Abstract
To analyze the impact of perigastric lipolymphatic tissue grouping by the surgeon on the number of pathologic sampled lymph nodes and to explore the appropriate lymph node delivery process.
The authors collected the medical records of gastric cancer patients who were hospitalized in Wuhan Union Hospital during the period January 2016 to January 2018. The authors selected 126 patients and divided them into experimental group and control group, 63 cases in each group. Samples of standard complete gastrectomy or distal gastrectomy +D2 lymph node dissection was performed. In experimental group, the fresh en bloc specimen was treated by the surgeon before the formalin fixation. The perigastric lipolymphatic tissue was divided into the lymph node grouping according to JSGC guideline III. Then the stomach and each group of lipolymphatic tissue were fixed and then transferred to the pathologic department, then the lymph nodes were harvested by the pathological technician. In control group, the whole en bloc specimen was fixed with formalin and then lymph nodes were detected by palpation and thin slice inspection, and then harvested by the pathological technician. The lymph node acquisition was compared in 2 groups.
The total number of lymph nodes in experimental group is 2611, the number of negative lymph nodes is 2273; the total number of lymph nodes in control group is 1643, the number of negative lymph nodes is 1351; the comparison difference in 2 groups was statistical sense (P < .01); patients with lymph node which reach 25 pieces/person of experimental group could reach a ratio of 90.1%, and that is 47.6% in the control group, the comparison difference in 2 groups was statistical sense (P < .01), the number of positive lymph nodes did not increase significantly compared with the control group, and there was no statistical significance in the 2 groups.
Dissecting the perigastric lipolymphatic tissue into lymph node groups by the surgeon might improve the total number of lymph node harvested by the pathological technician, and increase the rate of cases with >25 lymph nodes. Our results also implicated that, when the routing harvested lymph nodes were more than 20, the increasing number by perigastric lipolymphatic tissue grouping might result from more negative lymph nodes detected and might not result in stage migrating.
Keywords: lymph node, radical gastrectomy, subarea examination, subarea marker
1. Introduction
Gastric cancer is 1 of the 10 most common malignant tumors in China, of which the incidence of this disease is the highest in the digestive tract cancer. Although its incidence of this disease is decreasing, the mortality rate is still high.[1] The metastasis is mainly through the lymphatic pathway, and even in early gastric cancer, the lymph node metastasis rate can reach 8% to 15%.[2] For gastric cancer, operation is still the only possible cure. Local lesion excision and regional lymph node dissection are still the standard surgical procedures for the current gastric cancer operation. It is generally accepted that the number of positive lymph nodes (NPLN) is an important basis for clinical staging of malignant tumors and the important criterion to reflect radical operation, and it is also the precondition for clinicians to stage the clinical stage of malignant tumor, the results directly affect the selection of treatment options for malignant tumors. The negative nodes number was not indexed in the staging system but it had prognosis significance, therefore should be sorted out as many as possible. Surgical removal of metastatic lymph nodes is a good treatment for malignant tumors. It has important significance for preventing postoperative recurrence and improving the survival rate of patients. However, in China, because of the large population base, the operation of gastric cancer is relatively large, and few clinicians of most hospitals involve in the lymph node sorting work while the lymph node sorting work is mainly finished by the professional and technical personnel by touching and slice inspection, but it is difficult to realize the fine sorting due to the limited technical level and workload factors, which lead to affecting the number of lymph nodes detected, it fails to realize the accurate staging for patients, and also may affect the treatment strategies of patients after operation.
In the pathological department of our hospital, the average lymph node number of distal partial gastrectomy with D2 lymphadenectomy was 22 in year 2013. The total lymph node number (TLNN) is much higher than the lower limit for an N staging, but lower than the average in Japan, by different N stages. We noticed when we participate the CLASS01 that when surgeon grouped the perigastric lipolymphatic tissue, the average lymph nodes were higher. At present, there is no uniform definition of lymph node detection in gastric cancer patients, nor a systematic detection procedure. Thereafter, we conducted this retrospective paired study to explore if the perigastric lipolymphatic tissue grouping by surgeon interfered the lymph nodes number harvested by pathological technician, and to explore the rational flow of lymph node detection, which can provide reference for the development of related research.
2. Materials and methods
2.1. Baseline data
We collected the medical records of gastric cancer patients who were hospitalized in Wuhan Union Hospital for gastrointestinal surgery from December 2016 to December 2017 and met the inclusion criteria and exclusion criteria. The patients who were involved in the sorting perigastric lipolymphatic tissue grouping by the surgeon were set up as the experimental group (63 cases); we selected the same sex patients who were hospitalized in the same period, similar condition, similar age, the same operation method, and the patients who were directly selected by pathological technicians after operation were allocated to the control group (63 cases). Inclusion criteria: aged 18 to 80 years old, the patients were treated by endoscopic biopsy pathological diagnosis for gastric cancer; ultrasound or computed tomography found no other organ metastasis, no serious heart, liver, lung, kidney, and other important organ dysfunction; without preoperative neoadjuvant therapy, preoperative physical status score of ECOG 0/1 is expected to implement the full stomach or subtotal gastrectomy and D2 lymph node dissection. The results of R0 operation were obtained. Exclusion criteria: pregnant or lactating women, with severe mental illness; dysfunction associated with heart, lung, kidney, and other important organs; upper abdominal operation history (except the history of laparoscopic cholecystectomy), stomach operation (including in gastric cancer endoscopic submucosal dissection/endoscopic mucosal resection); preoperative imaging diagnosis of distant metastasis no, the possibility of radical operation; and the relevant information is incomplete. All participating subjects were fully informed of the study and the associated risks before signing an informed consent form, and the principles outlined by the declaration of Helsinki (2013) were adopted in this study.
2.2. Methods
All patients underwent laparoscopic radical gastrectomy for gastric cancer + D2 lymphadenectomy. In control group, the whole en-bloc specimen was fixed with formalin and then lymph nodes were detected by palpation and thin slice inspection, and then harvested by the pathological technician. In observation group, the surgeon demarcated the lymph node group with titanium clip during the lymph node dissection process and the fresh en-bloc specimen was treated by the surgeon before the formalin fixation. The perigastric lipolymphatic tissue was divided into the lymph node grouping according to JSGC guideline III. Then the stomach and each group of lipolymphatic tissue were fixed and then transferred to the pathologic department, then the lymph nodes were harvested by the pathological technician. The total number of lymph nodes harvested and the N stage were compared in 2 groups.
2.3. Observation index
Compare age, sex, tumor size, tumor location, operation mode, pathological stage, tumor differentiation, NPLN, number of negative lymph nodes (NNLN), total number of lymph nodes, lymph node negative rate (LNNR), etc., between the 2 groups.
2.4. Statistical analysis
All data were analyzed using the statistical package SPSS version 23.0. All measurement data were represented as 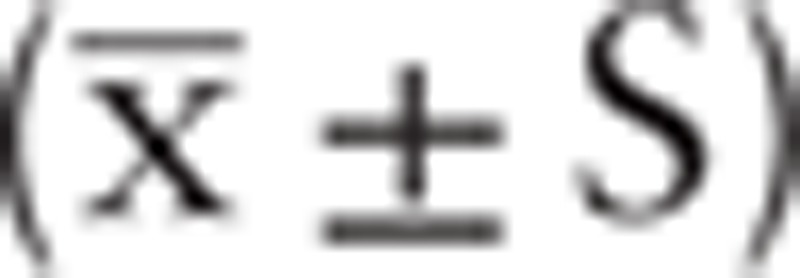 . The paired t test was used in the group, and the analysis of variance was used in the group. The count data were expressed in percentage (%), and the data were processed by chi-squared test, and P < .05 thought the difference was statistically significant.
. The paired t test was used in the group, and the analysis of variance was used in the group. The count data were expressed in percentage (%), and the data were processed by chi-squared test, and P < .05 thought the difference was statistically significant.
3. Results
3.1. Basic feature
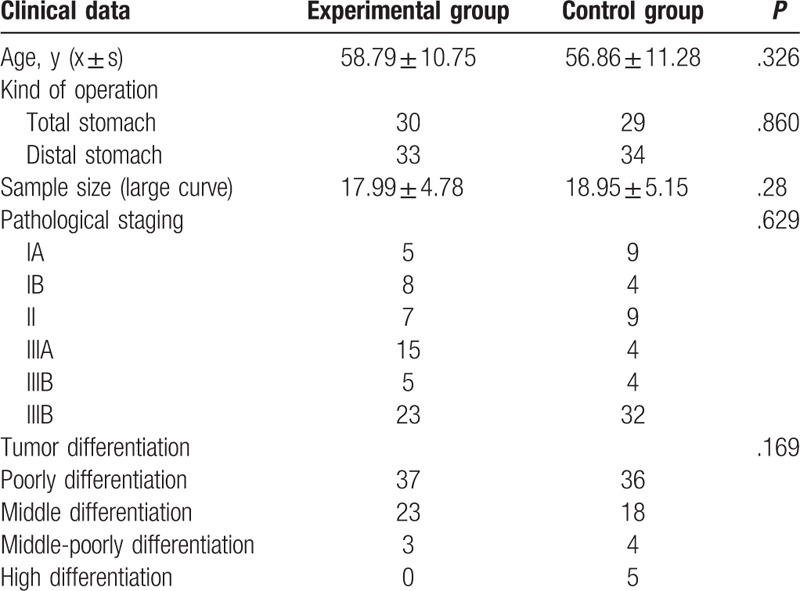
Experimental group 63 patients, in which male has 37 cases, female has 26 cases, age is in 28 to 82 years old, average age 58.79 ± 10.75; control group 63 cases, male 37 cases, female 26 cases, aged 27 to 81 years, mean age 56.86 ± 11.28. There is no statistically significant differences in age, sex, tumor size, tumor location, operative way and tumor differentiation between 2 groups (P > .05) (Table 1).
Table 1.
Comparison of clinicopathological data in the 2 groups (n = 63).
3.2. Clinicopathological results
There are no statistically significant differences in the NPLN, LNNR between the 2 groups. For comparison of the TLNNs of 2 groups of patients’, the experimental group has 2611 pieces, the control group has 1643 pieces, 2 groups had the significant differences (P < .001), and there is statistically significant differences in negative lymph node number between 2 groups (P < .001). The ratio of the experimental group patients with lymph node is more than 25 pieces/case (90.1%), and the control group is 47.6%, there is significant differences between 2 groups (P < .001) (Table 2).
Table 2.
Comparison of the number of lymph nodes of 2 groups, n = 63, example (%).
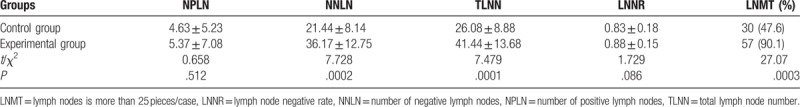
4. Discussion
Lymph node metastasis is one of the most important prognostic factors for gastric cancer patients.[2] The number of resected lymph nodes is closely related to postoperative pathological staging and prognosis, which has been taken seriously these years. In addition, the lower limit of how many lymph nodes should be pathologically inspected for an accurate and reasonable clinical staging has been under debate, an insufficient number of lymph nodes inspected will impact the N staging. Bouvier et al[3] investigated 749 cases of patients with gastric cancer and reported that when the number of checked lymph node was fewer than 10, then the pathology misdiagnosis rate reach 47.1%. It implicated that the staging would be not reliable before inspecting enough lymph nodes. Karpeh et al[4] investigated 1038 gastric cancer patients who had undergone gastrectomy, and found that the patients with more than 15 lymph node harvested had a better survival advantage over than the patients with less lymph nodes removed. The 8th edition of UICC and AJCC cancer staging guidelines proposed at least 15 lymph nodes should be pathologically inspected,[5] and it was also suggested that pathologists should find at least 16 lymph nodes[6,7] for each gastric cancer radical resection specimen, and for more accurate evaluation, the number of lymph nodes inspected should be more than 30 pieces.[8] Statistically speaking, if the number of lymph nodes inspected in the same case increased, more positive lymph nodes might be detected, and the N stage might shifted. In recent years, with the improvement of lymph node retrieval methods, the total number of lymph nodes retrieval had been increasing gradually,[9] and the lower limit of lymph nodes inspected for N staging in the NCCN gastric cancer guidelines had been promoted from 12 to 15 to avoid stage migration.
Although “at least 15 pieces” was recommended by the guideline, it was still controversial that if 15 pieces of lymph nodes was an good standard.[10] It is worth noting that radical lymph node dissection was not the same in total gastrectomy and distal gastrectomy. However, there was no individuation in guideline.[11] Lu et al[12] supposed that the lower limit of lymph nodes inspected in radical distal gastrectomy should ≥16 pieces, in radical total gastrectomy should ≥21 pieces. In our study, the average number of lymph nodes was 41.4 pieces in experimental group, but the number of positive lymph nodes did not increase significantly, so there was no significant change in postoperative pathological stage.
The influence factors of numbers of harvested and positive lymph nodes mainly include the following items: (1) operative factors: extended lymphadenectomy range could significantly improve the number of lymph nodes detected during operation, D2 lymph node dissection was an operation standard and 15 pieces of lymph node were generally guaranteed (the average number reached 25.2 pieces).[13] The surgeon's performance was shown to directly impact the number of lymph nodes harvested by several studies.[14–16] A Meta-analysis comparing minimally invasive operation with open operation had shown that laparoscopic surgery did not reduce the harvests of lymph node detection compared to open surgery.[17] (2) The demological and oncological factors: it was showed that obesity could affect the numbers of lymph nodes harvested in gastric cancer patients, due to the increased difficulty of a thorough lymphadenectomy during operation and lymph nodes detection in specimens.[18] However, there was also study shown that the patient's age, sex, BMI, and preoperative risk index had no significant impact on the number of lymph nodes harvested.[19] In addition, the oncologic features of the tumor also had impact on the harvest of lymph nodes. The higher the T staging was, the more the number of lymph nodes might be harvested.[20] (3) Postoperative pathological examination factors: the individual habits of the pathologists and the lymph node search methods were considered to very important factors influence the numbers of lymph nodes detected.[20] While, there was not yet a canonical standard of the lymph node detection , each medical center and even each pathologist used individual way to detect the lymph nodes. An easy measure to improve the harvest of lymph nodes after the surgery was surely meaningful for the pathological staging.
The detection and harvest of lymph nodes has been an important part of postoperative pathological examination for gastric cancer, generally completed by pathologist or pathological technician. While in Japan, it was often done by surgeon.[21] The detection method mainly include: (1) nude eye inspection together with finger palpation; (2) the chemical method including degreasing and methylene blue staining. This method could significantly improve the detection rate of lymph nodes; (3) the lymph node tracer method applied during the operation. It could stain some nodes and help the detection of tiny lymph node. Methylene blue staining, carbon nano tracer could all improve the detection rate of lymph nodes.[22–24] But the degreasing, staining or tracing technologies were not ordinarily used because of cost. Therefore, a suitable way to improve the lymph node harvested should be simple and low cost. In our hospital, the lymph nodes had been routinely detected by pathological technician with palpation and slicing inspection. While the numbers of lymph nodes harvested increased merely if the perigastric lipolymphatic tissue had been dissected into lymph node groups by the surgeon.
In our study, there was no statistically significant difference in age, sex, tumor size, tumor location, operative way and tumor differentiation between the two groups (P > 0.05). In the experimental group, the total number of lymph nodes was 41.44 ± 13.68, the negative lymph nodes was 36.17 ± 12.75, while in the control group the total number of lymph nodes was 26.08 ± 8.88, and the negative lymph nodes was 21.44 ± 8.14. The results showed that the total number of lymph node and negative lymph nodes in the observation group were significantly higher than those in the control group (P <0.001). The rate of patients in experimental group with lymph nodes more than 25 pieces was 90.1%, in the control group the ratio was 47.6% (P < 0.001).
Dissecting the perigastric lipolymphatic tissue into lymph node groups by the surgeon, might improve the total number of lymph nodes harvested by the pathological technician, there was no significant difference in the numbers of positive lymph nodes, the increase mainly result from more negative lymph nodes detected, there for no obvious stage migration was found.
Although the surgeon's grouping of perigastric lipolymphatic tissue only resulted in increase of negative nodes and did not resulted in an stage shift, it might yet had an prognostic value. Chen et al[25] analyzed 1363 cases of gastric cancer patients after surgery, found that the number of lymph nodes detected and N staging were independent prognostic factors, in patients with detected lymph nodes ≥25 pieces/case, the 5 year survival rates were 58–59% and 32–77% for N2 and N3 stages respectively, significantly better than the patients with the same N stage but less lymph nodes, 15–24 pieces of per case, whose 5 year survival rates were 52–48% and 21–67% for N2 and N3. But in N0 gastric cancer patients, a tendency of a tendency of better prognosis with more lymph node number was also noticed when the patients were grouped according the number of harvested lymph nodes (1–15 pieces, 16–20 pieces, 21–25 pieces, 26–30 pieces, >30 pieces).
The grouping of perigastric lipolymphatic tissue was easy but difficult for precision due to the lack of demarcation between stations. And the lymph node station differentiation had not been advocated, here we suggested the grouping for it might be a feasible and easy method to improve the pathological examination of lymph node.
5. Conclusion
Dissecting the perigastric lipolymphatic tissue into lymph node groups by the surgeon, might improve the total number of lymph node harvested by the pathological technician, and increase the rate of cases with more than 25 lymph nodes. Our results also implicated that, when the routing harvested lymph nodes were more than 20, the increasing number by perigastric lipolymphatic tissue grouping might result from more negative lymph nodes detected and might not result in stage migrating.
Author contributions
Shenghe Deng, Liming Shen, Jiang Li, Ke Wu, and Jiliang Wang contributed to the study design, literature search, collection of the data, and data analysis. Yinghao Cao and Lijuan Xiong contributed to the literature search and the writing of the manuscript. KaiXiong Tao, Guobin Wang, and Kailin Cai contributed to the review and revise of the manuscript.
Conceptualization: Yinghao Cao, Guobin Wang.
Data curation: Yinghao Cao, Lijuan Xiong, Shenghe Deng, Jiang Li, Jiliang Wang.
Formal analysis: Yinghao Cao, Jiliang Wang.
Investigation: Ke Wu.
Methodology: Shenghe Deng, Ke Wu, Jiliang Wang.
Project administration: Liming Shen, KaiXiong Tao.
Resources: KaiXiong Tao, Kailin Cai.
Software: Liming Shen, Ke Wu, Kailin Cai.
Supervision: Guobin Wang, Kailin Cai.
Validation: Jiang Li, Guobin Wang, Kailin Cai.
Visualization: Kailin Cai.
Writing – original draft: Kailin Cai.
Writing – review and editing: Lijuan Xiong, Kailin Cai.
Footnotes
Abbreviations: LNNR = lymph node negative rate, NNLN = number of negative lymph nodes, NPLN = number of positive lymph nodes, TLNN = total lymph node number.
YC and LX contributed equally to this work.
This study was supported by grants from the National Natural Science Foundation of China (No. 81272655), the Research Fund for Public Welfare in the Health Industry, Health Ministry of China (No. 201402015), and Clinical Research Physician Program of Tongji Medical College, HUST.
The authors have no conflicts of interest to disclose.
References
- [1].Roviello F, Marrelli D, Morgagni P, et al. Survival benefit of extended D2 lymphadenectomy in gastric cancer with involvement of second level lymph nodes: a longitudinal multicenter study. Ann Surg Oncol 2002;9:894–900. [DOI] [PubMed] [Google Scholar]
- [2].Adachi Y, Suematsu T, Shiraishi N, et al. Perigastric lymph node status as a prognostic indicator in patients with gastric cancer. Br J Surg 1998;85:1281–4. [DOI] [PubMed] [Google Scholar]
- [3].Bouvier AM, Haas O, Piard F, et al. How many nodes must be examined to accurately stage gastric carcinomas? Results from a population based study. Cancer 2002;94:2862–6. [DOI] [PubMed] [Google Scholar]
- [4].Karpeh MS, Leon L, Klimstra D, et al. Lymph node staging in gastric cancer: is location more important than number? An analysis of 1,038 patients. Ann Surg 2000;232:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual [M]. 8th ed.2016;New York, NY: Springer, 203–220. [Google Scholar]
- [7].Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017;67:93–9. [DOI] [PubMed] [Google Scholar]
- [8].Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, Version 2.2013. J Natl Compr Canc Netw 2013;11:531–46. [DOI] [PubMed] [Google Scholar]
- [9].Lee E, Chae Y, Kim I, et al. Prognostic relevance of immunohistochemically detected lymph node micrometastasis in patients with gastric carcinoma. Cancer 2002;94:2867–73. [DOI] [PubMed] [Google Scholar]
- [10].Kim YI. Does the retrieval of at least 15 lymph nodes confer an improved survival in patients with advanced gastric cancer? J Gastric Cancer 2014;14:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang PP, Xi HQ, Zhang KC, et al. Factor analysis and method exploring for lymph nodes harvest in gastric cancer. Zhonghua Wai Ke Za Zhi 2017;55:255–9. [DOI] [PubMed] [Google Scholar]
- [12].Lu J, Wang W, Zheng CH, et al. Influence of total lymph node count on staging and survival after gastrectomy for gastric cancer: an analysis from a two-institution database in China. Ann Surg Oncol 2017;24:486–93. [DOI] [PubMed] [Google Scholar]
- [13].Smith BR, Stabile BE. Aggressive D2 lymphadenectomy is required for accurate pathologic staging of gastric adenocarcinoma. Am Surg 2006;72:849–52. [PubMed] [Google Scholar]
- [14].Kang SY, Lee SY, Kim CY, et al. Comparison of learning curves and clinical outcomes between laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Gastric Cancer 2010;10:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhao LY, Zhang WH, Sun Y, et al. Learning curve for gastric cancer patients with laparoscopy-assisted distal gastrectomy: 6-year experience from a single institution in western China. Medicine (Baltimore) 2016;95:e4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhou D, Quan Z, Wang J, et al. Laparoscopic-assisted versus open distal gastrectomy with D2 lymph node resection for advanced gastric cancer: effect of learning curve on short-term outcomes. A meta-analysis. J Laparoendosc Adv Surg Tech A 2014;24:139–50. [DOI] [PubMed] [Google Scholar]
- [17].Straatman J, van der Wielen N, Cuesta MA, et al. Minimally invasive versus open total gastrectomy for gastric cancer: a systematic review and meta-analysis of short-term outcomes and completeness of resection: surgical techniques in gastric cancer. World J Surg 2016;40:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kodera Y, Ito S, Yamamura Y, et al. Obesity and outcome of distal gastrectomy with D2 lymphadenectomy for carcinoma. Hepatogastroenterology 2004;51:1225–8. [PubMed] [Google Scholar]
- [19].Hanna GB, Amygdalos I, Ni M, et al. Improving the standard of lymph node retrieval after gastric cancer surgery. Histopathology 2013;63:316–24. [DOI] [PubMed] [Google Scholar]
- [20].Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- [21].Kodera Y. Surgery for gastric cancer: has the East versus West issue been solved? Dig Surg 2013;30:92–5. [DOI] [PubMed] [Google Scholar]
- [22].Aoyama T, Fujikawa H, Cho H, et al. A methylene blue-assisted technique for harvesting lymph nodes after radical surgery for gastric cancer: a prospective, randomized, controlled study. Am J Surg Pathol 2015;39:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Candela FC, Urmacher C, Brennan MF. Comparison of the conventional method of lymph node staging with a comprehensive fat-clearing method for gastric adenocarcinoma. Cancer 1990;66:1828–32. [DOI] [PubMed] [Google Scholar]
- [24].Li Z, Ao S, Bu Z, et al. Clinical study of harvesting lymph nodes with carbon nanoparticles in advanced gastric cancer: a prospective randomized trial. World J Surg Oncol 2016;14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen HN, Chen XZ, Zhang WH, et al. Necessity of harvesting at least 25 lymph nodes in patients with stage N2-N3 resectable gastric cancer: a 10-year, single-institution cohort study. Medicine (Baltimore) 2015;94:e620. [DOI] [PMC free article] [PubMed] [Google Scholar]


