Abstract
Background:
The influence of the subglottic secretion drainage (SSD) on the microorganisms of ventilator associated pneumonia (VAP) is still unclear.
A meta-analysis focusing on the influence of the SSD on the microorganisms of VAP.
Methods:
A comprehensive search was conducted through the online studies of PubMed, Embase, Cochrane Library, Google scholar, CNKI (China National Knowledge Infrastructure), and VIPI (Database for Chinese Technical Periodicals) using specific search terms.
Included studies were randomized controlled trials (RCTs) that compare the microorganisms of VAP between SSD and standard endotracheal tube care in mechanically ventilated adults.
Results:
Nine RCTs were eligible. There was no significant difference in the rate of VAP caused by nonfermentative bacteria and enterobacteria between SSD group and control group (OR = 0.73, 95%CI, 0.53–1.01; P = .06). The episodes of VAP caused by Gram-positive cocci and Haemophilus influenzae organisms were lower in the SSD group (OR = 0.29, 95%CI, 0.18–0.48; P<0.00001). Less mean volume of SSD daily was observed in VAP group (OR = −16.97, 95%CI, −29.87–4.08; P = .010).
Conclusion:
We found SSD to be associated with significant decreases in VAP caused by Gram-positive cocci and H influenzae organisms but no significant differences in VAP caused by nonfermentative bacteria and enterobacteria. Less mean volume of SSD daily was observed in VAP group.
Keywords: meta-analysis, microorganisms, subglottic secretion drainage, ventilator associated pneumonia
1. Introduction
Ventilator associated pneumonia (VAP) is very common nosocomial infection in the patients who require invasive mechanical ventilation.[1] A number of prophylactic interventions advocated by various organizations include vigilant hand washing, early enteral feeding, sinusitis prophylaxis, head of the bed elevation, preservation of a normal gastric PH, selective digestive decontamination, conscientious oral care protocol, and subglottic secretion drainage (SSD).[2]
The concept of SSD is based on the hypothesis that decreasing aspiration of bacteria pooled above the cuff of the endotracheal tube into the lower respiratory tract reduces the risk of VAP. SSD using a specially designed endotracheal tube with a separate dorsal lumen which opens immediately above the endotrcheal cuff, has been developed to avoid the progression of subglottic secretions into the lower respiratory tract. SSD is widely used to prevent VAP. A survey from United States hospitals found that 55% of patients routinely use SSD.[3] Guidelines from United States, Canada and Europe recommend SSD.[4–6]
Many meta-analyses suggest that SSD can reduce VAP rates but does not clearly decrease duration of mechanical ventilation, mortality or ICU length of stay,[7–11] but the influence of the SSD on the microorganisms of VAP still required further data prove. For the limitations of prior meta-analyses, we undertook a meta-analysis focusing on the influence of the SSD on the microorganisms of VAP.
2. Methods
2.1. Search strategies
We searched all studies comparing SSD with standard endotracheal tubes and included at least microorganisms of ventilator associated pneumonia as an outcome. A comprehensive search was conducted through the online studies of PubMed, Embase, Cochrane Library, Google scholar, CNKI (China National Knowledge Infrastructure), and VIPI (Database for Chinese Technical Periodicals) from inception to August 2016. The following medical subject headings (MeSH) were searched: subglottic drainage, subglottic secretion, subglottic aspiration, ventilator associated pneumonia, and microorganisms. We traced the bibliographies of all retrieved trials and other related publications. We applied no language restriction. For our study is a meta-analysis so the ethical approval was not necessary.
2.2. Inclusion criteria/exclusion criteria
All randomized control trails (RCTs) comparing SSD with standard endotracheal tubes in the patients who require invasive mechanical ventilation were eligible. We also reviewed the references of all selected publications to ensure we had not missed any studies that could be included in our study. If there were more than one eligible publication from one publication from one author, the one with higher quality or the most recent publication date would be included. In addition, if the primary outcome was not the microorganisms of ventilator associated pneumonia, these studies were excluded.
2.3. Data extraction
Two reviewers independently evaluated the included studies and extracted data into RevMan. Any disagreement was resolved by discussion with a third reviewer. If still more data were required, communication through e-mail would be carried out with the authors.
2.4. Outcome measures
The primary endpoint of this meta-analysis was the VAP caused by nonfermentative bacteria and enterobacteria or VAP caused by Gram-positive cocci and Haemophilus influenzae organisms were measured from clinical features confirmed with endotracheal / bronchoscopically obtained cultures or by a good clinical response to antibiotic agents. The secondary endpoint was the mean volume of SSD daily between VAP group and non-VAP group was measured by accounted the volume of subglottic secretions aspirated every day.
2.5. Quality assessment
Firstly, all studies were assessed with the Jada Scale Scoring System.[12] In which the best study quality is scored 5 points. Studies with a score ≥3 points were considered as high quality research and were enrolled. Secondly, studies were also classified by agreement of 2 authors as having a low risk of bias, an unclear risk of bias, or a high risk of bias based on the Cochrane tool. This tool takes into account random sequence generation, concealment of the allocation sequence, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome, and selective reporting.
2.6. Statistical analysis
For each included study, odds ratio (OR) and 95% confidence intervals (CI) were calculated for dichotomous outcomes, and weighted mean differences (WMD) and 95% CI were calculated for continuous outcomes. Statistical heterogeneity was assessed using the I2 value ≤50% were considered as no statistical heterogeneity and used fixed-effects model to estimate the overall summary effect sizes. Otherwise, random-effects model was used a subgroup analysis or sensitivity analysis would be carried out. Risk of publication bias was assessed by visual inspection of funnel plots of effect sizes versus SE. We used Review Manager software (RevMan 5.3) and P value <.05 was considered as significant.
3. Results
3.1. Result of the search
The 2016 update the search strategy that identified a total of 9 published RCTs were included in the final analysis[13–21]. The details of search and exclusion criteria are displayed in the flow diagram (Fig. 1).
Figure 1.
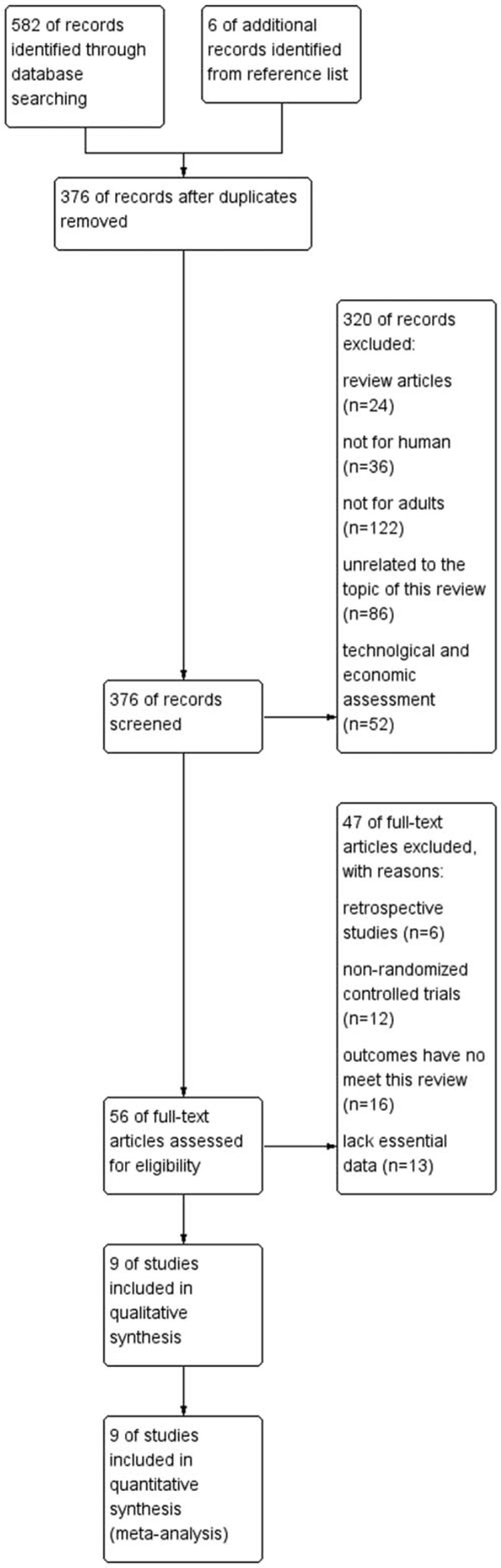
The graph shows a flow diagram of details search and exclusion criteria.
3.2. Characteristics of selected studies
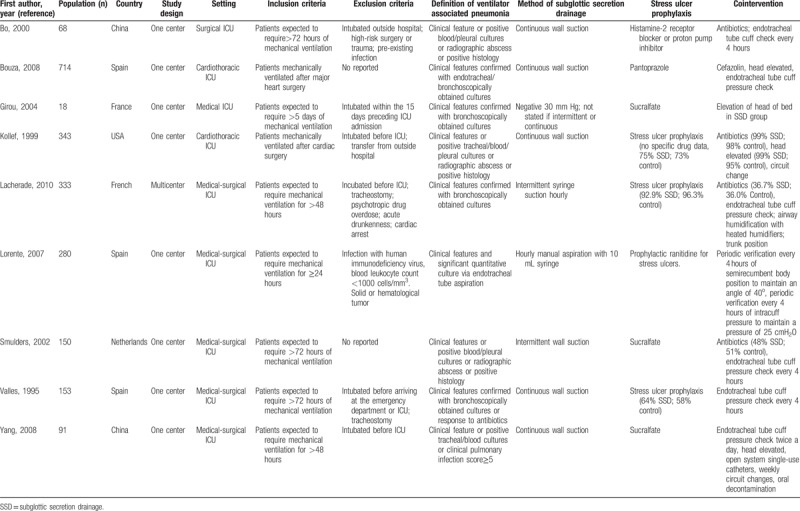
We identified 9 RCTs. All selected studies in our meta-analysis were published from 1995 to 2010. The selected study characteristics are summarized in Table 1.
Table 1.
Characteristics of included studies.
3.3. Risk of bias
The detailed risk of bias abut methodological quality of the included studies are elaborated and summarized, respectively, in Figure 2 and Figure 3.
Figure 2.
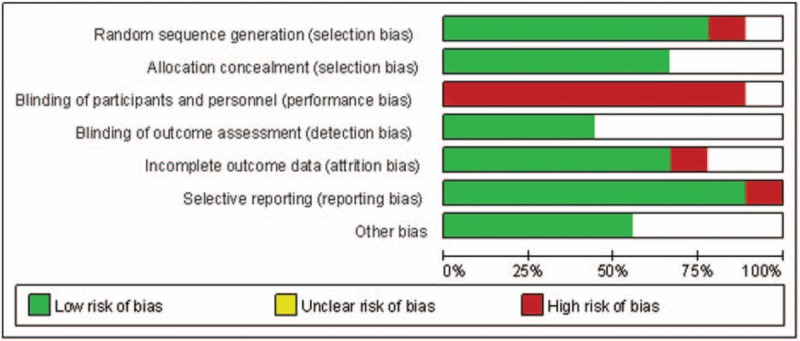
The graph shows a risk of bias graph.
Figure 3.

The graph shows a risk of bias summary.
3.4. Meta-analysis results
3.4.1. The primary endpoint
The primary endpoint “the episodes of VAP caused by nonfermentative bacteria and enterobacteria” was reported in all 9 studies. A total of 1042 patients in the SSD group and 1084 patients in the control group were available to compare the episodes of VAP. The pooled results showed that there was no significant difference in the rate of VAP caused by nonfermentative bacteria and enterobacteria between SSD group and control group (OR = 0.73, 95%CI, 0.53–1.01; P = .06) (Fig. 4).
Figure 4.
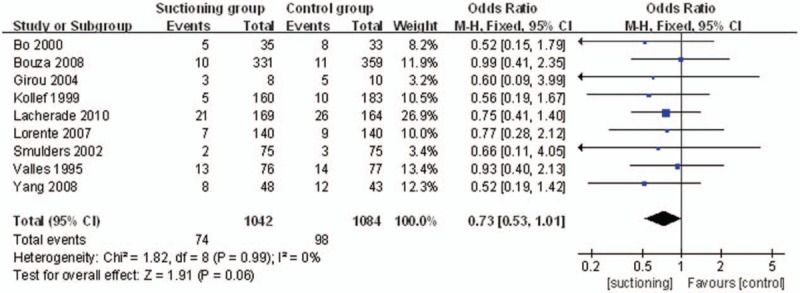
The graph shows a forest plot of relative risk with confidence interval for VAP caused by nonfermentative bacteria and enterobacteria. VAP = ventilator associated pneumonia.
The endpoint “the episodes of VAP caused by Gram-positive cocci and H influenzae organisms” was reported in all 9 studies. A total of 1042 patients in the SSD group and 1084 patients in the control group were available to compare the episodes of VAP. The episodes of VAP caused by Gram-positive cocci and H influenzae organisms were lower in the SSD group. (OR = 0.29, 95%CI, 0.18–0.48; P<.00001) (Fig. 5).
Figure 5.
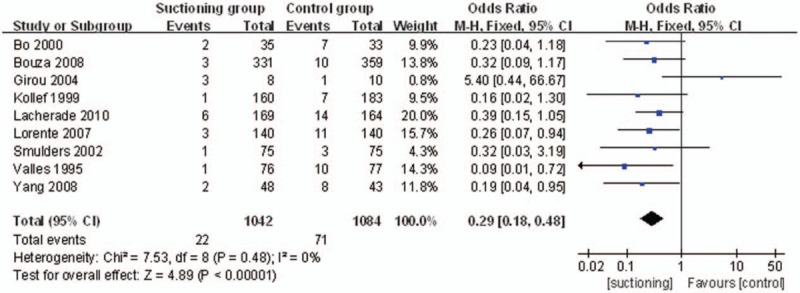
The graph shows a forest plot of relative risk with confidence interval for VAP caused by Gram-positive cocci and H influenzae organisms. VAP = ventilator associated pneumonia.
3.4.2. The secondary endpoint
The secondary endpoint “the mean volume of SSD daily between VAP group and non-VAP group” was reported in 3 studies. A total of 34 patients in the VAP group and 125 patients in the non-VAP group were available to compare the mean volume of SSD daily. Due to the high heterogeneity (P = .007, I2 = 80%), random-effects model was adopted to pool the data. Less mean volume of SSD daily was observed in VAP group (OR = −16.97, 95%CI, −29.87–4.08; P = .010) (Fig. 6).
Figure 6.
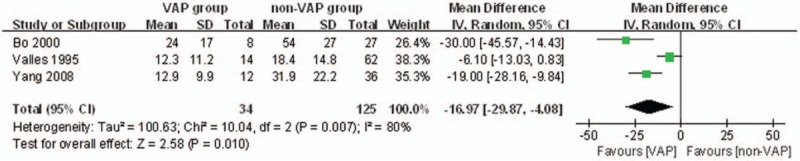
The graph shows a forest plot of relative risk with confidence interval for the mean volume of SSD daily between VAP group and non-VAP group. SSD = subglottic secretion drainage, VAP = ventilator associated pneumonia.
4. Discussion
The prevention of VAP is important for its impact on patient outcomes, costs and need for additional antibiotic. Many prevention measures are currently available. The combined use of SSD and continuous control of endotracheal tube cuff pressure reduced the incidence of ventilator-associated respiratory infection and saved health care costs.[22] We performed a meta-analysis to determine the effectiveness of SSD to prevent VAP in patients undergoing mechanical ventilation.
We found SSD to be associated with significant decreases in VAP caused by Gram-positive cocci and H influenzae organisms but no significant differences in VAP caused by nonfermentative bacteria and enterobacteria. Safdari et al[23] found a significant reduction in the incidence of early-onset VAP by using SSD with inspiratory pause maneuver among intubated patients. We know that early onset VAP which is related to micro-inhalation is more often caused by Gram-positive cocci and H influenzae. Meanwhile Gram-positive cocci and H influenzae are also frequently retrieved in head trauma patients and severe subarachnoid hemorrhage patients. The risk of micro-inhalation due to unconsciousness is often advocated. We suspect VAP caused by Gram-positive cocci and H influenzae organisms is more often associated with microinhalation, so it can be decreased by SSD. But the increased use of antibiotics, which may contribute to nonfermentative bacteria and enterobacteria infection in VAP patients, so the VAP caused by nonfermentative bacteria and enterobacteria can not be decreased by SSD.
The minimum inoculum needed for development of Gram-positive cooci or H influenzae pneumonia is higher than the inoculum needed for nonfermentative bacteria and enterobacteria pneumonias. This could explain the decrease of Gram-positive cooci or H influenzae pneumonias in SSD group. We also found the volume of subglottic secretions aspirated in patients with pneumonia was lower than that in patients without pneumonia. This may indicate an increase in the volume of secretions aspirated due to the reduction in the effectiveness of subglottic suction. This finding also told us the importance of maintaining adequate cuff pressure to prevent microaspirations to the bronchial tract. If the cuff pressure do not maintain adequate it will reduce the volume of subglottic secretions, and patients have more chance to get VAP. And if we do not aspirate subglottic secretion effectively it can cause the volume of subglottic secretions aspirated decrease, and also increase VAP rate.
In Lacherade 2010 study it is found that more patients got postextubation laryngeal dyspnea in SSD group than control group. In Girou 2004 study, 2 patients developed laryngeal edema immediately after extubation in SSD group, but no patient developed laryngeal edema in control group. It was reported that 40% of the patients under continuous suctioning developed laryngeal edema and tracheal mucosal erythema, haemorrhage or necrosis.[24] Many studies chose intermittent instead of continuous subglottic suctioning because of the risk of damaging the tracheal wall. But Frost et al[10] did not find significant adverse events in their meta-analysis from using continuous suction. Berra and Girou have found some adverse effects on the tracheal wall from continuous suction not only in animals but also in humans.[16,25] So subglottic secretion though manual suction may be better.
One limitation is that in Lorente 2007 study, we were not able to discriminate the independent influence of SSD and polyurethane cuff in the microorganisms of ventilator associated pneumonia. The other limitation is that not all included studies diagnosis of VAP used quantitative cultures of lower respiratory secretion though bronchoscope but just used tracheal secretion culture. Study form Browne has found that use of tracheal secretion culture to diagnosis VAP is as sensitive as bronchoscope.[26]
5. Conclusions
SSD is associated with significant reductions in VAP caused by Gram-positive cocci and H influenzae organisms but no significant differences in VAP caused by nonfermentative bacteria and enterobacteria. Less mean volume of SSD daily was observed in VAP group.
Author contributions
Conceptualization: Xu An Huang, Yan Ping Du.
Data curation: Xu An Huang, Yan Ping Du.
Formal analysis: Yan Ping Du, Liu Xia Li.
Investigation: Yan Ping Du.
Methodology: Xu An Huang, Yan Ping Du, Liu Xia Li.
Software: Bin Bin Fu, Xu An Huang.
Writing – review & editing: Xu An Huang, Yan Ping Du.
Footnotes
Abbreviations: CI = confidence intervals, CNKI = China National Knowledge Infrastructure, MeSH = medical subject headings, OR = odds ratio, RCTs = randomized controlled trials, SSD = subglottic secretion drainage, VAP = ventilator associated pneumonia, VIPI = Database for Chinese Technical Periodicals, WMD = weighted mean differences.
Conflict of interest: We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
References
- [1].Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009;302:2323–9. [DOI] [PubMed] [Google Scholar]
- [2].Kalil AC, Metersky ML, Klompas M, et al. Executive summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Krein SL, Fowler KE, Ratz D, et al. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf 2015;24:385–92. [DOI] [PubMed] [Google Scholar]
- [4].Klompas M, Branson R, Eichenwald EC, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Contr Hosp Epidemiol 2014;35(suppl 2):S133–54. [DOI] [PubMed] [Google Scholar]
- [5].Muscedere J, Dodek P, Keenan S, et al. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: diagnosis and treatment. J Crit Care 2008;23:138–47. [DOI] [PubMed] [Google Scholar]
- [6].Alvarez Lerma F, Sanchez Garcia M, Lorente L, et al. Guidelines for the prevention of ventilator-associated pneumonia and their implementation. The Spanish “Zero-VAP” bundle. Med Intensiva 2014;38:226–36. [DOI] [PubMed] [Google Scholar]
- [7].Caroff DA, Li L, Muscedere J, et al. Subglottic secretion drainage objective outcomes: a systematic review meta-analysis. Crit Care Med 2016;44:830–40. [DOI] [PubMed] [Google Scholar]
- [8].Muscedere J, Rewa O, McKechnie K, et al. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med 2011;39:1985–91. [DOI] [PubMed] [Google Scholar]
- [9].Dezfulian C, Shojania K, Collard HR, et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a meta-analysis. Am J Med 2005;118:11–8. [DOI] [PubMed] [Google Scholar]
- [10].Frost SA, Azeem A, Alexandrou E, et al. Subglottic secretion drainage for preventing ventilator associated pneumonia: a meta-analysis. Aust Crit Care 2013;26:180–8. [DOI] [PubMed] [Google Scholar]
- [11].Wang F, Bo L, Tang L, et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia: an updated meta-analysis of randomized controlled trials. J Trauma Acute Care Surg 2012;72:1276–85. [DOI] [PubMed] [Google Scholar]
- [12].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [13].Lorente L, Lecuona M, Jimenez A, et al. Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia. Am J Respir Crit Care Med 2007;176:1079–83. [DOI] [PubMed] [Google Scholar]
- [14].Lacherade JC, De Jonghe B, Guezennec P, et al. Intermittent subglottic secretion drainage and ventilator-associated pneumonia: a multicenter trial. Am J Respir Crit Care Med 2010;182:910–7. [DOI] [PubMed] [Google Scholar]
- [15].Bo H, He L, Qu J. Influence of the subglottic secretion drainage on the morbidity of ventilator associated pneumonia in mechanically ventilated patients. Chin J Tuberculosis Resp Dis 2000;23:472–4. [PubMed] [Google Scholar]
- [16].Girou E, Buu-Hoi A, Stephan F, et al. Airway colonisation in long-term mechanically ventilated patients. Effect of semi-recumbent position and continuous subglottic suctioning. Intensive Care Med 2004;30:225–33. [DOI] [PubMed] [Google Scholar]
- [17].Valles J, Artigas A, Rello J, et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Int Med 1995;122:179–86. [DOI] [PubMed] [Google Scholar]
- [18].Kollef MH, Skubas NJ, Sundt TM. A randomized clinical trial of continuous aspiration of subglottic secretions in cardiac surgery patients. Chest 1999;116:1339–46. [DOI] [PubMed] [Google Scholar]
- [19].Smulders K, van der Hoeven H, Weers-Pothoff I, et al. A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation. Chest 2002;121:858–62. [DOI] [PubMed] [Google Scholar]
- [20].Bouza E, Perez MJ, Munoz P, et al. Continuous aspiration of subglottic secretions in the prevention of ventilator-associated pneumonia in the postoperative period of major heart surgery. Chest 2008;134:938–46. [DOI] [PubMed] [Google Scholar]
- [21].Yang CS, Qiu HB, Zhu YP, et al. Effect of continuous aspiration of subglottic secretions on the prevention of ventilator-associated pneumonia in mechanically ventilated patients: a prospective, randomized, controlled clinical trial. Zhonghua Nei Ke Za Zhi 2008;47:625–9. [PubMed] [Google Scholar]
- [22].Lorente L, Lecuona M, Jimenez A, et al. Subglottic secretion drainage and continuous control of cuff pressure used together save health care costs. Am J Infect Control 2014;42:1101–5. [DOI] [PubMed] [Google Scholar]
- [23].Safdari R, Yazdannik A, Abbasi S. Effect of intermittent subglottic secretion drainage on ventilator-associated pneumonia: a clinical trial. Iran J Nurs Midwifery Res 2014;19:376–80. [PMC free article] [PubMed] [Google Scholar]
- [24].Hallais C, Merle V, Guitard PG, et al. Is continuous subglottic suctioning cost-effective for the prevention of ventilator-associated pneumonia? Infect Control Hosp Epidemiol 2011;32:131–5. [DOI] [PubMed] [Google Scholar]
- [25].Berra L, De Marchi L, Panigada M, et al. Evaluation of continuous aspiration of subglottic secretion in an in vivo study. Crit Care Med 2004;32:2071–8. [DOI] [PubMed] [Google Scholar]
- [26].Browne E, Hellyer TP, Baudouin SV, et al. A national survey of the diagnosis and management of suspected ventilator-associated pneumonia. BMJ Open Resp Res 2014;1:e000066. [DOI] [PMC free article] [PubMed] [Google Scholar]


