Abstract
Background:
Pre-transplantation serum ferritin (SF) has been considered to be a potential prognostic biomarker in patients undergoing allogeneic hematopoietic stem cell transplantation (allogeneic HSCT), but this conclusion remains controversial. Thus, we performed a meta-analysis to investigate the prognostic significance of pre-transplantation SF in patients undergoing allogeneic HSCT.
Methods:
We systematically searched PubMed, Embase, and Web of Science up to September 2017, and finally identified a total of 25 eligible studies with 4545 patients.
Results:
The pooled results of our meta-analysis showed that high pre-transplantation SF was markedly related to worse overall survival (OS) [hazard ratio (HR) = 1.82; 95% confidence interval (95% CI): 1.47–2.26; P < .001], nonrelapse mortality (NRM) (HR = 2.28; 95% CI: 1.79–2.89; P < .001), and progression-free survival (PFS) (HR = 1.72; 95% CI: 1.27–2.33; P < .001). In addition, high pre-transplantation SF was closely associated with a lower incidence of chronic graft versus host disease (cGVHD) (OR = 0.74, 95% CI: 0.58–0.96; P < .05), and a higher incidence of blood stream infections (BSIs) (OR = 1.67, 95% CI: 0.93–3.01; P = .09). However, no significance relationship was found between elevated pre-transplantation SF and acute graft versus host disease (aGVHD) (OR = 1.08, 95% CI:.72–1.62; P = .70).
Conclusion:
In patients undergoing allogeneic HSCT for hematological malignancies, elevated pre-transplantation SF was significantly associated with worse OS and PFS, higher incidence of NRM and BSI, and lower incidence of cGVHD, but it had no effect on aGVHD. Considering the limitations in our meta-analysis, more prospective and homogeneous clinical studies are needed to further confirm our findings.
Keywords: hematopoietic stem cell, meta-analysis, serum ferritin, transplantation
1. Introduction
Allogeneic hematopoietic stem cell transplantation (allogeneic HSCT) has been widely considered as an effective treatment for hematological malignancies, but favorable outcomes after allogeneic HSCT may be neutralized by several transplant-associated morbidities and mortality.[1] Hence, it is urgent to develop practical prognostic tools for predicting outcomes in patients with allogeneic HSCT, to encourage physicians appropriately to decide whether to treat individual patients with allogeneic HSCT, or to make preventive therapeutic schedules to mitigate relevant risks. A high iron burden is a common pre-transplantation abnormality, which might be partly attributed to multiple blood transfusions and hemolysis and can lead to liver function damage, hepatic sinusoidal obstruction syndrome, infection, and other problems, thus substantially influencing transplant-associated mortality and long-term survival.[2–5] Although liver biopsy is the gold standard for evaluating iron overload, serum ferritin (SF) is commonly used to assess the body's iron stores, due to its easy availability and the high procedural risks of liver biopsy. In addition, a recent study indicated that SF measured shortly before allogeneic HSCT is a reliable biomarker for iron overload, despite the fact that it is an acute-phase protein and its serum level can be influenced by acute infections, inflammations, and even malignant status.[6]
Furthermore, many studies have reported that pre-transplantation SF is a predictive biomarker for outcomes of patients with allogeneic HSCT. For instance, numerous studies indicated that elevated pre-transplantation SF was associated with inferior overall survival (OS)[7–9] and progression-free survival (PFS),[6,10,11] as well as a higher risk of nonrelapse mortality (NRM)[5,8,12] and blood stream infection (BSI).[9,13] In addition, several studies showed that there was an inverse relationship between raised pre-transplantation SF and chronic graft-versus-host disease (cGVHD).[14,15] Nevertheless, several studies on this topic reported conflicting results, indicating that high pre-transplantation SF might not be an independent prognostic marker in patients with allogeneic HSCT.[16–18] Considering the limited sample sizes of single studies regarding this topic, it is necessary to conduct a meta-analysis to further assess the prognostic value of elevated pre-transplantation SF in patients with allogeneic HSCT. A meta-analysis has been performed previously in this regard and indicated that elevated SF was correlated with lower OS and a higher incidence of NRM.[19] However, the previous meta-analysis did not include many recently published studies and only assessed the relationship of SF to OS and the NRM rate, but not PFS and post-transplantation GVHD and BSI, which increase the risk of transplant-related mortality and long-term survival. Furthermore, the previous meta-analysis did not separate allogeneic HSCT from autologous HSCT, which might introduce substantial heterogeneity to the pooled results. Therefore, we conducted this updated meta-analysis to more comprehensively investigate the prognostic significance of pre-transplantation elevated SF level in patients with hematological malignancies undergoing allogeneic HSCT.
2. Materials and methods
2.1. Ethics and dissemination
Ethical approval and informed consent are not required, as the study will be a literature review and will not involve direct contact with patients or alterations to patient care.
2.2. Study search strategy
We systematically searched PubMed, Embase, and Web of Science using the terms “ferritin” or “iron overload,” and “stem cell transplantation” from January 2000 to September 2017. We restricted the search to English published studies and human studies. Two independent reviewers performed the literature research.
2.3. Study selection criteria
The inclusion criteria were as follows: only allogeneic HSCT, ferritin level must be measured before allogeneic HSCT, OS or PFS or NRM or acute graft versus host disease (aGVHD)/cGVHD or BSIs were reported, and hazard ratio (HR), or odds ratio (OR) and their 95% confidence intervals (95% CIs) could be obtained directly, or sufficient data or survive curves were available to calculate the above estimates. The exclusion criteria were as follows: in vitro studies, case reports, conference abstracts, editorials, and reviews, and studies on patients with autologous HSCT.
2.4. Data extraction and quality assessment
The following information was extracted: the first author's name, country of research, study type, recruitment time, mean age of patients, disease type, case number, cut-off for SF, follow-up, OS, PFS, NRM, and aGVHD/cGVHD or BSI. The outcomes of interest included OS, PFS, NRM aGVHD/cGVHD, and BSI. If the studies did not directly provide HRs for OS, PFS, or NRM, the Engauge Digitizer version 4.1 (http://digitizer.sourceforge.net/, freely downloaded software) was used to extract the survival data from Kaplan–Meier curves.[20]
The Newcastle–Ottawa quality scale (NOS) was applied to assess the quality of the included studies. It evaluated the included studies in terms of the selection of participants, comparability, and ascertainment of outcome. The NOS score ranged from 0 (minimum) to 9 (maximum). A higher final score indicated a better methodological quality. A study with a score of 6 or higher was considered high-quality.
2.5. Statistical analysis
The statistical analyses of this meta-analysis were performed using Stata version 12.0 (Stata Corporation, College Station, TX). The pooled HRs and ORs and their corresponding 95% CIs were calculated to assess the association between SF and patient outcomes. The heterogeneity across the included studies was tested by the Cochran Q and Higgins I2 statistics. P < .05 and I2 > 50% indicated significant heterogeneity, whereas I2 < 25%, and 25% < I2 < 50%, indicated no heterogeneity and moderate heterogeneity, respectively. A random effects model was applied when statistical heterogeneity was detected; Otherwise, the fixed effects model was used. HR > 1 (low SF used as reference) indicated a higher risk of poor outcomes for high SF, and it was considered statistically significant if the 95% CI did not include 1 and P < .05. A sensitivity analysis was performed by sequentially deleting a single study in each step. The pooled results were considered stable if the HR did not significantly change with exclusion of the individual study. Publication bias was evaluated by Begg test and Egger tests, with funnel plot analysis. P < .05 with funnel plot asymmetry was considered a statistically significant publication bias.[21,22]
3. Results
3.1. Study search and study characteristics
The detailed process of study selection is described in Fig. 1. A total of 2197 studies were identified from PubMed, Embase, and Web of science after the initial literature search. After checking titles and abstracts, we eliminated 316 duplicated studies. In addition, 1814 studies including case reports, reviews, animal studies, irrelevant, and non-English studies were excluded, leaving 47 full-text articles for further evaluation. After that, 3 studies published by the same institution, 3 studies that enrolled patients undergoing autologous HSCT, 5 studies without extractive data, and 36 studies published in conference abstracts were excluded. Finally, a total of 25 studies were included in our meta-analysis.[4–18,23–32]
Figure 1.
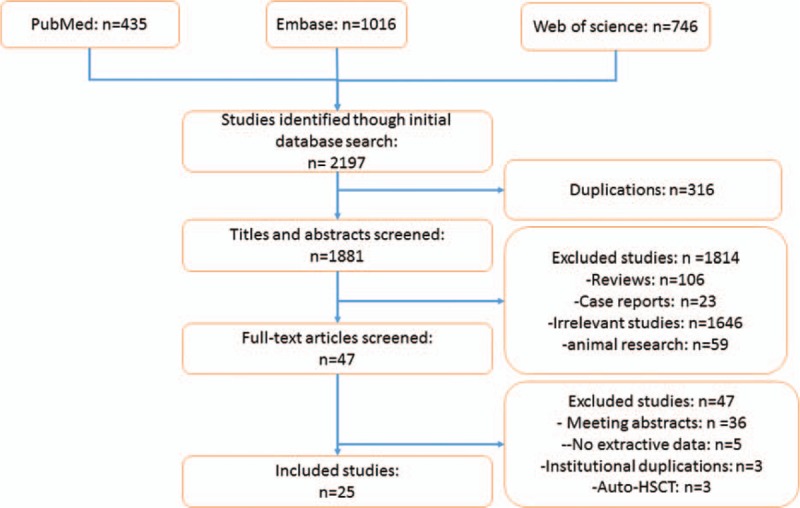
The study flow of study selection process.
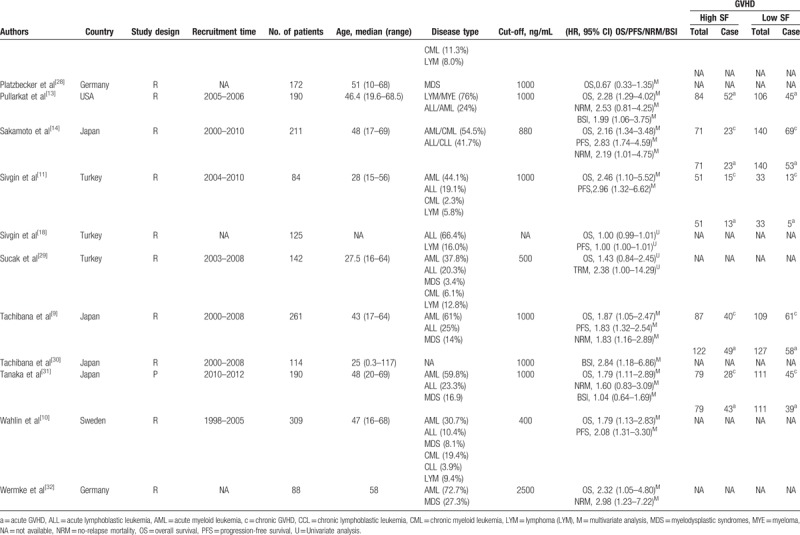
In all the included studies, a total of 4545 patients were enrolled between 1988 and 2013. Most of the included studies were retrospective, and only 1 study was prospective. Among the included studies, 22 enrolled mixed groups of patients, who suffered from acute myeloid leukemia (AML), acute lymphoblastic leukemia, chronic myeloid leukemia, chronic lymphoblastic leukemia, or myelodysplastic syndromes (MDS), 3 studies involved MDS only, and 1 study was involved AML only. More detailed information concerning the main characteristics of the included studies is presented in Table 1 . The scores for included study quality ranged from 5 to 7 according to the NOS (Table 2).
Table 1.
The main characteristics of the included studies.
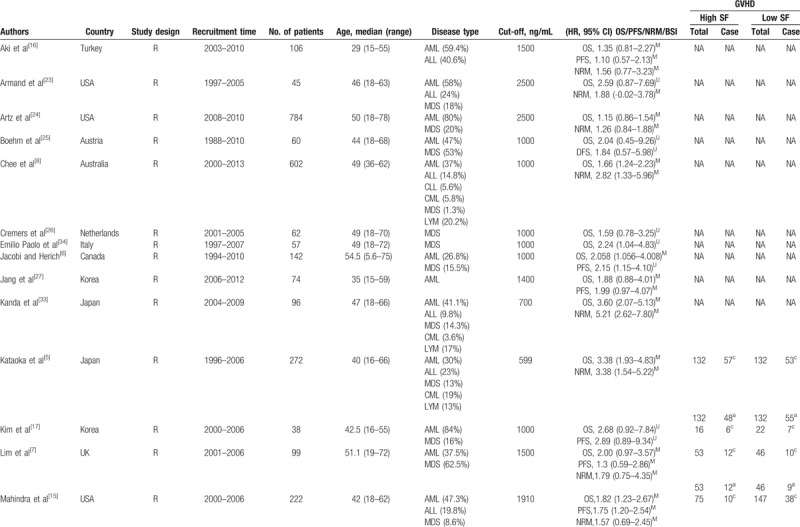
Table 1 (Continued).
The main characteristics of the included studies.
3.2. The prognostic significance of high SF in overall survival
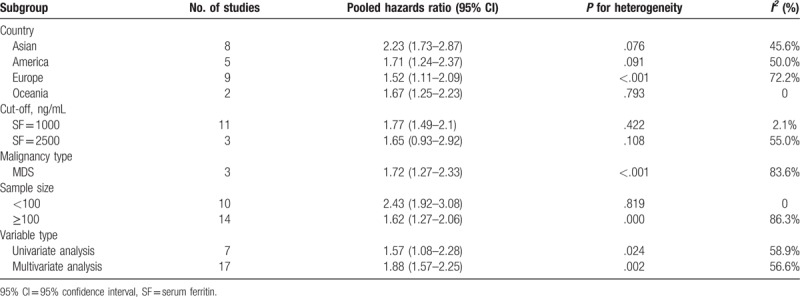
Twenty-four studies analyzed the relationship between SF and OS.[4–18,23–29,31,32] Considering the severe heterogeneity (I2 = 85.1%, P < .001), we calculated the HR and 95% CI using a random-effects model. The result from our meta-analysis showed that high SF was significantly related to worse OS (HR = 1.82; 95% CI: 1.47–2.26; P < .001) (Fig. 2). Furthermore, we performed stratified analyses to investigate the possible sources of heterogeneity according to region, cut-off value, hematological malignancy type, sample size, and variable type. The results of subgroup analyses indicated that the HR for the association between high SF with OS did not alter significantly in any of the following subgroups: Asian group (HR = 2.23; 95% CI: 1.73–2.87; P = .08), American group (HR = 1.71; 95% CI: 1.24–2.37; P = .09), European group (HR = 1.52; 95% CI: 1.11–2.09; P < .001), Oceania group (HR = 167; 95% CI: 1.25–2.23; P = .79), cut-off values (SF = 1000 or 2500 ng/mL; HR = 1.77; 95% CI: 1.49–2.10; P = .42 or HR = 1.65; 95% CI:.93–2.92; P = .11), malignancy type (MDS, HR = 1.72; 95% CI: 1.27–2.33; P < .001), sample size (<100 or ≥100; HR = 2.43; 95% CI: 1.92–3.08; P = .82 or HR = 1.62; 95% CI: 1.27–2.06; P < .001), or variable type (univariate analysis or multivariate analysis; HR = 1.57; 95% CI: 1.08–2.26; P < .05 or HR = 1.88; 95% CI: 1.57–2.25; P < .01) (Table 3).
Figure 2.
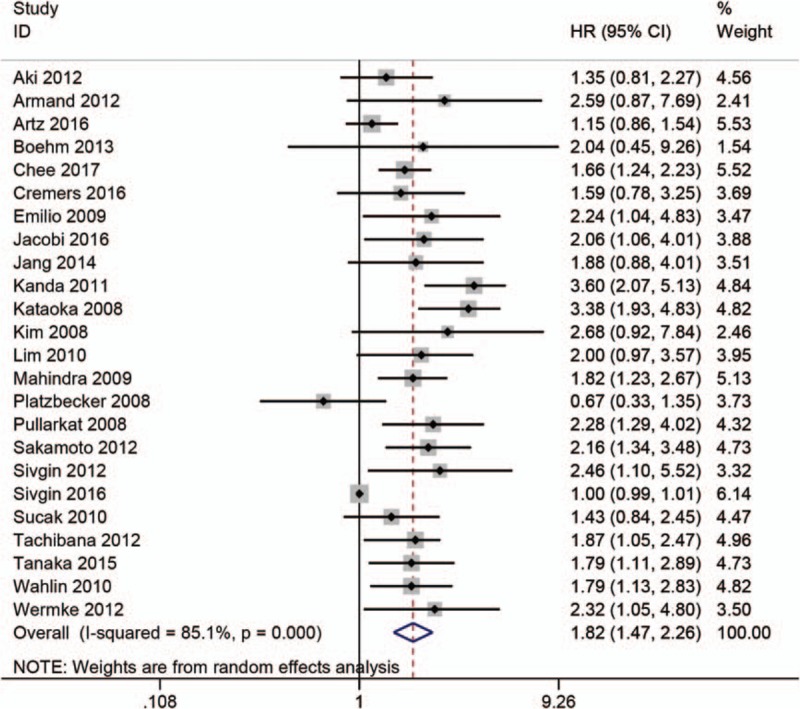
Meta-analysis of the prognostic significance of serum ferritin in overall survival.
Table 2.
The Newcastle–Ottawa Scale (NOS) quality assessment of the included studies.
3.3. The prognostic significance of high SF in progression-free survival
The relationship between high SF and PFS was mentioned in 12 studies.[6,7,9,10,14–18,25,27,29] The random-effects model was used to calculate the pooled HR with 95% CI due to obvious heterogeneity. The results indicated that there was an obvious connection between high SF and worse PFS (HR = 1.72; 95% CI: 1.27–2.33, P < .001) (Fig. 3). In order to explore the roots of heterogeneity, we performed subgroup analyses by region, cut-off value, sample size, and variable type. From the results, we observed no significant alterations of the pooled HR in any of the following subgroups: Asian group (HR = 1.82; 95% CI: 1.34–2.47; P = .19), American group (HR = 1.85; 95% CI: 1.34–2.55, P = .56), European group (HR = 1.59; 95% CI:.92–2.75; P < .001), Oceania group (HR = 1.84; 95% CI:.57–5.96), cut-off value (SF = 1000, HR = 1.90; 95% CI: 1.48–2.45; P = .69), sample size (<100 or ≥100; HR = 1.78; 95% CI: 1.24–2.57; P = .58 or HR = 1.70; 95% CI: 1.16–2.48; P < .001), or variable type (univariate analysis or multivariate analysis; HR = 1.00; 95% CI: 1.00–1.01; P = .48 or HR = 1.92; 95% CI: 1.62–2.27; P = .45) (Table 4).
Figure 3.
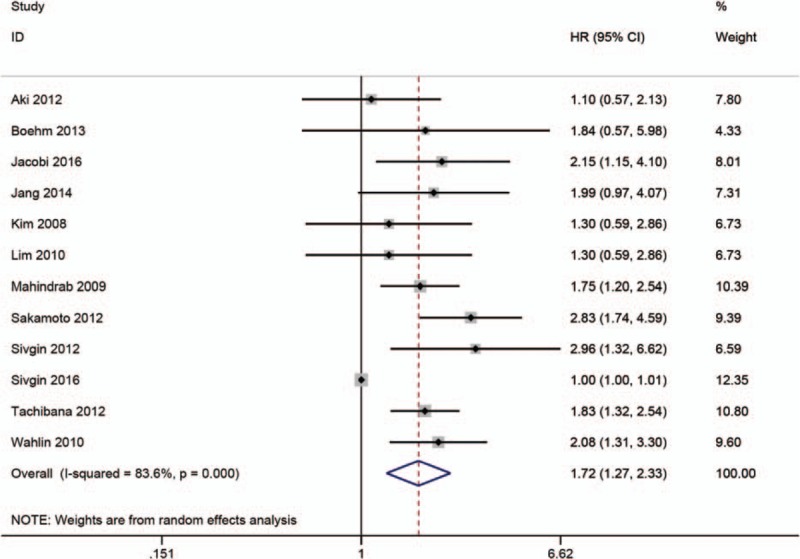
Meta-analysis of the prognostic significance of SF in progression-free survival.
Table 3.
Results of stratified analysis for the impact of SF on overall survival.
3.4. The prognostic significance of high SF in nonrelapse mortality
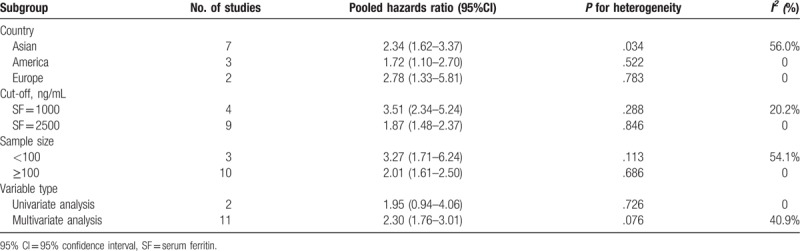
A total of 13 studies reported about NRM.[5,7–10,12–16,24,29,31] The pooled HR and 95% CI was calculated using a random-effects model due to severe heterogeneity. The results showed that patients with high SF experienced higher NRM (HR = 2.28; 95% CI: 1.79–2.89; P < .001) (Fig. 4). Furthermore, to investigate the roots of heterogeneity, we performed subgroup analyses by region, cut-off value, sample size, and variable type. From the results, no significant alterations of the pooled HR were observed in any of the following subgroups: Asian group (HR = 2.34; 95% CI: 1.62–3.37; P = .03), America, group (HR = 1.72; 95% CI: 1.10–2.70; P = .52), European group (HR = 2.78; 95% CI: 1.33–5.81; P = .78), cut-off value (SF = 1000 or 2500 ng/mL; HR = 3.51; 95% CI: 2.34–5.24; P = .29 or HR = 1.87; 95% CI: 1.48–2.37; P = .29), sample size (<100 or ≥100; HR = 3.27; 95% CI: 1.71–6.24; P = .11 or HR = 2.01; 95% CI: 1.61–2.50; P = .69), variable type (univariate analysis or multivariate analysis; HR = 1.95; 95% CI: 0.94–4.06; P = .73 or HR = 2.30; 95% CI: 1.76–3.01; P = .08) (Table 5).
Figure 4.
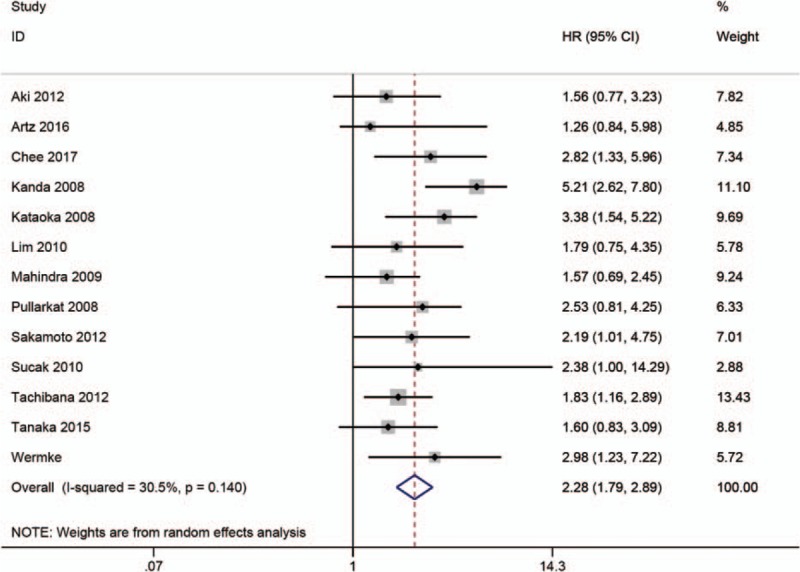
Meta-analysis of the prognostic significance of SF in nonrelapse mortality.
Table 4.
Results of stratified analysis for impact of SF on progression-free survival.
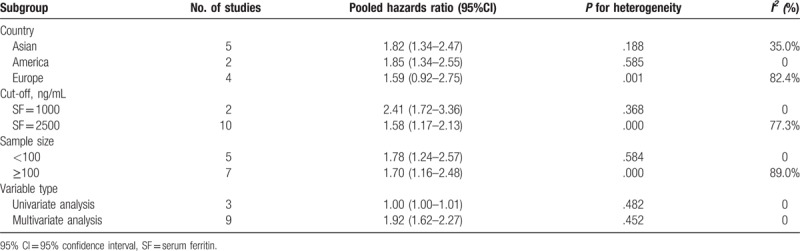
Table 5.
Results of stratified analysis for impact of SF on nonrelapse mortality.
3.5. The association of high SF with acute/chronic graft versus host disease
The relationship between SF and aGVHD/cGVHD was reported in 9 studies.[5,7,9,11,13–15,17,31] The pooled analysis of our meta-analysis showed that high SF was markedly related to cGVHD (OR = 0.74, 95% CI: 0.58–0.96; P < .05) (Fig. 5), but no significance was detected in aGVHD (OR = 1.08, 95% CI: 0.72–1.62; P = .70) (Fig. 6).
Figure 5.
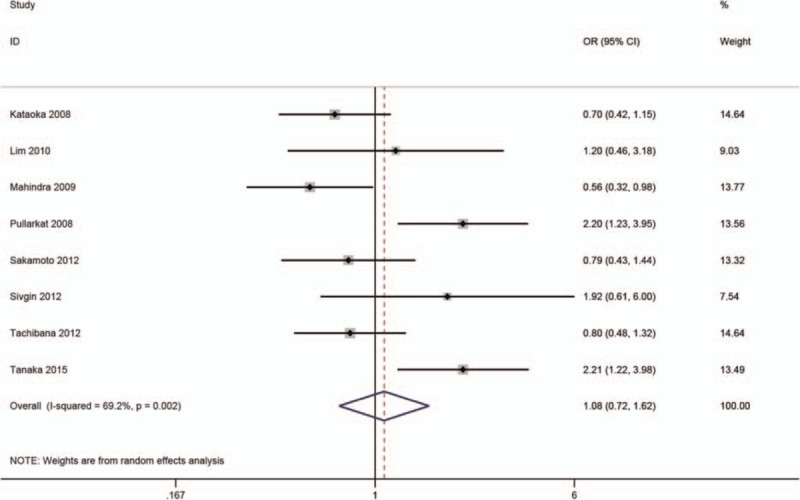
Meta-analysis of the association of SF with chronic graft versus host disease.
Figure 6.
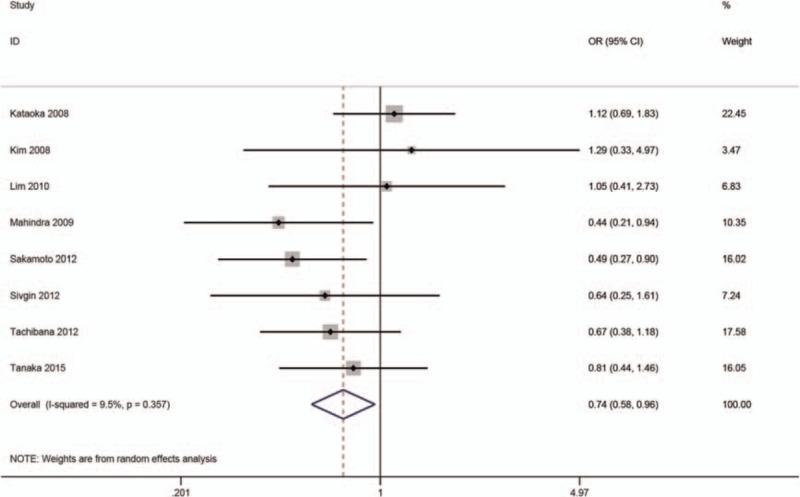
Meta-analysis of the association of SF with acute graft versus host disease.
3.6. The association of high SF with blood stream infections
Only 3 studies mentioned the association of high SF with BSI.[13,30,31] The results of our meta-analysis showed that high SF was significantly associated with a higher incidence of BSI (OR = 1.67, 95% CI: 0.93–3.01; P = .09) (Fig. 7).
Figure 7.
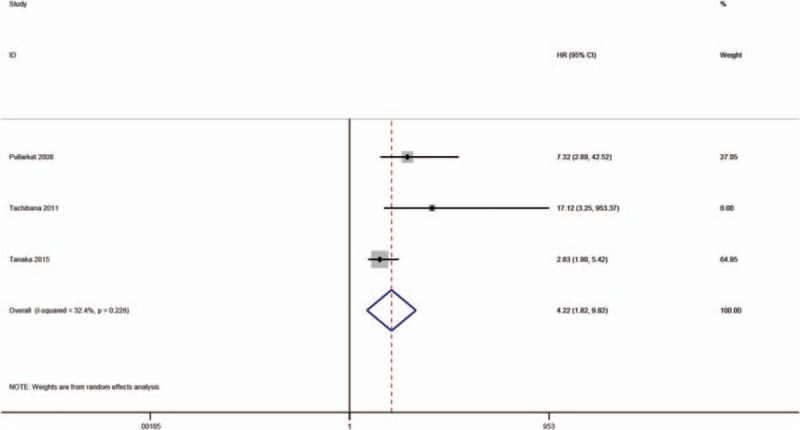
Meta-analysis of the association of SF with blood stream infections.
3.7. Sensitivity analysis
The sensitivity analyses were performed by omitting a single study per step to investigate the influence of individual studies on the pooled HRs of OS, PFS, and NRM. The results showed that the HR in each step did not alter substantially (Fig. 8A–C), indicating that our pooled results of OS, PFS, and NRM were robust to a degree.
Figure 8.
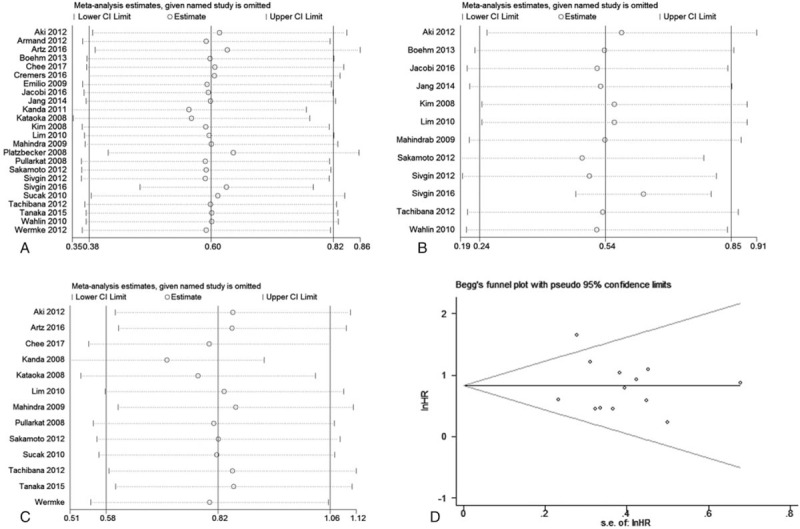
The sensitivity analyses for the pooled HRs of overall survival (A), progression-free survival (B), and nonrelapse mortality (C). The funnel plot for publication bias about the correlation serum ferritin with nonrelapse mortality (D).
3.8. Publication bias
Egger and Begg tests with funnel plots were performed to assess potential publication bias in our meta-analysis. The results of Egger and Begg test with funnel plots showed that that there was no obvious publication bias for NRM (Begg test: P = .95; Egger test: P = .62; Fig. 8D), but significant publication bias was observed for OS (Begg test: P = .08; Egger test: P < .001; Fig. 9A) and PFS (Begg test: P = .41; Egger test: P < .001; Fig. 9B).
Figure 9.
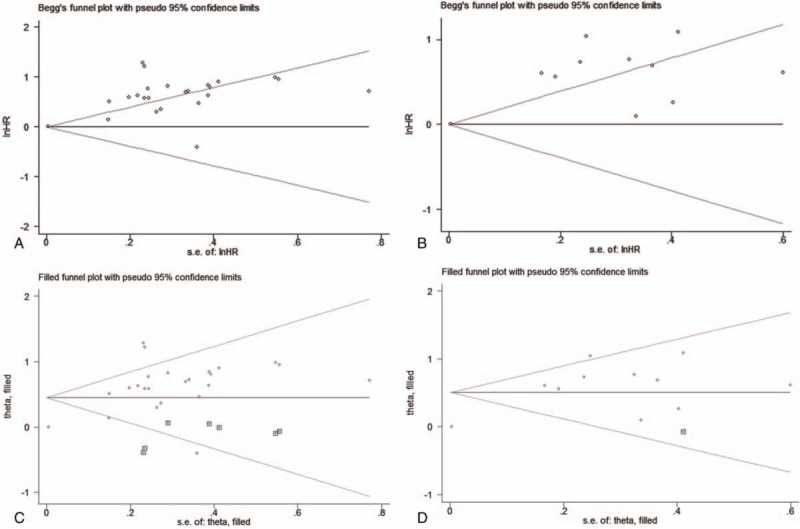
The funnel plots for publication bias about the correlation of SF with overall survival (A) and progression-free survival (B). The updated funnel plots for publication bias after trim-and-fill analysis about the correlation of SF with OS (C) and progression-free survival (D).
To explore whether the publication bias for OS and PFS substantially affected the stability of the pooled HRs in our meta-analysis, we further performed a trim-and-fill analysis. The results showed that the reasonable number of included studies should be 31 when adding 7 missing studies for OS, and 13 with 1 missing study for PFS; the updated pooled funnel plots for publication bias concerning the association of SF with OS (Fig. 9C) and PFS (Fig. 9D) were relatively symmetrical. More importantly, the updated pooled HRs also did not change significantly (HR = 1.566, 95% CI: 1.307–1.876; P < .001) and (HR = 1.657, 95% CI: 1.242–2.209; P < .001), suggesting that the pooled HRs of OS and PFS in our meta-analysis were still stable, although the publication bias for the association of SF with OS and PFS was significant in our meta-analysis.
4. Discussion
Consistent with the results of a previous meta-analysis,[19] the results of our study showed that elevated pre-transplantation SF was closely associated with worse OS and a higher incidence of NRM. In addition, we found that there was a substantial relationship between elevated pre-transplantation SF and worse PFS and a higher risk of BSI. Paradoxically, our meta-analysis showed that there was a significant association between high pre-transplantation SF and a lower incidence of cGVHD.
Currently, improving the OS and PFS of patients who undergo allogeneic HSCT is one of leading aims of hematologists. Considering the positive association of high pre-transplantation SF level with worse OS and PFS, pre-transplantation SF might be incorporated in prognostic models to guide physicians to make reasonable decisions about whether to treat individual patients with allogeneic HSCT, and decreasing the pre-transplantation SF level might be an effective strategy to improve OS and PFS. For instance, it has been reported that some drugs targeting mitigating iron overload were able to improve the outcomes in patients who underwent allogeneic HSCT.[33] However, the exact mechanisms underlying the relationship between SF and long-term survival have not been fully elucidated. It has been hypothesized that elevated pre-transplantation SF might negatively affect pro-oxidative/antioxidative homeostasis, which probably worsens the long-term survival of patients undergoing allogeneic HSCT.[35] Mortality not related relapse is also a serious issue for hematologists, and infection and GVHD are the 2 most common causes of NRM. The application of allogeneic HSCT is in part limited by the high mortality related to the procedure. In particular, it is often difficult for family members to understand and accept when a patient who undergoes HSCT succumbs to NRM. Hence, reducing the NRM rate is another leading goal of hematologists. Inconsistent results were reported in all 13 publications included in our meta-analysis.[7,8,12–16,24,29–32] Although some studies indicated that elevated pre-transplantation SF level was not related to a higher risk of NRM,[7,15,16,24,31] in our meta-analysis, the pooled HR suggested that elevated pre-transplantation SF level substantially increase the incidence of NRM. Therefore, decreasing pre-transplantation SF levels might help reduce the incidence of NRM. Similarly, our meta-analysis indicated that elevated pre-transplantation SF level was significantly related to BSI, which might partly explain the effect of elevated pre-transplantation SF on NRM. Some possible mechanisms responsible for the association between elevated pre-transplantation SF level and infection have been suggested. It was hypothesized that high SF levels could damage cellular immunity by affecting phagocytosis of immune cells.[36,37] In addition, the high SF might provide an advantageous environment for the growth of some opportunistic bacteria and fungi that are closely dependent on free iron.[36,37]
As mentioned above, our meta-analysis indicated that elevated pre-transplantation SF level impaired OS and PFS. However, a paradoxical result was found that elevated pre-transplantation SF level was associated with a lower incidence of cGVHD in patients undergoing allogeneic HSCT for hematological malignancies. Several relevant mechanisms might be in place to account for the superficially paradoxical results regarding long-term survival and cGVHD. It was reported that ferritin could play immunosuppressive roles in vitro and in vivo, and ferritin receptors were expressed on both T and B cells.[38,39] In addition, heavy chain ferritin could inhibit the proliferation of T cells in response to mitogen[40] and might play a critical role in immune-related diseases.[41] Thus, on the one hand, high ferritin levels might decrease the incidence of cGVHD via an immunosuppressive effect. On the other hand, in turn, an immunosuppressive effect from high SF might contribute to disease relapse and impairing OS and PFS in patients who undergo allogeneic HSCT for hematological malignancies. Regarding aGVHD, it has been reported that the decrease in aGVHD obtained by comprehensive preventive strategies was not able to reduce incidence of cGVHD in patients with allogeneic HSCT,[42,43] which might suggest that there is no association between aGVHD and c GVHD. In accordance with that, our meta-analysis showed that elevated pre-transplantation SF did not affect the incidence of aGVHD, but it still significantly decreased the incidence of cGVHD. Chronic GVHD is recognized as an immune-mediated syndrome, and its clinical manifestation is often similar to that of autoimmune disorders, but the pathophysiological mechanism responsible for cGVHD remains poorly elucidated. As to the pathophysiological mechanism underlying aGVHD, it has been hypothesized that a group of proinflammatory cytokines and chemokines released from damaged host tissues could activate host antigen-presenting cells, which are capable of promoting the proliferation and differentiation of infused donor T lymphocytes, causing target tissue destruction.[44] Thus, the difference between the pathophysiological mechanisms of acute and chronic GVHD might be an explanation for the results of our meta-analysis, which showed that pre-transplantation SF level exerted no substantial effect on the development of aGVHD, but decreased the incidence of cGVHD.
To the best of our knowledge, the present study is the most comprehensive meta-analysis investigating the effect of pre-transplantation SF on outcomes in patients undergoing allogeneic HSCT. However, our results should be interpreted with caution, considering that there are several potential limitations in our meta-analysis. First, the most important limitation is that the robustness of our conclusions might be challenged by the sound publication bias, although our trim-and-fill analysis and sensitivity analysis in this meta-analysis showed that the pooled results did not change significantly. Second, the level of SF is highly correlated with the inflammatory state of the patients. Therefore, when our conclusions are applied to clinical practice, the clinicians should exclude the influence of inflammatory state of patients on the level of SF. Third, only studies published in English were included, and some high-quality articles published in other languages might have been excluded, which would increase the publication bias. Fourth, other than the separate investigation of patients with MDS, patients with different kinds of hematological malignancies were mixed in our study for the combined analysis, which might introduce substantial heterogeneity and a degree of distrust in our results. Fifth, the majority of the included studies in our meta-analysis were retrospective in design, which inevitably increases the risk of bias and affects the reliability of the combined results. Sixth, the cut-offs for SF were not consistent among the included studies, which might also introduce significant heterogeneity. Furthermore, the pooled results of studies with different cut-offs limited this study's reliability and the practicability of clinical guidance. At last, the duration of follow-up in the included studies differed considerably, which might affect the reliability of the pooled HRs for OS and PFS.
In conclusion, in patients undergoing allogenic HSCT for hematological malignancies, elevated pre-transplantation SF was significantly associated with worse OS and PFS, a higher incidence of NRM and BSI, and a lower incidence of cGVHD, but it had no effect on aGVHD. Considering the above limitations, more prospective and homogeneous clinical studies are demanded to further confirm our findings.
Acknowledgment
We sincerely thank the Editage for helping us edit our manuscript professionally.
Author contributions
Data curation: Zhengwei Yan, Xianying Chen, Lihong Chen.
Formal analysis: Peilin Wu.
Formal analysis: Xianying Chen.
Investigation: Huiping Wang.
Resources: Yaling Chen, Lihong Chen.
Software: Huiping Wang, Yaling Chen, Lihong Chen, Peilin Wu.
Supervision: Wei Wang.
Validation: Wei Wang.
Writing – original draft: Zhengwei Yan.
Writing – review and editing: Wei Wang.
Footnotes
Abbreviations: aGVHD = acute graft versus host disease, AML = acute myeloid leukemia, BSI = blood stream infection, cGVHD = chronic graft versus host disease, CI = confidence interval, HR = hazard ratio, HSCT = allogeneic hematopoietic stem cell transplantation, MDS = myelodysplastic syndromes, NRM = nonrelapse mortality, OR = odds ratio, OS = overall survival, SF = serum ferritin.
The authors report no conflicts of interest.
References
- [1].Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol 2011;29:805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jastaniah W, Harmatz P, Pakbaz Z, et al. Transfusional iron burden and liver toxicity after bone marrow transplantation for acute myelogenous leukemia and hemoglobinopathies. Pediatr Blood Cancer 2008;50:319–24. [DOI] [PubMed] [Google Scholar]
- [3].Atilla E, Toprak SK, Demirer T. Current review of iron overload and related complications in hematopoietic stem cell transplantation. Turk J Haematol 2017;34:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alessandrino EP, Della Porta MG, Bacigalupo A, et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica 2010;95:476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kataoka K, Nannya Y, Hangaishi A, et al. Influence of pretransplantation serum ferritin on nonrelapse mortality after myeloablative and nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009;15:195–204. [DOI] [PubMed] [Google Scholar]
- [6].Jacobi N, Herich L. Measurement of liver iron concentration by superconducting quantum interference device biomagnetic liver susceptometry validates serum ferritin as prognostic parameter for allogeneic stem cell transplantation. Eur J Haematol 2016;97:336–41. [DOI] [PubMed] [Google Scholar]
- [7].Lim ZY, Fiaccadori V, Gandhi S, et al. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res 2010;34:723–7. [DOI] [PubMed] [Google Scholar]
- [8].Chee L, Tacey M, Lim B, et al. Pre-transplant ferritin, albumin and haemoglobin are predictive of survival outcome independent of disease risk index following allogeneic stem cell transplantation. Bone Marrow Transplant 2017;52:870–7. [DOI] [PubMed] [Google Scholar]
- [9].Tachibana T, Tanaka M, Numata A, et al. Pretransplant serum ferritin has a prognostic influence on allogeneic transplant regardless of disease risk. Leuk Lymphoma 2012;53:456–61. [DOI] [PubMed] [Google Scholar]
- [10].Wahlin A, Lorenz F, Fredriksson M, et al. Hyperferritinemia is associated with low incidence of graft versus host disease, high relapse rate, and impaired survival in patients with blood disorders receiving allogeneic hematopoietic stem cell grafts. Med Oncol 2011;28:552–8. [DOI] [PubMed] [Google Scholar]
- [11].Sivgin S, Baldane S, Kaynar L, et al. Pretransplant serum ferritin level may be a predictive marker for outcomes in patients having undergone allogeneic hematopoietic stem cell transplantation. Neoplasma 2012;59:183–90. [DOI] [PubMed] [Google Scholar]
- [12].Kanda J, Mizumoto C, Ichinohe T, et al. Pretransplant serum ferritin and C-reactive protein as predictive factors for early bacterial infection after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2011;46:208–16. [DOI] [PubMed] [Google Scholar]
- [13].Pullarkat V, Blanchard S, Tegtmeier B, et al. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2008;42:799–805. [DOI] [PubMed] [Google Scholar]
- [14].Sakamoto S, Kawabata H, Kanda J, et al. Differing impacts of pretransplant serum ferritin and C-reactive protein levels on the incidence of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Int J Hematol 2013;97:109–16. [DOI] [PubMed] [Google Scholar]
- [15].Mahindra A, Bolwell B, Sobecks R, et al. Elevated pretransplant ferritin is associated with a lower incidence of chronic graft-versus-host disease and inferior survival after myeloablative allogeneic haematopoietic stem cell transplantation. Br J Haematol 2009;146:310–6. [DOI] [PubMed] [Google Scholar]
- [16].Aki SZ, Suyani E, Bildaci Y, et al. Prognostic role of pre-transplantation serum C-reactive protein levels in patients with acute leukemia undergoing myeloablative allogeneic stem cell transplantation. Clin Transplant 2012;26:E513–21. [DOI] [PubMed] [Google Scholar]
- [17].Kim YR, Kim JS, Cheong JW, et al. Transfusion-associated iron overload as an adverse risk factor for transplantation outcome in patients undergoing reduced-intensity stem cell transplantation for myeloid malignancies. Acta Haematol 2008;120:182–9. [DOI] [PubMed] [Google Scholar]
- [18].Sivgin S, Nazlim S, Zararsiz G, et al. Increased bone marrow iron scores are strongly correlated with elevated serum ferritin levels and poorer survival in patients with iron overload that underwent allogeneic hematopoietic stem cell transplantation: a single center experience. Clin Lymphoma Myeloma Leuk 2016;16:582–7. [DOI] [PubMed] [Google Scholar]
- [19].Guo W, Dong A, He M, et al. A meta-analysis for effects of elevated pre-transplantation serum ferritin on the outcomes in patients undergoing hematopoietic stem cell transplantation. Cancer Invest 2016;34:340–7. [DOI] [PubMed] [Google Scholar]
- [20].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [22].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Armand P, Sainvil MM, Kim HT, et al. Does iron overload really matter in stem cell transplantation? Am J Hematol 2012;87:569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Artz AS, Logan B, Zhu X, et al. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica 2016;101:1426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Boehm A, Sperr WR, Kalhs P, et al. Long-term follow-up after allogeneic stem cell transplantation in patients with myelodysplastic syndromes or secondary acute myeloid leukemia: a single center experience. Wien Klin Wochenschr 2014;126:23–9. [DOI] [PubMed] [Google Scholar]
- [26].Cremers EM, van Biezen A, de Wreede LC, et al. Prognostic pre-transplant factors in myelodysplastic syndromes primarily treated by high dose allogeneic hematopoietic stem cell transplantation: a retrospective study of the MDS subcommittee of the CMWP of the EBMT. Ann Hematol 2016;95:1971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jang JE, Kim SJ, Cheong JW, et al. Early CMV replication and subsequent chronic GVHD have a significant anti-leukemic effect after allogeneic HSCT in acute myeloid leukemia. Ann Hematol 2015;94:275–82. [DOI] [PubMed] [Google Scholar]
- [28].Platzbecker U, Bornhauser M, Germing U, et al. Red blood cell transfusion dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndrome (MDS). Biol Blood Marrow Transplant 2008;14:1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sucak GT, Yegin ZA, Ozkurt ZN, et al. Iron overload: predictor of adverse outcome in hematopoietic stem cell transplantation. Transplant Proc 2010;42:1841–8. [DOI] [PubMed] [Google Scholar]
- [30].Tachibana T, Tanaka M, Takasaki H, et al. Pretransplant serum ferritin is associated with bloodstream infections within 100 days of allogeneic stem cell transplantation for myeloid malignancies. Int J Hematol 2011;93:368–74. [DOI] [PubMed] [Google Scholar]
- [31].Tanaka M, Kanamori H, Matsumoto K, et al. Clinical significance of pretransplant serum ferritin on the outcome of allogeneic hematopoietic SCT: a prospective cohort study by the Kanto Study Group for Cell Therapy. Bone Marrow Transplant 2015;50:727–33. [DOI] [PubMed] [Google Scholar]
- [32].Wermke M, Schmidt A, Middeke JM, et al. MRI-based liver iron content predicts for nonrelapse mortality in MDS and AML patients undergoing allogeneic stem cell transplantation. Clin Cancer Res 2012;18:6460–8. [DOI] [PubMed] [Google Scholar]
- [33].Kanda J, Kawabata H, Chao NJ. Iron overload and allogeneic hematopoietic stem-cell transplantation. Expert Rev Hematol 2011;4:71–80. [DOI] [PubMed] [Google Scholar]
- [34].Emilio Paolo A, Della Porta MG, Bacigalupo A, et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica 2010;95:476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yegin ZA, Pasaoglu H, Aki SZ, et al. Pro-oxidative/antioxidative imbalance: a key indicator of adverse outcome in hematopoietic stem cell transplantation. Int J Lab Hematol 2011;33:414–23. [DOI] [PubMed] [Google Scholar]
- [36].Marx JJ. Iron and infection: competition between host and microbes for a precious element. Best Pract Res Clin Haematol 2002;15:411–26. [PubMed] [Google Scholar]
- [37].Barclay R. The role of iron in infection. Med Lab Sci 1985;42:166–77. [PubMed] [Google Scholar]
- [38].Gray CP, Arosio P, Hersey P. Heavy chain ferritin activates regulatory T cells by induction of changes in dendritic cells. Blood 2002;99:3326–34. [DOI] [PubMed] [Google Scholar]
- [39].Matzner Y, Hershko C, Polliack A, et al. Suppressive effect of ferritin on in vitro lymphocyte function. Br J Haematol 1979;42:345–53. [DOI] [PubMed] [Google Scholar]
- [40].Cardier J, Romano E, Soyano A. Effect of hepatic isoferritins from iron overloaded rats on lymphocyte proliferative response: role of ferritin iron content. Immunopharmacol Immunotoxicol 1995;17:719–32. [DOI] [PubMed] [Google Scholar]
- [41].Recalcati S, Invernizzi P, Arosio P, et al. New functions for an iron storage protein: the role of ferritin in immunity and autoimmunity. J Autoimmun 2008;30:84–9. [DOI] [PubMed] [Google Scholar]
- [42].Deeg HJ, Lin D, Leisenring W, et al. Cyclosporine or cyclosporine plus methylprednisolone for prophylaxis of graft-versus-host disease: a prospective, randomized trial. Blood 1997;89:3880–7. [PubMed] [Google Scholar]
- [43].Nash RA, Pineiro LA, Storb R, et al. FK506 in combination with methotrexate for the prevention of graft-versus-host disease after marrow transplantation from matched unrelated donors. Blood 1996;88:3634–41. [PubMed] [Google Scholar]
- [44].Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet 2009;373:1550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


