Abstract
Despite the importance of strengthening of the transversus abdominis (TrA) muscle in individuals with low back pain, the effect of real-time ultrasound imaging on maintenance in selective strengthening of abdominal hallowing exercise (AHE) performance has not been investigated. So, the aim of this study was to investigate the effects of AHE with real-time ultrasound imaging feedback on selective reinforcing the TrA muscle.
Twenty healthy subjects were enrolled prospectively and randomized to train AHE for 2 weeks either by conventional feedback (group A) or by visual feedback from real-time ultrasound imaging additional to conventional feedback (group B). The changes in thickness of TrA, internal oblique abdominal muscle (IO), and external oblique abdominal muscle (EO) were measured using the ultrasonography. The changes in muscle activities of TrA-IO and EO were measured using surface electromyography.
The thickness of TrA, IO, and EO muscles in resting was not significantly changed in both groups A and B. However, the ratio of root mean square (RMS) values of TrA-IO/EO muscles, which mirrors selective contraction of TRA-IO muscles against EO muscle, was significantly higher in group B than in group A.
In healthy subjects, training with AHE using real-time ultrasound imaging feedback may be a useful additional method to conventional feedback for strengthening the TrA muscles selectively.
Keywords: abdominal hollowing, low back pain, real-time ultrasound imaging, surface electromyography, transversus abdominis
1. Introduction
Chronic low back pain is considered a common medical problem. Since the cause of the pain remains unknown, it is also being called chronic nonspecific low back pain.[1] The main cause of chronic low back pain has been reported as the lumbar instability, which is caused by atrophy and decrease in activation of local muscles such as transversus abdominis (TrA) and multifidus, rather than direct injury on low back itself.[2]
Trunk stabilization relieves and prevents recurrence of low back pain by acting like a corset of trunk by balancing abdominal deep muscles and trunk extensor muscles.[3] The trunk stabilization exercise strengthens local muscles around low back, and it is effective for alleviating dysfunction caused by the lumbar instability.
The abdominal hollowing exercise (AHE) is a type of trunk stabilizing exercise that has recently been used to enhance TrA muscle effectively.[4–6] However, several issues of TrA hinder the contraction of the TrA muscle during AHE. Anterolateral abdominal muscles consist of TrA, internal oblique abdominal muscle (IO), and external oblique abdominal muscle (EO). The TrA muscle is located in the deepest part among the anterolateral abdominal muscles, so it is neither visible nor selectively palpable. Also, most people do not know how to contract TrA voluntarily apart from more superficial abdominal muscles.[7]
Until now, previous studies demonstrated that the use of real-time ultrasound imaging feedback is beneficial for facilitating consistency of TrA muscle contraction during AHE.[7,8] Despite the importance of maintenance of strengthening the selective TrA muscle in individuals with chronic low back pain during daily life, the effect of real-time ultrasound imaging on maintenance of AHE performance (the degree of selective TrA muscle strengthening after AHE) was not investigated in previous studies.[7,8] So, in this study, we aimed to investigate the effects of AHE with different 2 feedback methods on selective reinforcing the TrA muscle in healthy individuals; conventional feedback versus visual feedback from real-time ultrasound imaging in addition to conventional feedback.
2. Materials and methods
2.1. Subjects
A convenience sample of 20 healthy adults (age 29.00 ± 3.00 years, body mass index 22.14 ± 1.71 kg/m2) were recruited for this study. Exclusion criteria included history of low back pain in 6 months, previous abdomen or back surgery, spinal abnormality, known neuromuscular disease, pregnancy, and prior training in AHE or trunk stabilizing exercise (Fig. 1). All subjects were provided with oral explanation and procedural instructions regarding the purpose of the experiment. Then, the subjects signed informed consent form approved by our Institutional Review Board.
Figure 1.
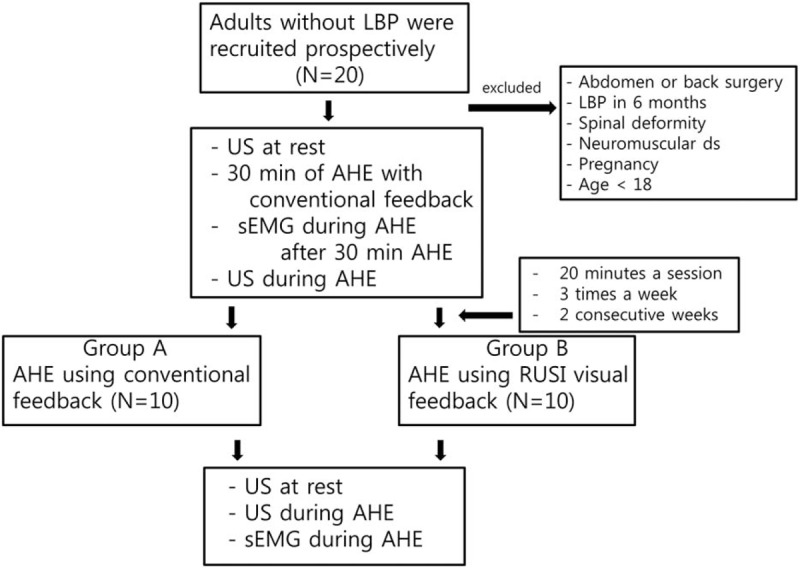
Flow chart of this study. LBP = low back pain.
2.2. Experimental design
On the first visit, the thickness of anterolateral abdominal muscles (TrA, IO, and EO) of all subjects at rest was measured with ultrasound image for the baseline assessment of muscle thickness. All subjects received education session about AHE with conventional (verbal and tactile) feedback from a physiatrist for 30 minutes. After the session, the baseline assessment of the muscles activity was recorded using the surface electromyography (EMG). This activity was recorded while each subject was performing the AHE. Then, they were randomized into 2 groups of A and B by 10 subjects each. Subjects in group A were trained AHE using conventional feedback from a physiatrist. For group B, in addition to the initial education about the conventional feedback, subjects were educated about visual feedback provided with real-time ultrasound imaging. Then, the subjects were trained AHE using visual feedback from real-time ultrasound imaging in addition to conventional feedback. Both groups were equally trained under the same condition: 20 minutes a session, 3 times a week, for 2 consecutive weeks. On the last visit, training effect was evaluated. With same method as the baseline assessment, the thickness and activities of anterolateral abdominal muscles in all subjects were measured by using ultrasound image and surface EMG.
2.3. Intervention
2.3.1. Conventional feedback
We called verbal and tactile feedback as conventional feedback for its easy accessibility in any environment. The guideline of the verbal feedback from a physiatrist is as follows: “In a supine hook-lying position, draw in the lower abdomen toward spine gently and slowly without both contracting the upper abdomen and moving back or pelvis, while comfortably breathing in and out.”[9] The guideline of the tactile feedback is as follows: when performing AHE, subjects placed their finger-tips 2 cm medial and caudal to the anterior superior iliac spine and felt contraction of the muscles on their fingertips. At the same time, the physiatrist positioned his or her fingertips together with the subject and confirmed whether the subject has properly positioned their fingertips and contracted muscles correctly[10] (Fig. 2A).
Figure 2.
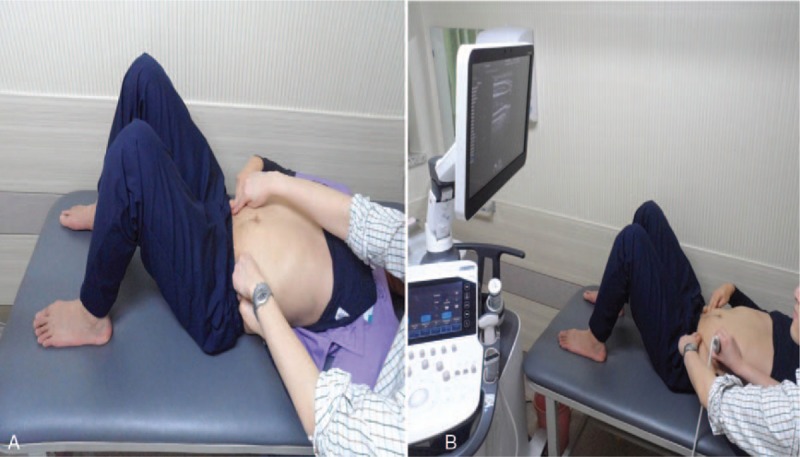
Performing abdominal hollowing exercise (AHE) with different feedback methods. A, AHE with conventional (verbal and tactile) feedback. B, AHE with visual feedback from real-time ultrasound image along with conventional feedback.
2.3.2. Visual feedback
As subject was in supine hook-lying position and relaxed, ultrasound (WS80A; Samsung Medison Inc, Seoul, South Korea) was employed to provide real-time visual feedback. The ultrasound monitor was set for subject to comfortably see the real-time image (Fig. 2B). For transducer, 5.0 cm 10 MHz linear transducer (L3–12A) with B (brightness)-mode was used. The transducer was located in subject's left anterolateral abdominal wall, which is lateral to the midline and halfway between iliac crest and the inferior border of the rib cage.[6,11] The medial edge of the probe was adjusted so that muscle-fascia junction of the TrA was 2 cm away from the medial side of ultrasound monitor (Fig. 3A). Before the AHE, the subject was asked to cough to show movement of his or her abdominal muscles on the monitor. Then, as subject was looking at the monitor, he or she performed the AHE with real time visual feedback as follows: lateral movement and thickening of the TrA muscle, thickening of the IO muscle, and avoiding contraction of the EO muscle[7] (Fig. 3B).
Figure 3.
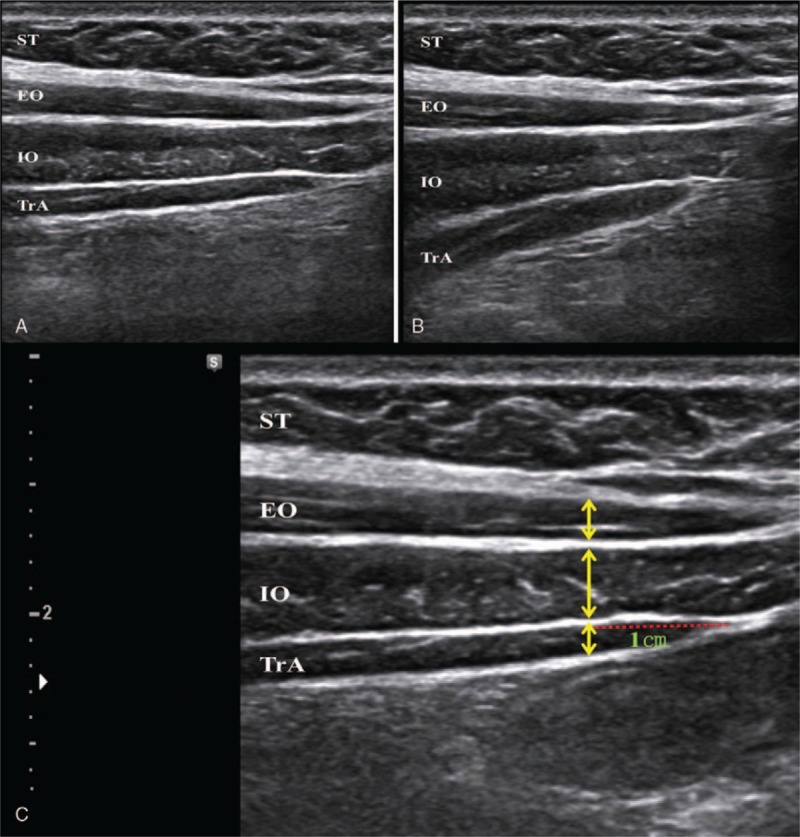
Real-time ultrasound image in anterolateral abdominal muscles. A, Relaxed state. B, Selective contraction of TrA muscle during abdominal hollowing exercise. EO = external oblique abdominal muscle, EO = external oblique abdominal muscle, IO = internal oblique abdominal muscle, IO = internal oblique abdominal muscle, ST = subcutaneous tissue, ST = subcutaneous tissue. C, Measurement in thickness of anterolateral abdominal muscles. Red dotted line indicates horizontal distance from muscle-fascia junction of the TrA. Yellow bidirectional arrows indicate the muscles thickness, TrA = transversus abdominis, TrA = transversus abdominis.
2.4. Data recording
2.4.1. Ultrasonography
To measure the thickness of the 3 muscles, the same transducer of the ultrasound was located in the same abdominal position as in visual feedback.[12] Then angle of the transducer was adjusted so that the image of fascia of TrA, IO, and EO appeared sharply.[13] The ultrasound image was captured at the end of expiration. Muscles thickness of the TrA, IO, and EO muscles was measured in the location horizontally 1 cm lateral to the muscle-fascia junction of the TrA[14] (Fig. 3C). To evaluate the inter-rater reliability, 2 trained physiatrists independently measured the abdominal muscles thickness.
2.4.2. Surface EMG
As the subject was lying on a flat table in relaxed supine position, 2 pairs of surface electrodes were attached. One pair of surface electrode was attached just below left eighth rib's angle, inferomedially toward the pubis,[15] to measure EO muscle activity. The other pair was attached approximately 2 cm medial and caudal to left anterior superior iliac spine. This recording was called TrA-IO signal. In this site, EO does not overlap but only TrA and IO are located, and these 2 muscles cannot be structurally differentiated.[16] The ground electrode was attached to lateral malleolus of left ankle. Both active electrode and reference electrode were attached after the skin of the site was shaved and disinfected by alcohol. The centers of 2 electrodes were 2 cm apart and they were parallel to muscle fiber[17] (Fig. 4).
Figure 4.
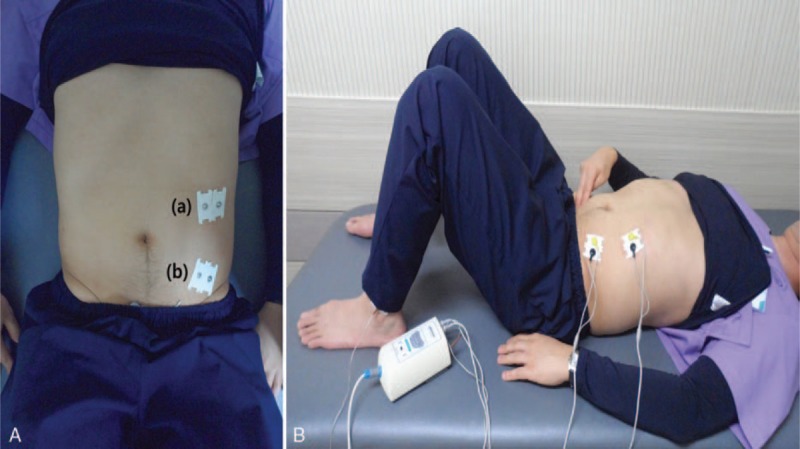
A, Attachment positions of surface electrodes: (a) external oblique abdominal muscle and (b) transversus abdominis-internal oblique abdominal muscle. B, Recording muscle activities of anterolateral abdominal muscles using surface electromyography during abdominal hollowing exercise.
All surface EMG signals were recorded using LXM3208-RF (Laxtha Inc, Daejeon, Korea). EMG data were collected 3 times to calculate their mean values, and they were analyzed (Fig. 4). The recorded EMG data was processed using Telescan 3.05 software (Laxtha Inc), sampled at 1024 Hz, and fixed gain at 300 per channel. The data were filtered at a band-pass of 10 to 450 Hz, rectified, and smoothened using a moving window at 100 ms.
Raw EMG data were converted into root mean square (RMS) values to quantify the muscle activities. Percentages of maximal voluntary contraction (% MVC) were calculated by normalization with MVC to evaluate how efficiently TrA-IO muscles were activated. MVC values of TrA-IO were obtained by maximally twisting upper-body to ipsilateral side against physiatrist's manual resistance.[15]
2.5. Statistical analysis
All the data were analyzed with SPSS ver. 21.0 for Windows (SPSS Inc, Chicago, IL). A Wilcoxon signed rank test was used to assess the mean differences: muscles thickness of the TrA, IO, and EO; RMS of TrA-IO, EO, and %MVC of TrA-IO between the pretraining and posttraining; difference in %MVC values of TrA-IO, RMS of TrA-IO and EO, and the thickness of TrA, IO, and EO muscles for each group. To evaluate intra and inter-rater reliability of ultrasonographic measurement, the intra and inter-rater reliability of ultrasonographic measurement were determined using intraclass correlation coefficients (ICC) with corresponding 95% confidence interval (CI). Following Portney and Watkins’ more rigid cut-off values for clinical measures, reliability was considered poor for ICCs <0.50, moderate for ICCs 0.50 to 0.75, good for ICCs 0.75 to 0.90, and excellent for values above 0.90.[18] A P value of <.05 was regarded as indicating statistical significance.
3. Results
3.1. Reliability of ultrasonographic measurement
We achieved the ICC for intra-rater reliability (ICC = 0.977; CI: 0.976–0.978), and ICC for inter-rater reliability (ICC = 0.973; CI: 0.970–0.975). With values above 0.90, intra-rater and inter-rater ICCs showed excellent levels of reliability.
3.2. Ultrasonographic data on muscle thickness
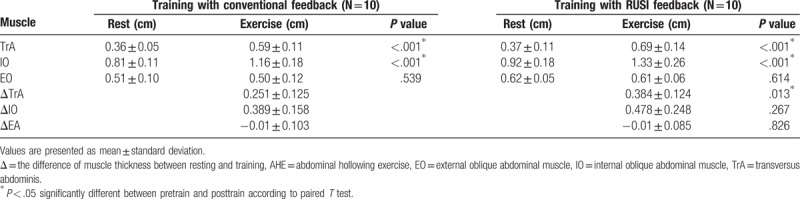
After 2 weeks of AHE training, the thicknesses of TrA, IO, and EO muscles in resting were not significantly changed in both groups A and B (Table 1). However, the thicknesses of contracted TrA and IO muscles during AHE were significantly increased than those of resting state in both of real-time ultrasound imaging and conventional feedback group (P < .05) (Table 2). When compared the difference in muscle thickness between the resting and contraction, the difference of TrA muscle thickness in real-time ultrasound imaging feedback group was significantly higher than conventional feedback group (P < .05) (Table 2). The difference of IO muscle thickness was not significantly different between real-time ultrasound imaging feedback group and conventional feedback group.
Table 1.
Comparison of muscle thickness in resting between pretraining and posttraining.
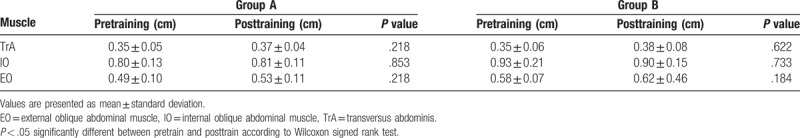
Table 2.
Comparison of muscle thickness during AHE between resting and training.
3.3. Surface EMG data
After 2 weeks of training, RMS and %MVC values in TrA-IO increased without statistical significance in both groups A and B. When compared the difference in %MVC values of TrA-IO during 2 weeks of training, however, the difference in %MVC value of TrA-IO was significantly higher in real-time ultrasound imaging feedback group than conventional feedback group (Table 3).
Table 3.
Comparison of electromyographic muscle activity between pretraining and posttraining.
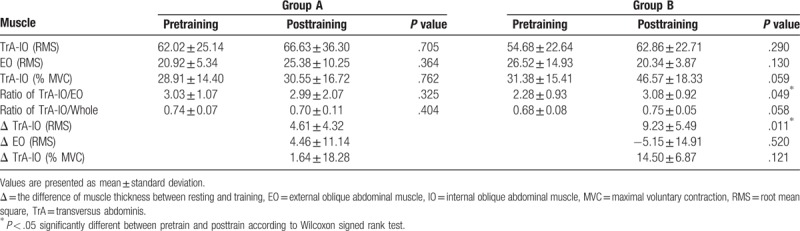
The ratio of RMS values of TrA-IO/EO muscles, which mirrors selective contraction of TRA-IO muscles against EO muscles, was significantly higher in group B than in group A (P < .05) (Table 3).
4. Discussion
Trunk muscles are mainly classified into local muscles and global muscles. Local muscles are defined as important muscles for lumbar spine stabilization, and these include TrA, and MF.[19] Global muscles such as EO and rectus abdominis (RA) are defined as muscles responsible for gross movements of the trunk.[20] Among the local muscles, TrA is the primary abdominal muscle associated with low back pain, and is controlled separately from other trunk muscles,[11] and it is activated preferentially prior to limb movement.[21,22] This muscle plays crucial role in the maintenance of trunk stability.[3] Therefore, it is important to enhance the TrA muscle. In our study, real-time ultrasound imaging feedback was provided on each time for 2 weeks of AHE training, and result showed that visual feedback from real-time ultrasound imaging may be useful additional method to conventional feedback for strengthening the TrA muscles more effectively in individuals without low back pain. To the best of our knowledge, there has been no study about the effect of AHE with real-time ultrasound imaging on the maintenance in selective strengthening of the TrA muscle, although previous studies demonstrated that the use of real-time ultrasound imaging feedback is beneficial for facilitating consistency of TrA muscle contraction during AHE.
When AHE was performed, the thicknesses of TrA and IO muscles during contraction were significantly increased in both of conventional biofeedback and real-time ultrasound imaging visual feedback. However, the thickness of EO muscle was not significantly changed in both of conventional biofeedback and real-time ultrasound imaging visual feedback. This implies that both of conventional biofeedback and real-time ultrasound imaging visual feedback contributed to selective contraction of TrA and IO muscles without contraction of EO muscle.
In the results of surface EMG, the ratio of RMS values of TrA-IO/EO muscles, which mirrors selective contraction of TRA-IO muscles against EO muscle, was significantly higher in real-time ultrasound imaging visual feedback group than conventional feedback group. When compared the differences in muscle thickness between the resting and contraction, the difference of TrA muscle thickness was significantly higher than those of IO muscle in real-time ultrasound imaging visual feedback group. These results show that in individuals without low back pain, training with AHE using real-time ultrasound imaging feedback in addition to conventional feedback enhances TrA muscle more selectively than AHE using conventional feedback. In other words, it is thought that the enhanced IO is more related to the characteristic of conventional feedback such as tactile feedback. Subject received tactile feedback on site where only TrA and IO muscles are located, which is small window of just medial to the anterior superior iliac spine.[7] In this site, where subject simultaneously felt direct contraction of IO and indirect contraction of TrA, subject could not differentiate contraction of 2 muscles from tactile feedback alone.
When compared the difference in %MVC values of TrA-IO during 2 weeks of training, the difference in %MVC value of TrA-IO was significantly higher in real-time ultrasound imaging feedback group than conventional feedback group. Considering more effective contraction of TrA muscles in real-time ultrasound imaging feedback group than conventional feedback group, this may imply that individuals without low back pain, training with AHE using real-time ultrasound imaging feedback may be more effective in selective strengthening TrA muscle than AHE using conventional feedback. Considering the importance of maintenance of strengthening the selective TrA muscle in individuals with chronic low back pain during daily life, training with AHE with real-time ultrasound imaging may be helpful for individuals with chronic low back pain.
Increased strength of TrA muscle in this study is thought to be neural adaption, not muscle hypertrophy, because the thicknesses of TrA and IO muscles in resting state were not significantly increased during 2 weeks of AHE training. These findings are in consistence to the previous study that demonstrated the early stage of strengthening is mainly due to neural adaption and muscle hypertrophy contributes strengthening after several weeks from start of strengthening exercise.[23]
This study has a few limitations. First, although statistically significant results were derived, the number of subjects was not enough to generalize the study result. Second, surface EMG was used instead of fine-wire EMG. For TrA that is located in the deeply, it was difficult to compare the muscle activity of TrA itself accurately with surface EMG between groups A and B. Since the fine-wire EMG is considered the gold standard for precise evaluation of muscle recruitment,[24] further researches with this EMG technique would be required for more accurate comparison. Last, we only investigated the effectiveness of AHE with real-time ultrasound imaging feedback for 2 weeks. So, we could not find any meaningful difference of muscle thickness in resting. Therefore, further researches that investigate long-term effects of AHE with real-time ultrasound imaging would be required.
5. Conclusion
In conclusion, training with AHE in healthy subjects using visual feedback may be a useful additional method to conventional feedback for strengthening the TrA muscles selectively.
Author contributions
Data curation: Dae Hee Lee, Seong Kyung Hong, Donghwi Park.
Formal analysis: Donghwi Park.
Funding acquisition: Donghwi Park.
Methodology: Seong Kyung Hong.
Software: Yang-Soo Lee, Chul-Hyun Kim, Jong Moon Hwang.
Supervision: Zeeihn Lee, Jong Min Kim.
Writing – original draft: Seong Kyung Hong, Donghwi Park.
Writing – review & editing: Donghwi Park.
Footnotes
Abbreviations: AHE = abdominal hallowing exercise, EMG = electromyography, EO = external oblique abdominal muscle, IO = internal oblique abdominal muscle, MVC = maximal voluntary contraction, RA = rectus abdominis, RMS = root mean square, TrA = transversus abdominis.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF- 2017R1D1A1B03033127).
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
The authors have no conflicts of interest to disclose.
References
- [1].Macedo LG, Maher CG, Latimer J, et al. Motor control exercise for persistent, nonspecific low back pain: a systematic review. Phys Ther 2009;89:9–25. [DOI] [PubMed] [Google Scholar]
- [2].Panjabi MM. Clinical spinal instability and low back pain. J Electromyogr Kinesiol 2003;13:371–9. [DOI] [PubMed] [Google Scholar]
- [3].Hides JA, Jull GA, Richardson CA. Long-term effects of specific stabilizing exercises for first-episode low back pain. Spine (Phila Pa 1976) 2001;26:E243–8. [DOI] [PubMed] [Google Scholar]
- [4].Hides J, Gilmore C, Stanton W, et al. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Man Ther 2008;13:43–9. [DOI] [PubMed] [Google Scholar]
- [5].Hodges PW, Richardson CA. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehabil 1999;80:1005–12. [DOI] [PubMed] [Google Scholar]
- [6].Souza GM, Baker LL, Powers CM. Electromyographic activity of selected trunk muscles during dynamic spine stabilization exercises. Arch Phys Med Rehabil V 82 2001;1551–7. [DOI] [PubMed] [Google Scholar]
- [7].Henry SM, Westervelt KC. The use of real-time ultrasound feedback in teaching abdominal hollowing exercises to healthy subjects. J Orthop Sports Phys Ther 2005;35:338–45. [DOI] [PubMed] [Google Scholar]
- [8].Hides JA, Miokovic T, Belavy DL, et al. Ultrasound imaging assessment of abdominal muscle function during drawing-in of the abdominal wall: an intrarater reliability study. J Orthop Sports Phys Ther 2007;37:480–6. [DOI] [PubMed] [Google Scholar]
- [9].Urquhart DM, Hodges PW, Allen TJ, et al. Abdominal muscle recruitment during a range of voluntary exercises. Man Ther 2005;10:144–53. [DOI] [PubMed] [Google Scholar]
- [10].Lee AY, Kim EH, Cho YW, et al. Effects of abdominal hollowing during stair climbing on the activations of local trunk stabilizing muscles: a cross-sectional study. Ann Rehabil Med 2013;37:804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hodges PW. Is there a role for transversus abdominis in lumbo-pelvic stability? Man Ther 1999;4:74–86. [DOI] [PubMed] [Google Scholar]
- [12].Razek AA, Fouda NS, Elmetwaley N, et al. Sonography of the knee joint(). J Ultrasound 2009;12:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Teyhen DS, Miltenberger CE, Deiters HM, et al. The use of ultrasound imaging of the abdominal drawing-in maneuver in subjects with low back pain. J Orthop Sports Phys Ther 2005;35:346–55. [DOI] [PubMed] [Google Scholar]
- [14].Whittaker JL. Ultrasound imaging of the lateral abdominal wall muscles in individuals with lumbopelvic pain and signs of concurrent hypocapnia. Man Ther 2008;13:404–10. [DOI] [PubMed] [Google Scholar]
- [15].Beith ID, Synnott RE, Newman SA. Abdominal muscle activity during the abdominal hollowing manoeuvre in the four point kneeling and prone positions. Man Ther 2001;6:82–7. [DOI] [PubMed] [Google Scholar]
- [16].Marshall P, Murphy B. The validity and reliability of surface EMG to assess the neuromuscular response of the abdominal muscles to rapid limb movement. J Electromyogr Kinesiol 2003;13:477–89. [DOI] [PubMed] [Google Scholar]
- [17].Ekstrom RA, Donatelli RA, Carp KC. Electromyographic analysis of core trunk, hip, and thigh muscles during 9 rehabilitation exercises. J Orthop Sports Phys Ther 2007;37:754–62. [DOI] [PubMed] [Google Scholar]
- [18].Cicchetti D, Bronen R, Spencer S, et al. Rating scales, scales of measurement, issues of reliability: resolving some critical issues for clinicians and researchers. J Nerv Ment Dis 2006;194:557–64. [DOI] [PubMed] [Google Scholar]
- [19].Bergmark A. Stability of the lumbar spine. A study in mechanical engineering. Acta Orthop Scand Suppl 1989;230:1–54. [DOI] [PubMed] [Google Scholar]
- [20].Norris CM. Abdominal muscle training in sport. Br J Sports Med 1993;27:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hodges PW, Richardson CA. Contraction of the abdominal muscles associated with movement of the lower limb. Phys Ther 1997;77:132–42. [DOI] [PubMed] [Google Scholar]
- [22].Hodges PW, Richardson CA. Transversus abdominis and the superficial abdominal muscles are controlled independently in a postural task. Neurosci Lett 1999;265:91–4. [DOI] [PubMed] [Google Scholar]
- [23].Moritani T, deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 1979;58:115–30. [PubMed] [Google Scholar]
- [24].Ferreira ML, Ferreira PH, Latimer J, et al. Comparison of general exercise, motor control exercise and spinal manipulative therapy for chronic low back pain: a randomized trial. Pain 2007;131:31–7. [DOI] [PubMed] [Google Scholar]


