Abstract
The authors retrospectively characterized the clinical outcomes of combining the Yeung endoscopic spine system (YESS) and transforaminal endoscopic surgical system (TESSYS) techniques during percutaneous transforaminal endoscopic discectomy (PTED) to treat multilevel lumbar disc herniation.
PTED using both YESS and TESSYS was performed on 52 patients with multilevel lumbar disc herniations who had shown no apparent response to previous conservative treatments. Postsurgical follow-ups were conducted at weeks 1, 26, and 48. Patients’ preoperative and postoperative performances were assessed by modified MacNab classification, Japanese Orthopedic Association (JOA) scores, Oswestry disability index (ODI), and visual analog scale (VAS), and compared with 34 and 45 patients who were treated only by YESS and TESSYS, respectively.
The postsurgery surgeon-performed assessment showed satisfactory results in 98% of the YESS + TESSYS-treated cases. The average operative time was 116 ± 23 minutes, intraoperative bleeding was 19 ± 12 mL, and bed stay was 3 days. No complications occurred, including infection, nerve injury, or spinal canal hematoma. One week after surgery, the modified MacNab classifications of the patients were excellent in 45, good in 6, fair in 1, and poor in 0 (98% were excellent or good). JOA, ODI, and VAS scores for low back pain significantly improved relative to the preoperative assessment (P < .01) and had remained stable at 26 and 48 weeks.
PTED that combined YESS and TESSYS techniques, depending on the predominant type of lumbar disc herniation at individual levels, is safe, minimally invasive, and effective.
Keywords: lumbar disc herniation, minimally invasive discectomy, percutaneous transforaminal endoscopic discectomy, transforaminal endoscopic surgical system, Yeung endoscopic spine system
1. Introduction
Sciatica describes symptoms of pain, numbness, and weakness of the lower back, leg, and foot, and is caused by compression or irritation of the sciatic nerve roots. This debilitating condition affects about 1.6% of the general population, and up to 43% of workers in selected occupations.[1,2] More than 90% of sciatica cases are due to lumbar disc herniation, that is, the protrusion of a portion of the spinal disc out of its normal position at one or multiple levels in the low back, that then presses the sciatic nerve.[3,4] Although the prognosis of sciatica has improved with advances in surgical techniques, a substantial number of patients continue to suffer pain for one or more years.[5,6] In addition, 5% to 15% of patients experience recurrence after lumbar discectomy.[7]
Lumbar disc herniation may be managed nonsurgically by conservative treatments such as traction, physiotherapy, and acupuncture. However, when the patient's condition proves intractable, surgery can be considered. Since the first reported open laminectomy and discectomy in 1934,[8] many surgical management techniques have been developed, with the basic goal of relieving the herniation-induced nerve root compression. Such techniques may be open or minimally invasive, using a posterior or posterolateral approach.[9] Especially in the last 30 years, the techniques and equipment for minimally invasive surgeries have developed rapidly.
The introduction of the microscope and endoscope has improved intraoperative vision, and blemish and muscle trauma has lessened.[10] Microdiscectomy reduces the incision from 5 to 3 cm,[11,12] and microendoscopic discectomy further reduces it to 2 cm.[13] Open procedures to manage herniation have been associated with potential iatrogenic morbidity that can be avoided through minimally invasive surgery.[9]
Techniques for implementing posterolateral endoscopic lumbar nerve decompression during percutaneous endoscopic lumbar discectomy include the intradisc Yeung endoscopic spine system (YESS) and the intracanal transforaminal endoscopic surgical system (TESSYS). These 2 techniques are fundamentally different. The YESS technique involves puncturing the disc space, facilitates access to the posterior epidural space, and uses a holmium–yttrium–aluminum–garnet laser to ablate bony and soft tissue for decompression.[14–16] The TESSYS technique enlarges the intervertebral foramen near the facet joint with special reamers, sequesters disc fragments, and decompresses foraminal stenosis.[17] Both YESS and TESSYS improve intraoperative visualization and better enable access to the pathological sites, and therefore cause less trauma, better outcomes, and faster recovery.
In this study, we retrospectively assessed the outcomes of 52 patients with multilevel lumbar disc herniation treated with combined YESS and TESSYS, appropriate to the type of herniation at individual levels, by comparing with patients managed by YESS or TESSYS alone.
2. Materials and methods
This study was approved by the Institutional Ethics Board of Guangzhou University of Chinese Medicine approved this study. Written informed consent from the involved patients was not obtained because of the retrospective nature of this study. All data were analyzed anonymously.
2.1. Patients
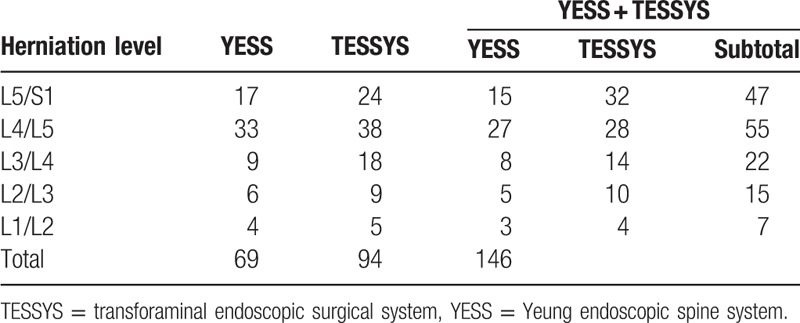
Fifty-two patients recruited from January 2011 to December 2013 (30 men and 22 women; aged 58.96 ± 10.28 years, range 45–89 years) were surgically treated by combining YESS and TESSYS (i.e., the YESS + TESSYS group) techniques, appropriate to the type of herniation at individual levels. The period from symptom initiation to surgery ranged from 3 to 18 months, averaging 8.45 ± 2.24 months. In these 52 patients, there were 146 herniated discs, intracanal and extracanal (foraminal and extraforaminal; Tables 1 and 2). The included herniation types were protrusion (124 discs), extrusion (13 discs), and sequestration (9 discs). There were 7, 15, 22, 55, and 47 herniations at levels L1/L2, L2/L3, L3/L4, L4/L5, and L5/S1, respectively (Table 3).
Table 1.
Cases of multilevel herniation managed by YESS, TESSYS, and YESS/TESSYS combination.
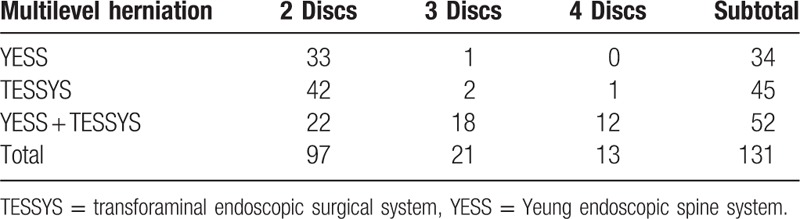
Table 2.
Lumbar disc herniation relative to the pedicle and spinal canal.
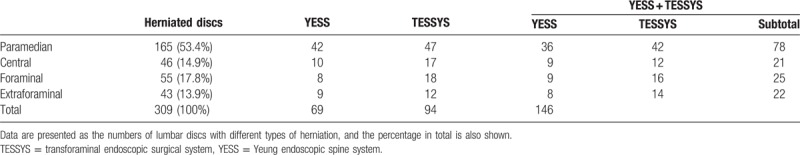
Table 3.
Location of lumbar disc herniation according to level.
The recoveries of these patients were compared with that of 34 patients treated by YESS alone and 45 patients treated by TESSYS alone before 2011 in our department. The clinicopathological properties are shown in Tables 1 to 3. All the surgeries were performed by a same group of doctors.
Patients enrolled in the study had not responded to ≥3 months of conservative therapy. Lumbar disc herniation was confirmed by preoperative computed tomography (CT) scanning and magnetic resonance imaging (MRI); herniation size was estimated and nerve root compression assessed. These patients had disc herniation at ≥2 levels, with symptoms on relevant nerves. All patients tested positive on straight leg raising or femoral nerve stretch, with obvious surgical indications. No contraindications were determined for either the YESS or the TESSYS techniques.[16,18]
The iliac crest height was measured by preoperative lumbar spine anterior–posterior and lateral dynamic flexion–extension radiography, to ensure that the iliac crest did not interfere with the surgical procedure. In addition, patients were excluded from the study for lumbar spinal tumor, infection, or deformity, or severe lumbar spinal stenosis or instability, or spondylolisthesis.
2.2. Preparation and surgery
Preoperative neurological examination was performed by electromyographic and electroneurophysiological monitoring, and dorsal root ganglion stimulation. Lumbar spine anterior–posterior and lateral dynamic flexion–extension radiography, CT scanning, and MRI were also performed before surgery.
The patients were placed prone on a radiolucent Wilson Frame (Mizuho Orthopedic Systems, Union City, CA) with their arms away from the side of the body (Fig. 1A). The abdomen was free to avoid pressure on the peritoneal vein, with spine, knee, and hip flexed to reduce lumbar lordosis (Fig. 1B). This position also reduced tension on the sciatic nerve and facilitated lordosis control. Epidural anesthesia was used by injecting 3 mL of 0.3% ropivacaine, with an additional 10 to 15 mL if spinal anesthesia was not achieved within 5 to 10 minutes after the first injection.
Figure 1.
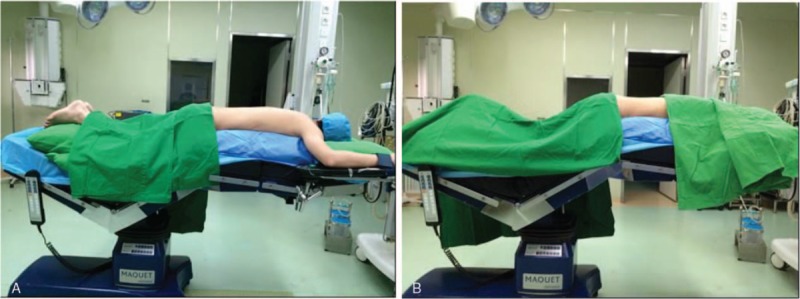
Patient positioning. Patient was positioned prone on a radiolucent Wilson Frame with the arms away from the side of the body (A) and spine, knee, and hip flexed during the surgery (B).
The surgery for the target disc using either YESS or TESSYS was performed as previously described.[15,17] Briefly, under the guidance of C-arm fluoroscopy, the target disc was defined and marked on the skin by a vertical line along the center of the spinous processes of the lumbar spine, and a transverse horizontal line across the center of the disc space. The anatomic disc center, identified by the intersections, was used as a reference point for the target disc during the operation. Under the guidance of C-arm fluoroscopy, taking anteroposterior and Ferguson views (by tilting 20° to 30°), the puncture point was identified, and an 18-G needle was inserted into the back 1/3 of the target disc.
The specific protocols used at individual disc levels depended on the sizes and types of herniation in the 52 patients managed by combining YESS and TESSYS. The YESS technique was used for central and some paracentral disc protrusions.[15] The TESSYS protocol was applied to treat the remaining types of paracentral, extrusion, and sequestration herniations, as well as for foraminal and extraforaminal lumbar disc herniations.[17] In the 2 control groups treated by YESS or TESSYS alone, YESS or TESSYS was performed regardless of the sizes and types of the herniation.
After the tip of the needle reached the correct position, a chromo-discography was performed using a mixture of iohexol and methylene blue (9:1 in volume) at an injection rate of 1 to 3 mL/disc, with careful monitoring of the pain induced by the contrast agent, and then a guidewire was introduced. The discograph was used to help identify precisely the target disc and further delineate the pathology. The needle insertion and discography were carried out sequentially from disc L5/S1 to L1/L2.
After all the target discs were identified, the surgery was continued sequentially from L5/S1 to L1/L2. A 6–7-mm incision was made at the needle insertion point, the needle removed, and the guidewire left in situ. A dilator was then inserted along the guidewire and confirmed to advance to the desired area. A 7-mm working cannula was then inserted into the herniated disc. With the assistant surgeon holding the working cannula and maintaining pressure, to avoid damage to the nerve root by accidental movement of the cannula, a Wolf 70° panorama endoscope (Richard Wolf, Knittlingen, Germany) was installed.
During installation of the TESSYS working cannula and prior to insertion of the endoscope, decompression was performed as needed near the transverse nerve and the ligamentum flavum-disc space, by dissecting obstructive bone and the side of the ligamentum flavum, using the smallest reamer and endoscopic forceps. By noting the visible radio-opacity on the discography images and the intraoperative light blue staining, the annular fissure was reached, and the pathologic nucleus removed.
2.3. Postoperative management
After surgery, the patients were kept on bed rest on a firm mattress for at least 6 hours. They were then allowed to mobilize for no longer than 20 minutes with the support of a lumbar brace. Low back muscle exercise was encouraged on the bed, and physiotherapy was introduced after stitch removal 10 days after the surgery to promote functional rehabilitation. Heavy lifting while bending at the waist was forbidden within 6 weeks after surgery. The lumbar brace was removed after 6 weeks.
2.4. Evaluation parameters and follow-ups
During the preoperative assessment and follow-ups at weeks 1, 26, and 48 after surgery, the Japanese Orthopedic Association (JOA) scoring system was used to evaluate low back pain, gait, standing, sitting, lifting, and sensory and motor disturbances. The Oswestry disability index (ODI) was used to evaluate patients’ low back pain, ability to perform personal care, ability to lift, walk, sit, and stand, and sleep quality. Visual analog scale (VAS) scoring was also used based on the patients’ feedback on the surgery, pain, and performance.
A MacNab scoring system was applied to assess the outcome of the surgery as excellent, good, fair, or poor, relative to the preoperative measurement. Standards for excellent outcome were normal straight leg raising (>70°), normal lower limb sensory and motor functions, normal muscle strength, and disappearance of low back pain. A good outcome was considered for straight leg raising increased by 30° but not to 70°, and sporadic slight low back pain that had no influence on work or life. A rating of fair required an improvement of 15° in straight leg raising but not to 70°, with significant relief of low back pain compared with before surgery, although occasional pain medication is needed. No obvious improvement or worsened symptoms, with patients requiring continuous pain medication, was rated as a poor outcome.
2.5. Statistics
All data were processed using SPSS 17.0 software (IBM China, Beijing, China), and are presented as mean ± standard deviation. The paired sample t test and rank sum test were performed, depending on the characteristics of the variables being compared.
3. Results
Representative surgical outcome of YESS + TESSYS is shown in Fig. 2, compared with that of YESS or TESSYS alone (Figs. 3 and 4). The outcomes of 52 patients, who were surgically treated with YESS and TESSYS, based on the type of herniation were retrospectively reviewed, in comparison with 34 and 45 patients treated by YESS and TESSYS alone, respectively. The average operative time was 116 ± 23 minutes, with an average operative bleeding of 5 ± 1 mL, and a representative surgical process and the outcome are shown in Fig. 1.
Figure 2.
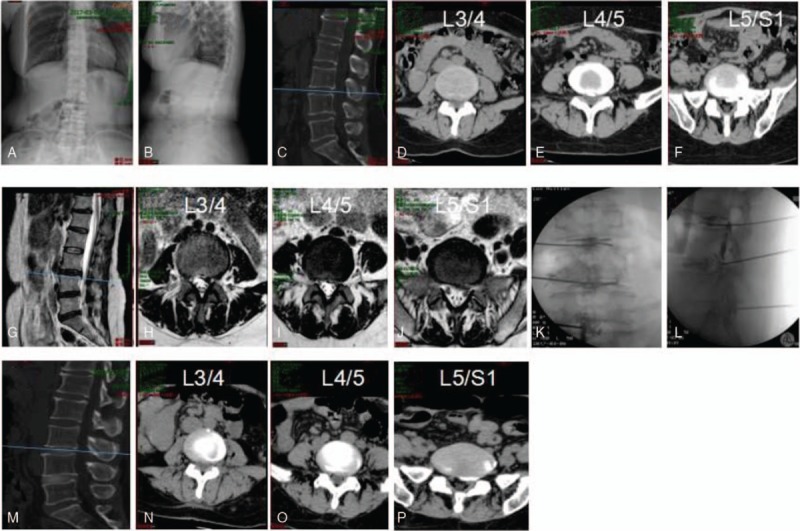
An example of percutaneous transforaminal endoscopic discectomy surgery combining YESS and TESSYS on a 51-year-old female patient presented with 20 years of numbness and pain in the left buttock and lower limbs. Diagnosed with disc herniation at L4/L5 and L5/S1 (A–F), the patient was treated by YESS at L4/L5 and TESSYS at L5/S1. Preoperative CT (A–C) and magnetic resonance imaging scanning (D–F) results are shown. Panels G and H show the position of puncturing needles. Postoperative CT images are shown in I–K. CT = computed tomography, TESSYS = transforaminal endoscopic surgical system.
Figure 3.
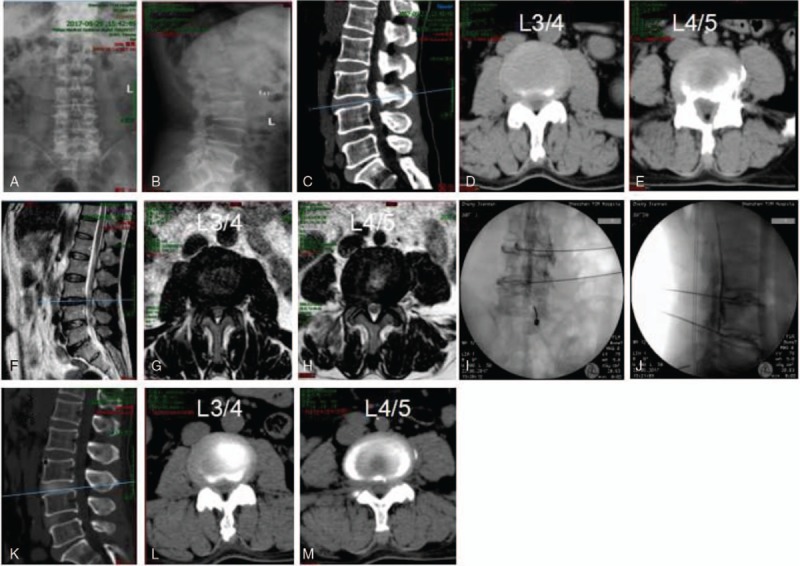
Representative percutaneous transforaminal endoscopic discectomy surgery by YESS on a 61-year-old female patient presented with 4 years of numbness and pain in the left buttock and lower limbs. Diagnosed with disc herniation at L3/L4 and L4/L5 (A–H), the patient was treated by YESS. Preoperative CT (A–E) and magnetic resonance imaging scanning (F–H) results are shown. Panels I and J show the position of puncturing needles. Postoperative CT images are shown in K–M. CT = computed tomography.
Figure 4.
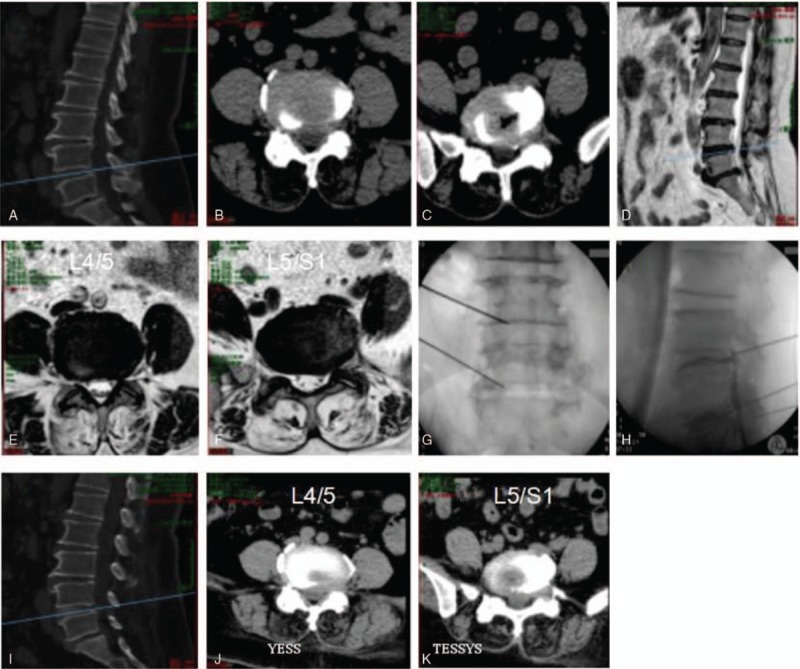
Representative percutaneous transforaminal endoscopic discectomy surgery by TESSYS on a 41-year-old female patient presented with 10 months of numbness and pain in the left buttock and lower limbs. Diagnosed with disc herniation at L3/L4, L4/L5, and L5/S1 (A–J), the patient was treated by TESSYS. Preoperative CT (A–F) and magnetic resonance imaging scanning (G–J) results are shown. Panels K and L show the position of puncturing needles. Postoperative CT images are shown in M–P. CT = computed tomography, TESSYS = transforaminal endoscopic surgical system.
All patients were assessed 1 week after surgery, using the modified MacNab classification system (Table 4); 98% of 52 patients in the YESS + TESSYS group were rated excellent or good and 2% fair or poor. No complications were observed, including infection, nerve damage, or intraspinal hematoma. The clinical symptoms of these 52 patients were significantly improved. The total JOA score at postoperative 1 week was 24.37 ± 0.47, which was significantly higher than the preoperative value (11.50 ± 0.41; P < .01; Table 5). Importantly, the patients in the YESS + TESSYS group recovered significantly better than did those managed by YESS alone (23.03 ± 0.51, P < .01) or TESSYS alone (23.01 ± 0.34, P < .01; Table 4). In addition, for patients in the YESS + TESSYS group the average ODI score at week 1 was 7.27 ± 1.39, which was significantly lower than that before surgery (26.17 ± 12.68, P < .01; Table 6). These patients scored significantly better than did those treated by YESS alone (score: 8.53 ± 1.52, P < .01) or TESSYS alone (score: 8.37 ± 1.45, P < .01). Evaluation with VAS scoring system also confirmed similar benefits of YESS + TESSYS to the patient (Table 7).
Table 4.
Modified MacNab classification of the outcomes at weeks 1 and 26 after the surgery.
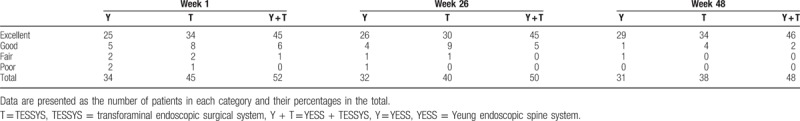
Table 5.
Japanese Orthopedic Association scores before and after surgery.
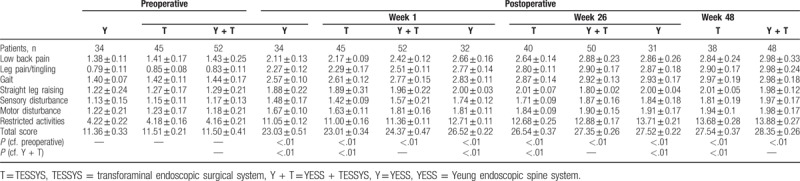
Table 6.
Oswestry disability index scores before and after surgery.
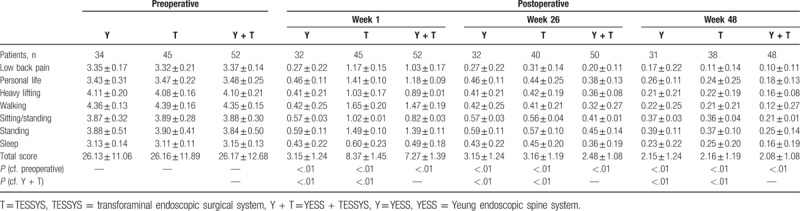
Table 7.
Visual analog scale scores before and after surgery.
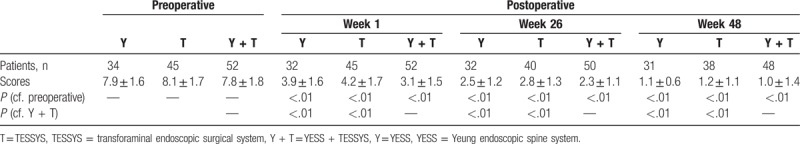
Importantly, the improvements in clinical symptoms in the YESS + TESSYS group were found to have remained stable at the 26-week follow-up; results of the modified MacNab classification showed that outcomes were excellent or good in 98% of cases, fair in 2%, and none were poor (Table 4). In agreement with this, the JOA, ODI, and VAS scores at the week-26 follow-up (27.35 ± 0.26, 2.48 ± 1.08, and 2.3 ± 1.1, respectively), significantly improved compared with those at week 1 (Tables 5 and 6). Similar performance was found for patients after 48 weeks. Of note, patients managed by combining YESS and TESSYS scored better according to the JOA, ODI, and VAS scales than did those treated by YESS or TESSYS alone (Tables 5–7).
4. Discussion
In the present retrospective study, the surgical outcomes of 52 cases of lumbar disc herniation using percutaneous transforaminal endoscopic discectomy (PTED) combining YESS and TESSYS techniques were assessed, in comparison with 34 and 45 patients treated by YESS or TESSYS. Both young and elderly patients who suffered from multilevel intracanal and extracanal herniation were enrolled. A satisfactory result rate of 98% was achieved and the clinical symptoms were significantly improved regardless of patient age and the forms of herniation. The favorable outcome remained stable at the 26- and 48-week follow-up period, suggesting that PTED combining the YESS and TESSYS techniques is an effective way to manage multilevel herniation.
Multilevel lumbar disc herniation is a long-term cumulative disease due to aging, or repetitive damage and repair of lumbar discs that occur more often in the aging population.[19] While lumbar discectomy in young patients usually has a good clinical outcome,[20,21] the effectiveness on aging patients is less documented.[22] It is commonly believed that for elderly patients, particularly those with a long history of pathology and recurrent disease, early surgical treatment can achieve an ideal prognosis.[22] However, it remains challenging to choose appropriate surgical approaches that help decompress the affected nerve, maintain spine stability, and recover spinal function.
Based on our and others’ clinical experience, the advantages of PTED techniques over other decompression approaches are obvious: less trauma, better visualization,[23,24] improved identification of the pathological tissues with contrast agent and methylene blue,[25,26] and less chance of iatrogenic lumbar instability and the formation of intracanal scar tissue.[9,24,27] However, PTED techniques also have their contraindications. YESS technique requires posterolateral endoscopic access which can be blocked by extruded sequestered or migrated disc herniations, epidural scarring-associated herniations, severe central canal stenosis, and hard calcified herniations.[28] The TESSYS procedure is not ideal for dorsally dislocated disc herniation, central stenosis, or tumors.[29] Therefore in this study, YESS and TESSYS were alternated according to the form of herniation at individual levels. The YESS technique was used to remove central and some of the paracentral disc protrusions, while TESSYS surgery was performed to treat migrated or sequestered herniations. This strategy allowed us to apply the 2 techniques to full advantage for the management of lumbar disc herniation, to decompress completely the affected nerve roots, and to promote the repair of intervertebral discs. Our strategy also avoided the excessive removal of the nucleus pulposus, to help maintain long-term disc function in the movement of the vertebrae.
Our results indicated a 98% satisfactory rate achieved in the 52 patients treated by PTED that combined the YESS and TESSYS techniques. This strategy proved to be superior to using YESS and TESSYS separately on the patients, by both the JOA and ODI scoring systems. The outcomes were also improved compared with the previously reported 89.2% satisfactory rate achieved when YESS was used alone, or the 88.2% reached in a restricted endoscopic series by Kambin et al.[30] However, it is noteworthy that this is a retrospective study in which YESS and TESSYS were applied appropriate to herniation forms. The follow-ups are relatively the short- and long-term effect of this combinational technique needs to be confirmed. In addition, further studies are required to compare this approach with novel technique, such as target puncture.
5. Conclusion
Our retrospective study suggests that in patients with multilevel lumbar disc herniation, using both YESS and TESSYS as appropriate to treat different types of herniation is a feasible and efficient approach to improve symptoms and, importantly, maintain the function of the lumbar spine. The combinational surgical approach leads to improved clinical outcome, compared with strategies with YESS or TESSYS alone.
Author contributions
Conceptualization: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Data curation: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Formal analysis: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Investigation: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Methodology: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Project administration: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Resources: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Software: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Supervision: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Validation: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Visualization: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Writing – original draft: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Writing – review and editing: Zhitao Sun, Shenghua He, Yeguang Wang, Dujun Ma, Weiwei Tan, Juyi Lai.
Footnotes
Abbreviations: CT = computed tomography, JOA = Japanese Orthopedic Association, MRI = magnetic resonance imaging, ODI = Oswestry disability index, PTED = percutaneous transforaminal endoscopic discectomy, TESSYS = transforaminal endoscopic surgical system, VAS = visual analog scale, YESS = Yeung endoscopic spine system.
ZS and SH have contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Jacobs WC, van Tulder M, Arts M, et al. Surgery versus conservative management of sciatica due to a lumbar herniated disc: a systematic review. Eur Spine J 2011;20:513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine (Phila Pa 1976) 2008;33:2464–72. [DOI] [PubMed] [Google Scholar]
- [3].Smith N, Masters J, Jensen C, et al. Systematic review of microendoscopic discectomy for lumbar disc herniation. Eur Spine J 2013;22:2458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Valat JP, Genevay S, Marty M, et al. Sciatica. Best Pract Res Clin Rheumatol 2010;24:241–52. [DOI] [PubMed] [Google Scholar]
- [5].Vroomen PC, de Krom MC, Slofstra PD, et al. Conservative treatment of sciatica: a systematic review. J Spinal Disord 2000;13:463–9. [DOI] [PubMed] [Google Scholar]
- [6].Weber H, Holme I, Amlie E. The natural course of acute sciatica with nerve root symptoms in a double-blind placebo-controlled trial evaluating the effect of piroxicam. Spine (Phila Pa 1976) 1993;18:1433–8. [PubMed] [Google Scholar]
- [7].Swartz KR, Trost GR. Recurrent lumbar disc herniation. Neurosurg Focus 2003;15:E10. [DOI] [PubMed] [Google Scholar]
- [8].Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med 1934;211:210–5. [Google Scholar]
- [9].Nellensteijn J, Ostelo R, Bartels R, et al. Transforaminal endoscopic surgery for symptomatic lumbar disc herniations: a systematic review of the literature. Eur Spine J 2010;19:181–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Blamoutier A. Surgical discectomy for lumbar disc herniation: surgical techniques. Orthop Traumatol Surg Res 2013;99:S187–96. [DOI] [PubMed] [Google Scholar]
- [11].Springer, Caspar W. A new surgical procedure for lumbar disc herniation causing less tissue damage through a microsurgical approach. Lumbar Disc Adult Hydrocephalus 1977;74–80. [Google Scholar]
- [12].Williams RW. Microlumbar discectomy: a conservative surgical approach to the virgin herniated lumbar disc. Spine 1978;3:175–82. [PubMed] [Google Scholar]
- [13].Destandau J. A special device for endoscopic surgery of lumbar disc herniation. Neurol Res 1999;21:39–42. [DOI] [PubMed] [Google Scholar]
- [14].Knight M, Vajda A, Jakab G, et al. Endoscopic laser foraminoplasty on the lumbar spine—early experience. Minim Invasive Neurosurg 1998;41:5–9. [DOI] [PubMed] [Google Scholar]
- [15].Yeung AT. The evolution of percutaneous spinal endoscopy and discectomy: state of the art. Mt Sinai J Med 2000;67:327–32. [PubMed] [Google Scholar]
- [16].Yeung AT, Yeung CA. Kim DH, Fessler RG, Regan JJ. Posterolateral selective endoscopic diskectomy: The YESS technique. Endoscopic Spine Surgery and Instrumentation. New York, NY: Thieme; 2004. 201–11. [Google Scholar]
- [17].Hoogland T, Schubert M, Miklitz B, et al. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine 2006;31:E890–7. [DOI] [PubMed] [Google Scholar]
- [18].Gore S, Yeung A. The “inside out” transforaminal technique to treat lumbar spinal pain in an awake and aware patient under local anesthesia: results and a review of the literature. Int J Spine Surg 2014;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kleinig TJ, Brophy BP, Maher CG. Practical neurology -3: back pain and leg weakness. Med J Aust 2011;195:454–7. [DOI] [PubMed] [Google Scholar]
- [20].Daneyemez M, Sali A, Kahraman S, et al. Outcome analyses in 1072 surgically treated lumbar disc herniations. Minim Invasive Neurosurg 1999;42:63–8. [DOI] [PubMed] [Google Scholar]
- [21].Padua R, Padua S, Romanini E, et al. Ten- to 15-year outcome of surgery for lumbar disc herniation: radiographic instability and clinical findings. Eur Spine J 1999;8:70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fujii K, Henmi T, Kanematsu Y, et al. Surgical treatment of lumbar disc herniation in elderly patients. J Bone Joint Surg Br 2003;85:1146–50. [DOI] [PubMed] [Google Scholar]
- [23].Alfen FM, Lauerbach B, Ries W. Developments in the area of endoscopic spine surgery. Eur Musculoskelet Rev 2006;1:3–6. [Google Scholar]
- [24].Jasper GP, Francisco GM, Telfeian AE. Endoscopic transforaminal discectomy for an extruded lumbar disc herniation. Pain Physician 2013;16:E31–5. [PubMed] [Google Scholar]
- [25].Ahn Y. Transforaminal percutaneous endoscopic lumbar discectomy: technical tips to prevent complications. Expert Rev Med Devices 2012;9:361–6. [DOI] [PubMed] [Google Scholar]
- [26].Tenenbaum S, Arzi H, Herman A, et al. Percutaneous posterolateral transforaminal endoscopic discectomy: clinical outcome, complications, and learning curve evaluation. Surg Technol Int 2011;21:278–83. [PubMed] [Google Scholar]
- [27].Karakasli A, Yildiz DV, Kumtepe E, et al. Biomechanical comparison of intact lumbar lamb spine and endoscopic discectomized lamb spine. Eklem Hastalik Cerrahisi 2013;24:33–8. [DOI] [PubMed] [Google Scholar]
- [28].Yeung CA, Kauffman CP, Yeung AT. Phillips FM, Lauryssen C. Percutaneous decompression. Lumbar Intervertebral Disc. New York, NY: Thieme; 2011. 92–103. [Google Scholar]
- [29].Iprenburg M. Transforaminal endoscopic surgery—technique and provisional results in primary disc herniation. Eur Musculoskelet Rev 2007;2007:73–6. [Google Scholar]
- [30].Kambin P, O’Brien E, Zhou L, et al. Arthroscopic microdiscectomy and selective fragmentectomy. Clin Orthop Relat Res 1998. 150–67. [PubMed] [Google Scholar]


