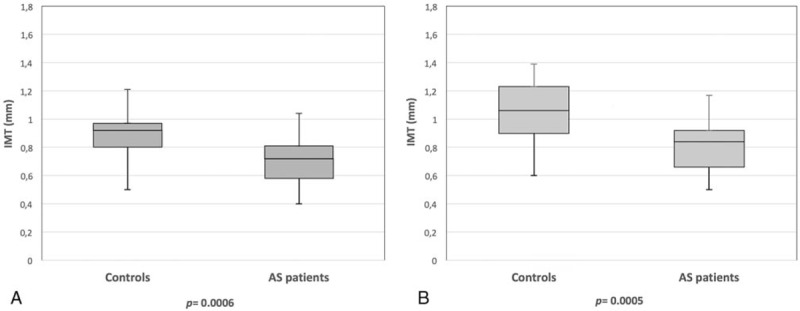
(A) Box plots of the mean intima-media thickness (IMT) of the carotid bulb in patients with ankylosing spondylitis (AS) treated with tumor necrosis factor-α (TNF-α) antagonists and in healthy controls. (B) Box plots of the maximum IMT of the carotid bulb in patients with AS treated with TNF-α antagonists and in healthy controls.
A significantly lower number of carotid plaques was observed in patients with AS than in healthy controls (P = .02). In both populations, no plaque reached a percentage of stenosis higher than 30% and no vulnerable plaques were observed.
4. Discussion
Our study designed to investigate whether there were differences in atherosclerosis between patients with AS treated with TNF-α antagonists without interruption for 2 years and nontreated healthy controls showed a significantly lower carotid atherosclerosis in patients with AS than in healthy controls. This result is corroborated by the fact that the study took into account both the IMT measurements and plaque presence, thus avoiding misclassification of cardiovascular disease risk. The result is very interesting because the 2 populations were overlapping as regards the presence of traditional cardiovascular risk factors (hypertension, hypercholesterolemia, diabetes, smoking, and BMI) and this seems to confirm that the administration of anti-TNF therapy may have vascular beneficial effects slowing the atherosclerosis progression. Why only in internal carotid there were no differences between the 2 populations is difficult to explain. Complex mechanisms underlie the IMT of the vessels and several mechanisms are implicated in the start and progression of atherosclerotic process in each vascular district. The geometry of the vessel, especially the presence of a large curvature and planarity, is certainly one of those mechanisms, having a great role in favoring the IMT growth, due to the complex and oscillatory transverse shear stress on the vessel wall.[29–33] In inflammatory arthritis, white blood cells and proinflammatory cytokines, inducing and maintaining high inflammatory levels, may be considered the actual cause of the atherosclerotic progression. Indeed, while in patients with AS with low disease activity, subclinical atherosclerosis is not accelerated[14] due to the low levels of inflammation, all of this changes when the disease is of moderate/severe activity as it was the case in our patients with AS.
A meta-analysis highlighted that the level of evidence on the use of TNF-α antagonists was still too low to draw any conclusion, despite it provided vascular beneficial effects in inflammatory arthritis and prevented subclinical atherosclerosis and arterial stiffness.[34] However, the anti-inflammatory effect of TNF-α antagonists is undeniable, like has been seen in psoriatic patients in which these drugs provided a significant decrease in myocardial infarction risk.[35] An improvement of the endothelial function was the precise suggested mechanism through which TNF-α antagonists were able to reduce the level of atherosclerosis.[36] Indeed, the current knowledge claims that the TNF-α antagonists are able to lower retinol-binding protein 4 level, an agent of vascular oxidative damage, apart from reducing serum insulin levels and insulin resistance that, when dysregulated, promote endothelial dysfunction.[37,38]
Since AS is associated with an increased atherosclerosis and risk of myocardial infarction and stroke,[10,11] our belief is that no other reasons than the treatment with TNF-α antagonists might explain the significant lower levels of carotid atherosclerosis in patients with AS respect to those observed in healthy controls. Probably, this was also the consequence of a long-term treatment of patients with AS with these drugs, whereas, in other studies in which this was not evident, the treatment could have been discontinued or only done for a short period of time.
Limitations of this study are the small sample size and the fact that we had no patients without TNF-α antagonist treatment as proper controls. On the contrary, it would not have been ethically correct to perform a placebo controlled randomized trial with TNF inhibitors in patients with AS.
5. Conclusion
Unequivocal evidence is that the patients with AS treated with TNF-α antagonists would have a lower carotid atherosclerosis than that of matched healthy controls.
Therefore, the TNF-α antagonists in addition to the reduction of inflammatory arthritis have also the ability to slowdown the progression of the carotid atherosclerosis.
Author contributions
Conceptualization: Enrico Maria Zardi, Domenico Maria Zardi, Antonella Afeltra.
Data curation: Enrico Maria Zardi, Maria Elena Pipita, Domenico Lichinchi.
Formal analysis: Enrico Maria Zardi, Maria Elena Pipita, Chiara Giorgi, Domenico Lichinchi, Domenico Maria Zardi.
Investigation: Enrico Maria Zardi, Antonella Afeltra.
Methodology: Enrico Maria Zardi, Chiara Giorgi, Domenico Lichinchi, Domenico Maria Zardi.
Project administration: Antonella Afeltra.
Supervision: Enrico Maria Zardi, Chiara Giorgi, Domenico Lichinchi.
Validation: Enrico Maria Zardi, Maria Elena Pipita, Domenico Maria Zardi.
Visualization: Maria Elena Pipita, Antonella Afeltra.
Writing – original draft: Enrico Maria Zardi, Domenico Maria Zardi.
Writing – review & editing: Enrico Maria Zardi, Chiara Giorgi, Antonella Afeltra.
Footnotes
Abbreviations: AS = ankylosing spondylitis, ASAS = Assessment of Spondylo Arthritis Society, BASDAI = Bath Ankylosing Spondylitis Disease Activity Index, BASFI = Bath Ankylosing Spondylitis Functional Index, BASMI = Bath Ankylosing Spondylitis Metrology Index, BMI = body mass index, ICC = intraclass correlation coefficient, IMT = intima-media thickness, TNF = tumor necrosis factor.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Petri M, Perez-Gutthann S, Spence D, et al. Risk factors for coronary disease in patients with systemic lupus erythematosus. Am J Med 1992;93:513–9. [DOI] [PubMed] [Google Scholar]
- [2].Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am J Epidemiol 1997;145:408–15. [DOI] [PubMed] [Google Scholar]
- [3].Zardi EM, Sambataro G, Basta F, et al. Subclinical carotid atherosclerosis in elderly patients with primary Sjögren syndrome: a duplex Doppler sonographic study. Int J Immunopathol Pharmacol 2014;27:645–51. [DOI] [PubMed] [Google Scholar]
- [4].Zardi EM, Basta F, Afeltra A. Levels of Vitamin D, disease activity and subclinical atherosclerosis in post-menopausal women with Sjögren's syndrome: does a link exist? In Vivo 2016;30:721–5. [PubMed] [Google Scholar]
- [5].Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, et al. The high prevalence of subclinical atherosclerosis in patients with ankylosing spondylitis without clinically evident cardiovascular disease. Medicine (Baltimore) 2009;88:358–65. [DOI] [PubMed] [Google Scholar]
- [6].Bodnár N, Kerekes G, Seres I, et al. Assessment of subclinical vascular disease associated with ankylosing spondylitis. J Rheumatol 2011;38:723–9. [DOI] [PubMed] [Google Scholar]
- [7].Skare TL, Verceze GC, Oliveira AA, et al. Carotid intima-media thickness in spondyloarthritis patients. Sao Paulo Med J 2013;131:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gupta N, Saigal R, Goyal L, et al. Carotid intima media thickness as a marker of atherosclerosis in ankylosing spondylitis. Int J Rheumatol 2014;839135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Verma I, Krishan P, Syngle A. Predictors of atherosclerosis in ankylosing spondylitis. Rheumatol Ther 2015;2:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hamdi W, Chelli Bouaziz M, Zouch I, et al. Assessment of preclinical atherosclerosis in patients with ankylosing spondylitis. J Rheumatol 2012;39:322–6. [DOI] [PubMed] [Google Scholar]
- [11].Mathieu S, Pereira B, Soubrier M. Cardiovascular events in ankylosing spondylitis: an updated meta-analysis. Semin Arthritis Rheum 2015;44:551–5. [DOI] [PubMed] [Google Scholar]
- [12].Sari I, Okan T, Akar S, et al. Impaired endothelial function in patients with ankylosing spondylitis. Rheumatology (Oxford) 2006;45:283–6. [DOI] [PubMed] [Google Scholar]
- [13].Erre GL, Sanna P, Zinellu A, et al. Plasma asymmetric dimethylarginine (ADMA) levels and atherosclerotic disease in ankylosing spondylitis: a cross-sectional study. Clin Rheumatol 2011;30:21–7. [DOI] [PubMed] [Google Scholar]
- [14].Arida A, Protogerou AD, Konstantonis G, et al. Subclinical atherosclerosis is not accelerated in patients with ankylosing spondylitis with low disease activity: new data and metaanalysis of published studies. J Rheumatol 2015;42:2098–105. [DOI] [PubMed] [Google Scholar]
- [15].Mathieu S, Joly H, Baron G, et al. Trend towards increased arterial stiffness or intima-media thickness in ankylosing spondylitis patients without clinically evident cardiovascular disease. Rheumatology (Oxford) 2008;47:1203–7. [DOI] [PubMed] [Google Scholar]
- [16].van Sijl AM, van Eijk IC, Peters MJ, et al. Tumour necrosis factor blocking agents and progression of subclinical atherosclerosis in patients with ankylosing spondylitis. Ann Rheum Dis 2015;74:119–23. [DOI] [PubMed] [Google Scholar]
- [17].Tam LS, Shang Q, Kun EW, et al. The effects of golimumab on subclinical atherosclerosis and arterial stiffness in ankylosing spondylitis - a randomized, placebo-controlled pilot trial. Rheumatology (Oxford) 2014;53:1065–74. [DOI] [PubMed] [Google Scholar]
- [18].Persson J, Formgren J, Israelsson B, et al. Ultrasound-determined intima-media thickness and atherosclerosis. Direct and indirect validation. Arterioscler Thromb Vasc Biol 1994;14:261–4. [DOI] [PubMed] [Google Scholar]
- [19].Zardi EM, Afeltra A. How to predict subclinical atherosclerosis in systemic lupus erythematosus. Rheumatology (Oxford) 2011;50:821–3. [DOI] [PubMed] [Google Scholar]
- [20].Zardi EM, Afeltra A. Endothelial dysfunction and vascular stiffness in systemic lupus erythematosus: are they early markers of subclinical atherosclerosis? Autoimmun Rev 2010;9:684–6. [DOI] [PubMed] [Google Scholar]
- [21].Pignoli P, Tremoli E, Poli A, et al. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986;74:1399–406. [DOI] [PubMed] [Google Scholar]
- [22].Di Geso L, Zardi EM, Afeltra A, et al. Comparison between conventional and automated software-guided ultrasound assessment of bilateral common carotids intima-media thickness in patients with rheumatic diseases. Clin Rheumatol 2012;31:881–4. [DOI] [PubMed] [Google Scholar]
- [23].Zardi EM, Di Geso L, Afeltra A, et al. An ultrasound automated method for non-invasive assessment of carotid artery pulse wave velocity. J Invest Med 2017;pii: jim-2017-000430. [DOI] [PubMed] [Google Scholar]
- [24].Rueda-Gotor J, Llorca J, Corrales A, et al. Carotid ultrasound in the cardiovascular risk stratification of patients with ankylosing spondylitis: results of a population-based study. Clin Exp Rheumatol 2016;34:885–92. [PubMed] [Google Scholar]
- [25].Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- [26].Braun J, Davis J, Dougados M, et al. ASAS Working Group. First update of the international ASAS consensus statement for the use of anti-TNF agents in patients with ankylosing spondylitis. Ann Rheum Dis 2006;65:316–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis 2012;34:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fox AJ. How to measure carotid stenosis. Radiology 1993;186:316–8. [DOI] [PubMed] [Google Scholar]
- [29].Kornet L, Lambregts J, Hoeks AP, et al. Differences in near-wall shear rate in the carotid artery within subjects are associated with different intima-media thicknesses. Arterioscler Thromb Vasc Biol 1998;18:1877–84. [DOI] [PubMed] [Google Scholar]
- [30].Tanaka H, Dinenno FA, Monahan KD, et al. Carotid artery wall hypertrophy with age is related to local systolic blood pressure in healthy men. Arterioscler Thromb Vasc Biol 2001;21:82–7. [DOI] [PubMed] [Google Scholar]
- [31].Augst AD, Ariff B, McG Thom SA, et al. Analysis of complex flow and the relationship between blood pressure, wall shear stress, and intima-media thickness in the human carotid artery. Am J Physiol Heart Circ Physiol 2007;293:H1031–7. [DOI] [PubMed] [Google Scholar]
- [32].Zhang C, Xie S, Li S, et al. Flow patterns and wall shear stress distribution in human internal carotid arteries: the geometric effect on the risk for stenoses. J Biomech 2012;45:83–9. [DOI] [PubMed] [Google Scholar]
- [33].Mohamied Y, Rowland EM, Bailey EL, et al. Change of direction in the biomechanics of atherosclerosis. Ann Biomed Eng 2015;43:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tam LS, Kitas GD, Gonzalez-Gay MA. Can suppression of inflammation by anti-TNF prevent progression of subclinical atherosclerosis in inflammatory arthritis? Rheumatology (Oxford) 2014;53:1108–19. [DOI] [PubMed] [Google Scholar]
- [35].Armstrong AW. Do TNF inhibitors reduce the risk of myocardial infarction in psoriasis patients? JAMA 2013;309:2043–4. [DOI] [PubMed] [Google Scholar]
- [36].Brezinski EA, Follansbee MR, Armstrong EJ, et al. Endothelial dysfunction and the effects of TNF inhibitors on the endothelium in psoriasis and psoriatic arthritis: a systematic review. Curr Pharm Des 2014;20:513–28. [DOI] [PubMed] [Google Scholar]
- [37].Genre F, López-Mejías R, Miranda-Filloy JA, et al. Antitumour necrosis factor α treatment reduces retinol-binding protein 4 serum levels in non-diabetic ankylosing spondylitis patients. Ann Rheum Dis 2014;73:941–3. [DOI] [PubMed] [Google Scholar]
- [38].Genre F, López-Mejías R, Miranda-Filloy JA, et al. Adipokines, biomarkers of endothelial activation, and metabolic syndrome in patients with ankylosing spondylitis. Biomed Res Int 2014;2014:860651. [DOI] [PMC free article] [PubMed] [Google Scholar]


