Abstract
Background:
To compare the efficacy of high- and low-energy extracorporeal shock wave therapy (ESWT) for patients with myofascial pain syndrome (MPS) of the upper trapezius.
Methods:
Thirty patients (3 men, 27 women) were randomly assigned to receive either high-energy ESWT (0.210 mJ/mm2) or low-energy ESWT (0.068 mJ/mm2). Both groups received 1500 pulses of ESWT once a week, for 2 weeks. Outcome measurement was assessed by verbal numeric pain scale (VNS), neck disability index (NDI), neck range of motion (ROM) (flexion, extension, rotation, lateral bending), and pressure threshold (PT). Statistical analysis was performed with significance level of P < .05.
Results:
No statistically significant differences of demographic and clinical characteristics existed between the 2 groups. VNS, NDI, neck ROM (rotation to sound side, lateral bending to affected side, lateral bending to sound side), and PT were improved in both groups. In contrast, statistically significant improvements in neck flexion and neck extension were observed only in the high-energy group. We also found significant differences in post-treatment NDI (4.20 ± 1.78 vs 6.47 ± 2.48) and post-treatment neck flexion ROM (65.47 ± 10.09 vs 55.93 ± 11.07) between high-energy and low-energy group.
Conclusion:
ESWT effectively improves VNS, NDI, neck ROM, and PT to patients with MPS of the upper trapezius. High-energy ESWT was more effective in improving NDI and neck flexion ROM compared to the low-energy ESWT, suggesting superiority in functional improvement. Further studies are required to specify the effect of ESWT by energy intensity.
Keywords: energy level, ESWT, myofascial pain syndrome, upper trapezius
1. Introduction
Myofascial pain syndrome (MPS) is a common cause of pain in clinical practice. Its prevalence has been estimated at 12%.[1] MPS is characterized by areas known as myofascial trigger points (TrPs) associated with a hyperirritable spot in a taut band of a skeletal muscle. It is a very small, localized area of muscle contraction that is hard to touch; very tender; painful on compression, stretch, overload, or contraction of the muscle; and usually has a distinct referred pain pattern.[2] The exact pathophysiology and etiology of TrP and MPS are still unknown.[3] However, many proposed mechanisms have been studied and reported in the literature. It has been suggested that myofascial pain is a complex form of neuromuscular dysfunction consisting of motor and sensory abnormalities involving both the peripheral and central nervous systems.[3–5]
The diagnosis of MPS, which manifests with 1 or more active TrP, is usually based on the patient's subjective symptoms and the presence of an active TrP characterized by tender spots in 1 or more palpable taut band, a referred pain pattern, local twitch response (LTR), and restricted range of motion (ROM).[6] Traditional therapeutic approaches for the treatment of MPS include pharmacotherapy (nonsteroidal anti-inflammatory drugs, steroids, tricyclic antidepressants, vasodilators, or oral skeletal muscle relaxants), injection therapy (TrP injection of local anesthetic with or without corticosteroid, or “dry” needling), physical therapy, and behavioral modification.[7]
More recently, the use of extracorporeal shock wave therapy (ESWT) has expanded to the treatment of MPS.[8–14] Müller-Ehrenberg demonstrated the efficacy of focused ESWT (800 impulses of energy level: 0.04 to 0.26 mJ/mm2; 6 Hz; average 7 treatments, 2 sessions per week) on TrP, alleviating pain in 95% of the 30 patients in his group at 3 months.[8] In another study, Ji used an electromagnetic device to demonstrate the same results on MPS of the upper trapezius compared to a placebo.[9] In this study, the treatments of the taut band (700 impulses) and that of the surrounding area (300 impulses) were performed separately at energy levels of 0.056 mJ/mm2, in 2 sessions per week for 2 weeks.[9] Although recent studies have shown some degree of the efficacy, it is not yet clear which energy level is the most effective in pain relief and clinical improvement of MPS after ESWT.[14] To our knowledge, no studies investigating the dose-dependent effectiveness of ESWT in MPS patients have been reported. Therefore, we conducted a prospective single-blind randomized clinical trial to compare the high-energy with low-energy ESWT.
2. Methods
2.1. Participants
This is a prospective single-blind randomized clinical trial to compare the high-energy with low-energy ESWT in terms of the efficacy for the patients with MPS of the upper trapezius.
After obtaining an approval and registering for a clinical trial with the institutional review board (IRB), subjects were recruited at the outpatient musculoskeletal pain clinic from September 1, 2016 to December 31, 2016. Upon consenting to be included in the study, the patients were asked blinded general questions regarding age, previous conservative treatments (physiotherapy, TrP injection), pain duration, current medicine intake, and average pain intensity over the previous week, by an interviewer. Diagnoses of MPS in the upper trapezius muscle were made by 2 physiatrists based on Simon's criteria.[15] That is, a well-defined, tender, hypersensitive, palpable nodule located within a taut band of the upper trapezius muscle; typical referred pain pattern; and LTR elicited by a snapping palpation of the TrP, with pain onset within 3 months and pain related to the ipsilateral shoulder and neck regions with an 11-point verbal numeric pain scale (VNS) score of 4 or more.
The exclusion criteria for this study included patients with severe diseases (heart disease, liver disease), severe psychiatric problems, systematic rheumatic disease, neurologic disorders such as cervical radiculopathy or stroke, bleeding tendency, history of side effects to local anesthetics, presence or suspicion of infection, patients using a pacemaker, those who have undergone any drug therapy (other than acetaminophen) or anticoagulant therapy, those aged under 19 or over 70 years, and pregnant or breastfeeding women, which might affect the results during the treatment or follow-up periods.
2.2. Interventions
A research assistant randomly assigned participants to the study groups by block randomization, using a table of random numbers. A simpler classification was used to distinguish between low-energy ESWT having an energy flux density (EFD) of less than 0.12 mJ/mm2 and high-energy ESWT having an EFD between 0.12 and 0.38 mJ/mm2.[16] The patients were randomly assigned to receive either high-energy ESWT (0.210 mJ/mm2), or low-energy ESWT (0.068 mJ/mm2). Both groups received 1500 pulses once a week for 2 weeks. All patients were required to discontinue pain medications, including Nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, antidepressants, and muscle relaxants, for a week before the first treatment with ESWT.
All ESWTs were performed by 2 physiatrists with over 3 years of relevant experience using a Dornier AR2 (MedTech, Munich, Germany). The patients were asked to sit on a chair without a backrest and lower the arm. For the target position, 2 physiatrists examined the TrP of the upper trapezius and applied focusing on the area (TrP) at which muscular twitching response or referred pain should be induced by appropriately adjusting the location of the localized probe. If MPS was diagnosed bilaterally, the more painful side was treated. To avoid the intense pain during the treatment, a subcutaneous infiltration of a local anesthetic, lidocaine (1%, 5 mL) was administered under sterile conditions. Local anesthesia was performed with the equal dosage of anesthetic (a total of 5 mL per 1 injection) in every therapy for all the participants of both groups. A transparent film (Tegaderm 3M, St. Paul, MN) was applied on the injected site, and coupling gel was used between the shock wave head and the TrP. Rescue medication was allowed throughout the entire study period if the pain became unbearable (2 g of acetaminophen per day for up to 2 weeks after the last treatment). No other conservative treatment (e.g., laser, acupuncture, ultrasound, other NSAID drug or corticosteroid, TPI) was allowed until after the 2-week follow-up period after the last treatment. The assignments were then delivered in sealed opaque envelopes. The patients as well as the follow-up evaluators were blinded to the treatment assignments.
2.3. Outcome measurement
The clinical follow-up examinations were performed by an independent observer, who had no information about the treatment protocol, and conducted during hospital visits at the baseline and 2 weeks after the last treatment. Outcome measurement was assessed by the VNS, neck disability index (NDI), neck ROM (flexion, extension, rotation, and lateral bending), and pain threshold. VNS was obtained at baseline and 2 weeks after the last treatment to weigh up the treatment efficacies. While using the VNS, the patients were asked to rate their pain on a scale from 0 to 10, where 0 and 10 represented “no pain” and “the worst pain possible,” respectively, in whole numbers with 11 integers including zero.[17] The degree of physical disability was measured with NDI, which is the most widely used survey questionnaire for assessing cervical spine abnormality. NDI was first developed to evaluate the degree of limitations in the daily lives of patients with severe cervical pains, especially for those with whiplash trauma.[16] NDI with 10 questionnaires is composed of 7 functional activity-related, 2 symptom-related, and 1 concentration-related questions. The final NDI score was obtained by adding all scores from the questions. Higher NDI score indicated increased functional disability related to cervical abnormality. The original developer, Vernon, suggested interpreting the scores by the following ranges from 0 to 50: 4 or lower = no disability, 5 to 14 = mild disability, 15 to 24 = moderate disability, 25 to 34 = severe disability, and 35 or over = complete disability.[18] The body positioning for ROM measurement included having each subject sit in a straight-back, wood-frame chair with an upright posture, low- and mid-back regions contacting the backrest, feet flat on the floor, and upper extremities positioned at the sides with the shoulders relaxed.[19] A physician assisted the patient in achieving maximum active motion, and the other measured active ROM in 4 directions (flexion, extension, rotation, and lateral bending) using a Dualer IQ inclinometer (JTECH Medical, Salt Lake City, UT). The measurements were repeated 3 times, and the results were expressed as averages.
Pressure threshold (PT) was defined as the minimum pressure (kg/cm2) that induced pain or discomfort.[20] PT was measured with an analog algometer—a force gauge fitted with a rubber disk with a surface area of 1 cm2. The rubber tip of the algometer was placed on the dominant upper trapezius muscle, 9-cm lateral to the C7 spinous process (consistent with the motor point of the upper trapezius), with the shaft vertical to the examined surface.[20] Pressure was then increased continuously by approximately 0.1 kg/s until the PT was obtained.
2.4. Statistical analysis
The chi-square test was used to compare the sex, treated side, and bilaterality between the 2 groups. Mann–Whitney U test was used to compare the age, duration of symptom, VNS, NDI, neck ROM, and PT between the 2 groups before the treatment. Mann–Whitney U test was also used to compare VNS, NDI, neck ROM, and PT between the 2 groups after the treatment. Wilcoxon's signed rank test was used for comparison of pretreatment outcomes and post-treatment outcomes in each group. Statistical analysis was performed with IBM SPSS Statistics 24 (24.0.0.0) with a significance level of P < .05.
3. Results
3.1. Subjects
Only 30 out of 37 participants were evaluated since 1 participant dropped due to follow-up loss and 6 participants decided not to participate because of symptom relief before intervention. There was no withdrawal due to side effects of the treatment. The final participants were 3 men and 27 women (Fig. 1).
Figure 1.
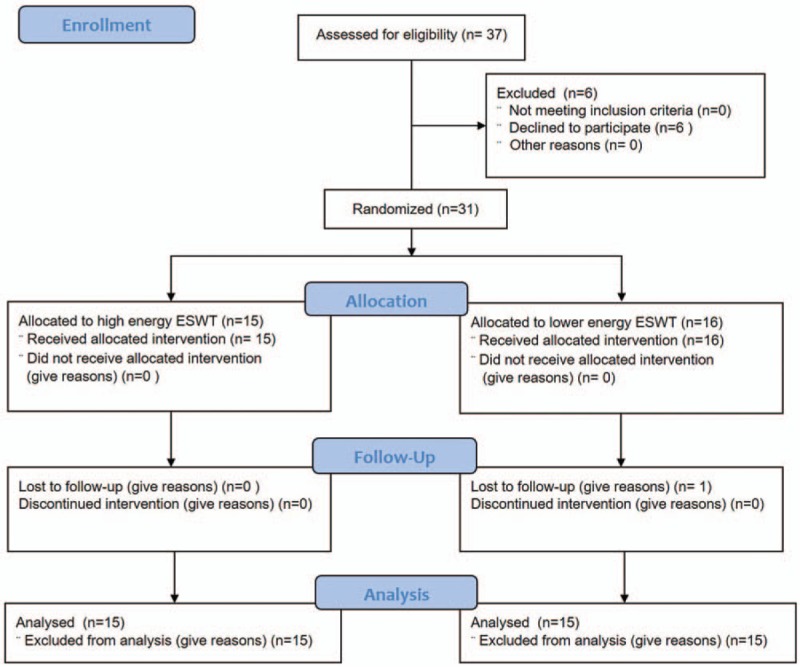
Flow diagram of subjects.
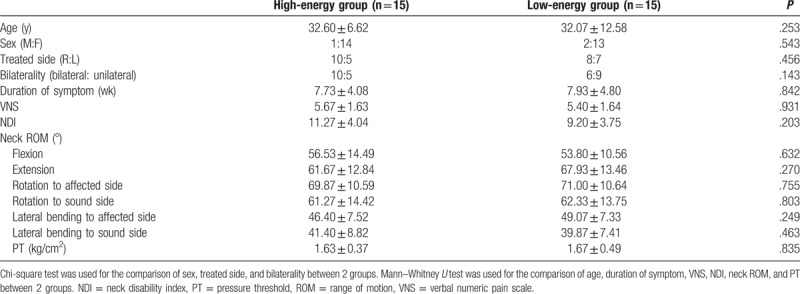
The subjects’ demographics and clinical characteristics are summarized in Table 1. There were no statistically significant differences of age, sex, treated side, bilaterality, and the mean duration of symptoms between the 2 groups.
Table 1.
Prehomogeneity test of the subjects’ demographics, clinical characteristics, and preliminary assessment.
3.2. Preliminary assessment
Table 1 additionally documents that there were no statistically significant differences of VNS, NDI, neck flexion ROM, neck extension ROM, neck rotation ROM to affected side, neck rotation ROM to sound side, neck lateral bending ROM to affected side, and neck lateral bending ROM to sound side between the groups (P value = .931, .203, .632, .270, .755, .803, .249, .463, and .835, respectively).
3.3. Comparison of outcome measures before and after treatment in each group
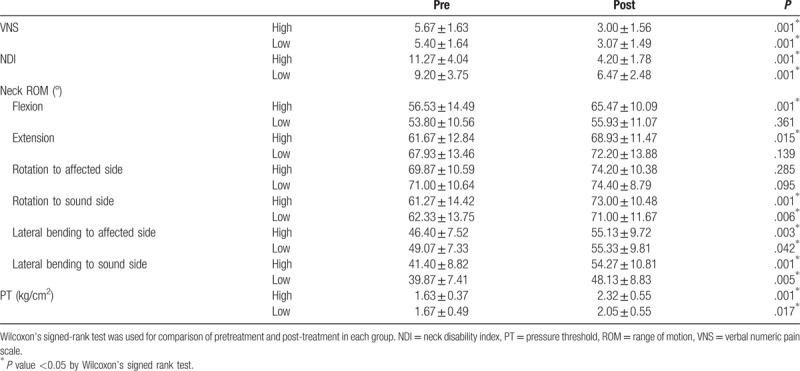
Table 2 shows that pre- and post-treatment VNS, NDI, neck ROM (rotation to sound side, lateral bending to affected side, lateral bending to sound side), and PT had statistically significant differences in each group. In contrast, statistically significant improvements in neck flexion ROM and neck extension ROM were observed only in the high-energy group. Meanwhile, pre- and post-treatment ROM of neck rotation to the affected side had no statistically significant differences in both high- and low-energy groups.
Table 2.
Comparison of outcome measures before and after treatment in each group.
3.4. Comparison of outcome measures between 2 groups after treatment
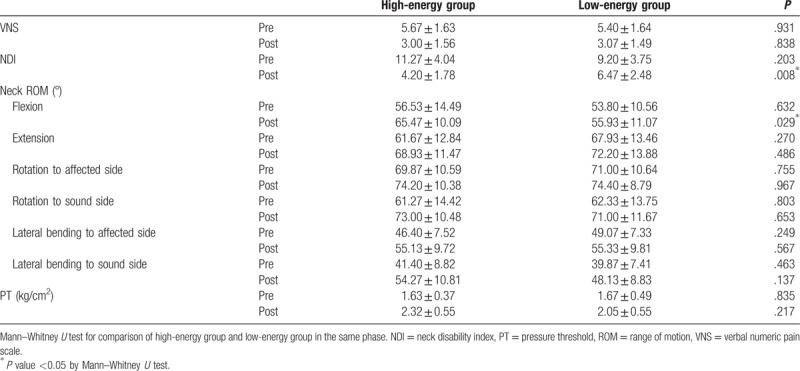
There were statistically significant differences between the groups in NDI (P value = .008) and ROM of neck flexion (P value = .029), respectively, after treatment.
There were no statistically significant differences between the groups in VNS (P value = .838), ROM of neck extension (P value = .486), ROM of neck rotation to the affected side (P value = .967), ROM of neck rotation to the sound side (P value = .653), ROM of neck lateral bending to the affected side (P value = .567), ROM of neck lateral bending to the sound side (P value = .137), and PT (P value = .217) after treatment.
The above results are summarized in Table 3.
Table 3.
Comparison of outcome measures between 2 groups in the same time period.
4. Discussion
In this study, we compared the VNS, NDI, neck ROM, and PT before and after 2 sessions of ESWT, and documented several significant differences. VNS, NDI, neck ROM (rotation to the sound side, lateral bending to the affected side, lateral bending to the sound side), and PT were improved in both groups. However, neck flexion ROM and neck extension ROM were improved only in the high-energy group with statistical significance. We also found the significant differences in the post-treatment NDI and post-treatment neck flexion ROM between the 2 groups.
Several studies have demonstrated the efficacy of ESWT on MPS. Müller-Ehrenberg and Licht reported that focused ESWT on MTPs relieved pain in 3 months for 95% of 30 patients (800 impulses of energy level: 0.04–0.26 mJ/mm2; 6 Hz; average 7 treatments, 2 sessions per week).[8] In Jeon's study, weekly focused ESWT (3 sessions of 1500 pulses with 0.10 mJ/mm2) was as effective as TENS and TrP injection for the pain control of the MPS in the trapezius muscle on 30 patients.[9] In another study, the combination of ESWT and shoulder stabilization exercises was effective in reducing pain and restoring function in MPS patiens.[11] Moghtaderi et al[12] reported that the application of ESWT to both plantar fascia and gastroc-soleus TrPs was more effective than solitary plantar fascia alone in the treatment of plantar fasciitis. In another randomized study, it has been demonstrated that low-energy ESWT (1000 pulses at 0.25 mJ/mm2) for MPS can be more effective when administered with regimen of 3 session treatments.[13]
Several previous studies investigated how ESWT has an impact on MPS. They concluded it by inferring certain hypotheses, so it still remains obscure. Considering the Energy Crisis Hypothesis and the mechanotransduction effect of ESWT in other diseases,[4,21,22] ESWT in MPS probably facilitates perfusion, helps the reformation of blood vessels (angiogenesis effect), facilitates connective tissue recovery, supplies adenosine triphosphate to the blood stream around the TrPs, and alters the pain signaling in ischemic tissues caused by calcium influx when applied deeply to the injured regions.[23] In addition, a recent animal study has tested the hypothesis that relief of chronic pain by ESWT is due to selective loss of nerve fibers in peripheral nerves. To confirm this hypothesis, high-energy ESWTs were applied to the ventral side of the right distal femur of rabbits. The femoral and sciatic nerves were investigated at the light and electron microscopic level after 6 weeks. ESWT caused a selective, substantial loss of unmyelinated nerve fibers within the femoral nerve of the treated hind limb, while the sciatic nerve of the treated hind limb remained unaffected. It probably implies that a relief of chronic pain by a transient dysfunction of nerve excitability at neuromuscular junction via selective partial denervation (degeneration of acetylcholine receptor in free nerve ending) does play an important role in the effects of ESWT application on the musculoskeletal system.[24]
We mentioned the probable mechanism of ESWT on pain reduction in the above paragraph. Then how does ESWT affect the ROM? An abovementioned study stated that ESWT was involved in improvement of ROM of the shoulder joints and recovery of cervical functions, as the ESWT gun confines unwanted motions in joint exercises and provides stability.[1] Although the study mentioned positive effects of combination of ESWT and stabilization exercise on VAS (pain), constant Murley scale score, and NDI (functional improvement), there were also statistically significant improvements of such outcome measures in the ESWT-only group.[11] Especially with regard to ROM and strength, ESWT-only group showed statistically significant improvement (P < .05) after the 4-week intervention, whereas exercise-only group did not. Unfortunately, the exact mechanism of neck ROM improvement has not been clearly investigated up to now. But we find that the improvement of neck ROM occurs secondary to the pain relief of the upper trapezius by ESWT when the anatomy and function of this muscle are taken into account. The upper trapezius originates from the occipital bone (superior nuchal line and external occipital protuberance) and the spinous processes of all cervical vertebrae via the nuchal ligament. It is inserted to lateral third of the clavicle. Its actions are to draw the scapula obliquely upward, to rotate the glenoid cavity inferiorly (acting with the inferior part of the serratus anterior), to tilt the head to the same side, and to rotate the head to the opposite side (with the shoulder girdle fixed).
In spite of the extensive use of ESWT worldwide, studies on dose-relationship between the intensity and the biological effects of ESWT remain inadequate. In addition, to our knowledge, there have been only few studies for MPS of such kind. Therefore, we decided to compare the high-energy ESWT and low-energy ESWT for MPS patients. Low-energy ESWT usually has little side effects and good effectiveness as per the results of laboratory researches.[25,26] Moreover, it does not require local anesthesia, which is known to prevent the interference of recovery of damaged tissues and rupture of tissues in high-energy ESWT.[25,27] It can also prevent the phenomenon of lowering of the level of adaptation of the patients or patients’ giving up of the treatment because of pain in the middle of the treatment course.[9] Low-energy ESWT is also known to show fewer local swelling and tenderness.[27] However, the treatment effects of high-energy ESWT are superior.[28–30] Previous studies demonstrated that higher intensity energy destroys more unmyelinated sensory nerve fibers, and thereby has a greater pain-reducing effect.[31,32] The histological reaction to the ESWT is known to be dose-dependent on the total energy delivered to the tissue (total effectiveness energy = EFD [mJ/mm2] × mm2 × number) (mJ).[25,33] A preliminary study was conducted to compare the immediate effects of high-energy versus low-energy ESWT on the cutaneous microcirculation in a real-time and noninvasive setting, using a rat standardized model. High-energy ESWT significantly increases parameters of cutaneous microcirculation immediately after application, resulting in higher tissue oxygen saturation, venous filling pressure, and blood velocity, which result in higher tissue perfusion with enhanced oxygen saturation, in contrast to low-energy as well as placebo ESWT.[34]
Our study showed greater improvement of NDI and neck flexion ROM in the high-energy group than that in the low-energy group by statistical significance. This phenomenon is related to functional improvement. Although our study showed it without suggesting the exact mechanism of ESWT application on functional improvement and it has not been studied exactly so far, the functional improvement by ESWT application is probably caused by the pain reduction effect of ESWT. In other words, functional improvement could follow pain reduction. In this manner, the patients in our study probably showed pain reduction and functional improvement.
The probable side effects of ESWT include cutaneous erythema, pain, small hematoma, syncope, headache, and so on. However, our patients did not experience major adverse effects.
The present study has several limitations. First, it has demographical limitations (only a small number of subjects, female predominance, and limited distribution of age). Second, as it was conducted for a short period of time, it could not fully assess the long-term effects of high-energy and low-energy ESWT. More precisely, although the neuromodulation effect could be reflected in the results of present study, the enhancement of tissue perfusion to ischemic tissue via angiogenesis could not be reflected in such a short period of time. Therefore, future studies should be conducted for a longer period of time to fully investigate the effects. Third, it does not have a control group to rule out the placebo effect. Fourth, the effectiveness of ESWT in improving the outcome measures are inferred solely based upon the past studies. Molecular, cellular, and histological research should have been conducted together with the present study. Especially, further in vivo studies are required to explain the pain-relieving mechanisms of ESWT. In addition, further investigations would allow us to understand the dose-dependent effectiveness of ESWT on MPS and its mechanisms. Fifth, as an application of local anesthesia inhibits the effects of ESWT on nociceptors,[35] local anesthesia in this study could have affected the findings of our research. However, as local anesthesia was performed with the same dosage of anesthetic in every therapy for all the participants of both groups, it could not have significantly affected the result.
5. Conclusion
ESWT was effective in improving VNS, NDI, neck ROM, and PT for patients with MPS of the upper trapezius. High-energy ESWT was especially effective in improving NDI and neck flexion ROM compared to the low-energy ESWT. In other words, high-energy ESWT has superiority for pain reduction and functional improvement for patients with MPS of the upper trapezius.
Considering the limitations mentioned above, further studies could specify the effects of ESWT by energy intensity.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C2319).
The authors have not received any other benefits from commercial sources for the work reported in the manuscript. The authors declare no conflicts of interest with regard to the work.
Author contributions
Conceptualization: Jae Ki Ahn, Yongbum Park.
Data curation: Ki Deok Park, Woo Yong Lee, Min-ho Park, Jae Ki Ahn.
Formal analysis: Ki Deok Park, Woo Yong Lee, Min-ho Park, Jae Ki Ahn.
Funding acquisition: Ki Deok Park.
Investigation: Ki Deok Park, Min-ho Park, Yongbum Park.
Methodology: Ki Deok Park, Woo Yong Lee, Min-ho Park, Yongbum Park.
Supervision: Yongbum Park.
Writing – original draft: Ki Deok Park, Woo Yong Lee, Min-ho Park.
Writing – review & editing: Ki Deok Park, Woo Yong Lee, Yongbum Park.
Footnotes
Abbreviations: EFD = energy flux density, ESWT = extracorporeal shock wave therapy, LTR = local twitch response, MPS = myofascial pain syndrome, NDI = neck disability index, PT = pressure threshold, ROM = range of motion, TrP = trigger point, VNS = verbal numeric pain scale.
KDP and WYL have contributed equally to this work as first authors.
Funding/support: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C2319).
The authors have not received any other benefits from commercial sources for the work reported in the manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Blasberg B, Chalmers A. Temporomandibular pain and dysfunction syndrome associated with generalized musculoskeletal pain: a retrospective study. J Rheumatol Suppl 1989;19:87–90. [PubMed] [Google Scholar]
- [2].Simons D. Clinical and etiological update of myofascial pain from trigger points. J Musculoskeletal Pain 1996;4:97–125. [Google Scholar]
- [3].Shah JP, Phillips TM, Danoff JV, et al. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol 2005;99:1977–84. [DOI] [PubMed] [Google Scholar]
- [4].Shah JP, Danoff JV, Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil 2008;89:16–23. [DOI] [PubMed] [Google Scholar]
- [5].Kuan TS, Hong CZ, Chen JT, et al. The spinal cord connections of the myofascial trigger spots. Eur J Pain 2007;11:624–34. [DOI] [PubMed] [Google Scholar]
- [6].Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol 2004;14:95–107. [DOI] [PubMed] [Google Scholar]
- [7].Ferrante FM, Bearn L, Rothrock R, et al. Evidence against trigger point injection technique for the treatment of cervicothoracic myofascial pain with botulinum toxin type A. Anesthesiology 2005;103:377–83. [DOI] [PubMed] [Google Scholar]
- [8].Muller-Ehrenberg H, Licht G. Diagnosis and therapy of myofascial pain syndrome with focused shock waves. Med Orthop Tech 2005;5:1–5. [Google Scholar]
- [9].Jeon JH, Jung YJ, Lee JY, et al. The effect of extracorporeal shock wave therapy on myofascial pain syndrome. Ann Rehabil Med 2012;36:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ji HM, Kim HJ, Han SJ. Extracorporeal shock wave therapy on myofascial pain syndrome of upper trapezius. Ann Rehabil Med 2012;36:675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cho YS, Park SJ, Jang SH, et al. Effects of the combined treatment of extracorporeal shock wave therapy (ESWT) and stabilization exercises on pain and functions of patients with myofascial pain syndrome. J Phys Ther Sci 2012;24:1319–23. [Google Scholar]
- [12].Moghtaderi A, Khosrawi S, Dehghan F. Extracorporeal shock wave therapy of gastroc-soleus trigger points in patients with plantar fasciitis. A randomized, placebo-controlled trial. Adv Biomed Res 2014;25:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gür A, Koca İ, Karagüllü H, et al. Comparison of the effectiveness of two different extracorporeal shock wave therapy regimens in the treatment of patients with myofascial pain syndrome. Arch Rheumatol 2014;29:186–93. [Google Scholar]
- [14].Ramon S, Gleitz M, Hernandez L, et al. Update on the efficacy of extracorporeal shockwave treatment for myofascial pain syndrome and fibromyalgia. Int J Surg 2015;24:201–6. [DOI] [PubMed] [Google Scholar]
- [15].Simons DG, Travell JG, Simons LS. Travell and Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual. 2nd ed.1999;Baltimore, MD: Williams & Wilkins, 278–307. [Google Scholar]
- [16].Ioppolo F, Tattoli M, Di Sante L, et al. Extracorporeal shock-wave therapy for supraspinatus calcifying tendinitis: a randomized clinical trial comparing two different energy levels. Phys Ther 2012;92:1376–85. [DOI] [PubMed] [Google Scholar]
- [17].Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain Pract 2003;3:310–6. [DOI] [PubMed] [Google Scholar]
- [18].Vernon H, Mior S. The neck disability index: a study of reliability and validity. J Manipulative Physiol Ther 1991;14:409–15. [PubMed] [Google Scholar]
- [19].Fletcher JP, Bandy WD. Intrarater reliability of CROM measurement of cervical spine active range of motion in persons with and without neck pain. J Orthop Sports Phys Ther 2008;38:640–5. [DOI] [PubMed] [Google Scholar]
- [20].Malanga GA, Gwynn MW, Smith R, et al. Tizanidine is effective in the treatment of myofascial pain syndrome. Pain Physician 2002;5:422–32. [PubMed] [Google Scholar]
- [21].Ottomann C, Hartmann B, Tyler J, et al. Prospective randomized trial of accelerated re-epithelization of skin graft donor sites using extracorporeal shock wave therapy. J Am Coll Surg 2010;211:361–7. [DOI] [PubMed] [Google Scholar]
- [22].Rompe JD, Hope C, Küllmer K, et al. Anlagesic effect of extracorporeal shock-wave therapy on chronic tennis elbow. J Bone Joint Surg Br 1996;78:233–7. [PubMed] [Google Scholar]
- [23].Hofmann A, Ritz U, Hessmann MH, et al. Extracorporeal shock wave-mediated changes in proliferation, differentiation and gene expression of human osteoblasts. J Trauma 2008;65:1402–10. [DOI] [PubMed] [Google Scholar]
- [24].Hausdorf J, Lemmens MA, Heck KD, et al. Selective loss of unmyelinated nerve fibers after extracorporeal shockwave application to the musculoskeletal system. Neuroscience 2008;155:138–44. [DOI] [PubMed] [Google Scholar]
- [25].McClure S, Dorfmuller C. Extracorporeal shock wave therapy: theory and equipment. Clin Tech Equine Pract 2003;2:348–57. [Google Scholar]
- [26].Ogden JA, Alvarez RG, Levitt RL, et al. Electrohydraulic high-energy shock-wave treatment for chronic plantar fasciitis. J Bone Joint Surg Am 2004;86:2216–28. [DOI] [PubMed] [Google Scholar]
- [27].Rompe JD, Meurer A, Nafe B, et al. Repetitive low-energy shock wave application without local anesthesia is more efficient than repetitive low-energy shock wave application with local anesthesia in the treatment of chronic plantar fasciitis. J Orthop Res 2005;23:931–41. [DOI] [PubMed] [Google Scholar]
- [28].Metzner G, Dohnalek C, Aigner E. High-energy extracorporeal shock-wave therapy for the treatment of chronic plantar fasciitis. Foot Ankle Int 2010;31:790–6. [DOI] [PubMed] [Google Scholar]
- [29].Liang HW, Wang TG, Chen WS, et al. Thinner plantar fascia predicts decreased pain after extracorporeal shock wave therapy. Clin Orthop Relat Res 2007;460:219–25. [DOI] [PubMed] [Google Scholar]
- [30].Theodore GH, Buch M, Amendola A, et al. Extracorporeal shock wave therapy for the treatment of plantar fasciitis. Foot Ankle Int 2004;25:290–7. [DOI] [PubMed] [Google Scholar]
- [31].Takahashi N, Ohtori S, Saisu T, et al. Second application of low-energy shock waves has a cumulative effect on free nerve endings. Clin Orthop Relat Res 2006;443:315–9. [DOI] [PubMed] [Google Scholar]
- [32].Ohtori S, Inoue G, Mannoji C, et al. Shock wave application to rat skin induces degeneration and reinnervation of sensory nerve fibers. Neurosci Lett 2001;315:57–60. [DOI] [PubMed] [Google Scholar]
- [33].Rompe JD, Kirkpatrick CJ, Kullmer K, et al. Dose-related effects of shock waves on rabbit tendo achillis. A sonographic and histological study. J Bone Joint Surg Br 1998;80:546–52. [DOI] [PubMed] [Google Scholar]
- [34].Kraemer R, Sorg H, Forstmeier V, et al. Immediate dose-response effect of high-energy versus low-energy extracorporeal shock wave therapy on cutaneous microcirculation. Ultrasound Med Biol 2016;42:2975–82. [DOI] [PubMed] [Google Scholar]
- [35].Klonschinski T, Ament SJ, Schlereth T, et al. Application of local anesthesia inhibits effects of low-energy extracorporeal shock wave treatment (ESWT) on nociceptors. Pain Med 2011;12:1532–7. [DOI] [PubMed] [Google Scholar]


