Abstract
The objective to assess the association between gastrectomy and the risk of pulmonary tuberculosis among patients without gastric cancer in Taiwan.
There were 762 subjects with newly performing gastrectomy as the gastrectomy group since 2000 to 2012, and 2963 randomly selected subjects without gastrectomy as the non-gastrectomy group. Subjects with history of pulmonary tuberculosis or gastric cancer before the index date were excluded. Both gastrectomy and non-gastrectomy groups were matched with sex, age, and comorbidities. The incidence of pulmonary tuberculosis was assessed in both groups. The multivariable Cox proportional hazards regression model was used to assess the hazard ratio and 95% confidence interval for risk of pulmonary tuberculosis associated with gastrectomy.
The overall incidence of pulmonary tuberculosis was 1.97-fold greater in the gastrectomy group than that in the non-gastrectomy group. The multivariable Cox proportional hazards regression analysis demonstrated that the adjusted HR of pulmonary tuberculosis was 1.97 for the gastrectomy group, compared with the non-gastrectomy group. Male sex, age (increase per 1 year), chronic obstructive pulmonary disease, and splenectomy were other factors that could be related to pulmonary tuberculosis.
Gastrectomy is associated with 1.97-fold increased risk of pulmonary tuberculosis among patients without gastric cancer.
Keywords: epidemiology, gastrectomy, pulmonary tuberculosis
1. Introduction
For several decades, the high extent of tuberculosis (TB) prevalence and its socioeconomic impact via associated health care costs have made it a major public health concern. World Health Organization (WHO) statistics indicated that in 2014 alone there was an estimated 10.4 million new cases.[1] Prevalence varies significantly between countries across the world and, for example, in Taiwan data from 2006 showed the incidence was 63 individual cases per 100,000 population.[2] Upon TB infection, the most prominent tissue affected are the lungs and a cluster of various risk factors for pulmonary TB are well characterized. These include characteristics such as aging, male sex, and lifestyle choices including tobacco smoking and excess alcohol consumption.[3,4] Other diseases are also associated with TB, including chronic obstructive pulmonary disease (COPD), pneumoconiosis,[5,6] chronic kidney disease (CKD), diabetes mellitus (DM), and human immunodeficiency virus (HIV).[7]
In addition to the risk factors stated above, gastrectomy in patients with peptic ulcers is a potential risk for TB, particularly pulmonary TB.[8–10] The articles mentioned the association between pulmonary TB and any degree of gastrectomy were scanty and most of them were time-honored and did not meet the trend. Based on these findings, the present study investigated the association of gastrectomy and increased risk of pulmonary TB using the Taiwan National Health Insurance Program database.
2. Methods
2.1. Study design and source of information
This population-based cohort study utilized material from the Taiwan National Health Insurance Program database which allowed access to information from approximately 99% of the population of 23 million individuals[11–23] since March 01, 1995. This database is well established and more detailed information can be found in previous studies.[24–29] The present study was approved by the Ethics Review Board of China Medical University in Taiwan (CMUH-104-REC2–115).
2.2. Participants
The gastrectomy group was included as subjects aged 20 to 84 years with newly performing gastrectomy between 2000 and 2012 (International Classification of Diseases [ICD] 9th Revision, ICD-9 procedure codes 43.5, 43.6, 43.7, 43.8, 43.81, 43.82, 43.89, 43.91, and 43.99). To increase the statistical power of the study, for each subject with gastrectomy, 4 subjects without gastrectomy were randomly selected as the non-gastrectomy group. The index date was defined as the date of performing gastrectomy. Both gastrectomy and non-gastrectomy groups were matched by sex, age (every 5-year span) and comorbidities. Subjects with a history of pulmonary TB, or gastric cancer prior to the index date were excluded from the study.
2.3. Comorbidities
Comorbidities which could be potentially related to pulmonary TB were included, as follows: alcohol-related disease, COPD, CKD, DM, pneumoconiosis, splenectomy, chronic liver disease including cirrhosis, hepatitis B infection, hepatitis C infection, and other chronic hepatitis. All comorbidities were diagnosed in accordance to the ICD-9 codes. The accuracy of ICD-9 codes has been examined in previous studies.[30–33]
2.4. Major outcome
In this study we monitored individuals until the end of 2013, with or without diagnosis of pulmonary TB. A principal and novel observation was diagnosis of pulmonary TB (ICD-9 codes 010, 011, 012, and 018) during the course of the follow-up period.
2.5. Statistical analysis
To statistically analyze changes between the gastrectomy and non-gastrectomy groups, we used the Chi-square test for categorized variables and the t test for continuous variables. These included sex, age, and various comorbidities as listed above. We defined incidence of pulmonary TB within each group as the number of pulmonary TB events identified during the follow-up period, divided by the total follow-up person-years. The univariable model was first used to analyze all variables and, subsequently, only those found to be significant were chosen for additional analysis using the multivariable model. To do so, multivariable Cox proportional hazards regression was used to assess the hazard ratio (HR) and 95% confidence interval (CI) for risk of pulmonary TB. All analyses were performed by the SAS software version 9.2 (SAS Institute, Inc., Cary, NC).
3. Results
3.1. Baseline characteristics of the study population
Table 1 demonstrates the baseline characteristics of the study population. There were 762 subjects in the gastrectomy group and 2963 subjects in the non-gastrectomy group, with similar distributions of sex and age. The mean age of the study subjects was 59.0 years (±16.2 years) for the gastrectomy group and 58.4 years (±16.2 years) for the non-gastrectomy group (t test; P = .4). The gastrectomy group had a higher proportion of splenectomy than the non-gastrectomy group (4.72% vs 2.4%; Chi-square test; P = .001). There was no significant difference in other comorbidities between the gastrectomy and non-gastrectomy groups (Chi-square test; P > .05).
Table 1.
Baseline characteristics between gastrectomy group and non-gastrectomy group.

3.2. Incidence of pulmonary TB in the study population stratified by sex and age
Table 2 demonstrates that the overall incidence of pulmonary TB was 1.97-fold greater in the gastrectomy group than that in the non-gastrectomy group (6.74 vs 3.42 per 1000 person-years, 95% CI 1.56, 2.49). In a sub-analysis, the partial gastrectomy group and the total gastrectomy group demonstrated higher incidences of pulmonary TB compared with the non-gastrectomy group (5.58 and 20.9 per 1000 person-years, respectively). The incidence of pulmonary TB, as stratified by sex and age, was higher in the gastrectomy group compared with the non-gastrectomy group. The gastrectomy group aged 65 to 84 years had the highest incidence of pulmonary TB (14.3 per 1000 person-years). Figure 1 demonstrates that the Kaplan–Meier cumulative incidence of pulmonary TB was higher in the gastrectomy group than that in the non-gastrectomy group (5.4% vs 4.0% at the end of follow-up; P < .001).
Table 2.
Incidence of pulmonary tuberculosis estimated by sex and age between gastrectomy group and non-gastrectomy group.
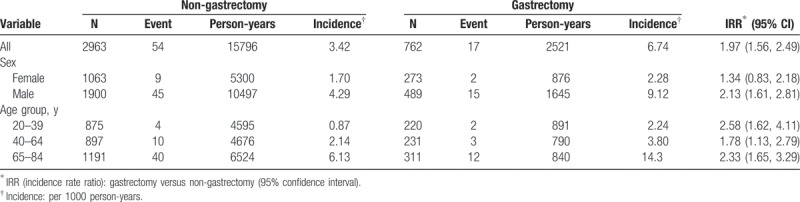
Figure 1.
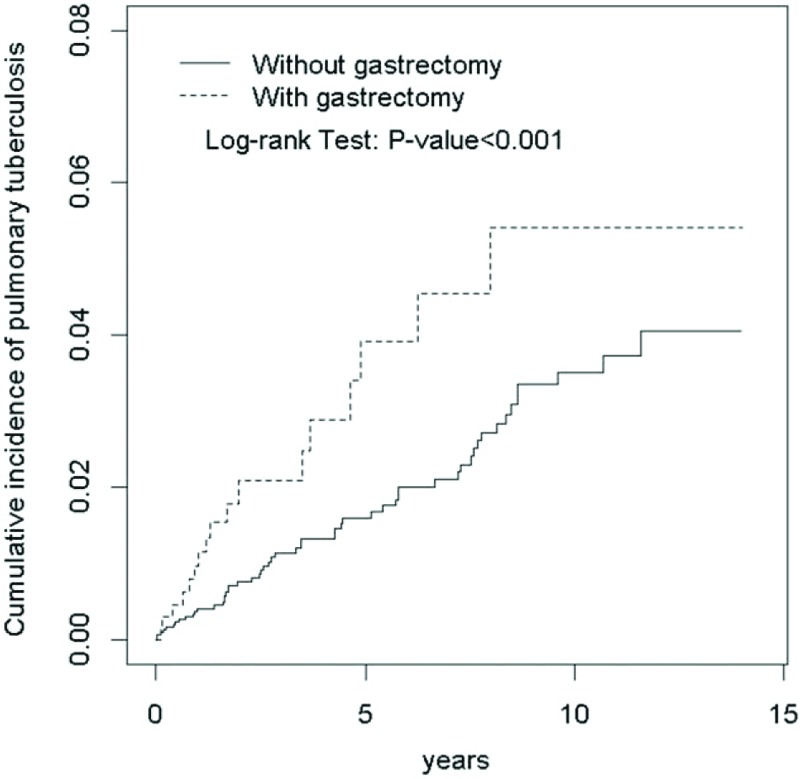
Cumulative incidence of pulmonary tuberculosis for subjects with gastrectomy and without gastrectomy (5.4% vs 4.0% at the end of follow-up; P < .001).
3.3. Pulmonary TB was associated with gastrectomy and comorbidities
Table 3 demonstrates the risk of pulmonary TB associated with gastrectomy and comorbidities. After analysis of all variables using the univariable model, only those found to be significant were then analyzed further using the multivariable model. After appropriate correction for covariables, the adjusted HR of pulmonary TB for the gastrectomy group was calculated to be 1.97 (95% CI 1.13, 3.42), compared with the non-gastrectomy group. In addition, male sex (adjusted HR 2.5; 95% CI 1.31, 4.77), age (increase per 1 year; adjusted HR 1.05; 95% CI 1.03, 1.07), COPD (adjusted HR 2.52; 95% CI 1.50, 4.24), and splenectomy (adjusted HR 6.1; 95% CI 2.73, 13.7) were other factors that could be related to pulmonary TB.
Table 3.
Cox model measured hazard ratio and 95% confidence interval of pulmonary tuberculosis associated with gastrectomy and comorbidities.

3.4. Interaction effect on pulmonary TB between gastrectomy and splenectomy
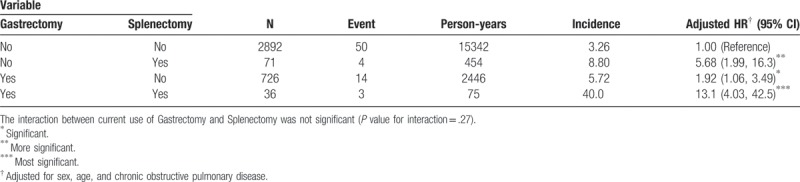
As a reference of subjects without gastrectomy and without splenectomy, the adjusted HR of pulmonary TB was 1.92 (95% CI 1.06, 3.49) for those subjects with gastrectomy and without splenectomy. The adjusted HR increased to 13.1 (95% CI 4.03, 42.5) for those subjects with gastrectomy and splenectomy (Table 4).
Table 4.
Interaction effect on pulmonary tuberculosis between gastrectomy and splenectomy.
4. Discussion
Previous literature revealed that there was a probable link between gastrectomy and pulmonary TB, on and off since 1954.[34] The published literature may be divided into 3 stages. Before 1954, the retrospective studies were on small populations,[35,36] therefore resulted in certain recall bias. Most authors were interested in the surgical sequel of post-gastrectomy resulting from any disease and provided only certain information in regards to the association of gastrectomy and pulmonary TB. Furthermore, Johnsson[37] was the first author to describe a search for pulmonary TB following partial gastrectomy, however, the results were not inclusive.
Between 1960 and 1990, certain literature was focused on the associations between gastrectomy and pulmonary TB, probably for the advanced treatment for active TB. The prevalence of gastrectomy among patients with pulmonary TB ranged from 1.7% to 12.3%[38–40] and the sample size was increased compared with previous studies. Thorn et al[10] indicated that the annual incidence of TB among these individuals was approximately 5 times the rate of same age in this area. In addition, the observation period ranged from 1.5 to 6.5 years in the present study, thus increased the specificity and sensitivity compared with other similar studies in this stage. After 1990, due to the rapid progress in statistics and related researches, certain studies were focused in Japan[8,41] and Taiwan.[2] A large scale randomized study was assigned and the results indicated that gastrectomy may be a risk factor for the reactivation of pulmonary TB. Huang et al[2] concentrated on the incidence rate of any spectrum of TB with gastrectomy after gastric cancer predominantly, ignoring gastrectomy after other diseases. To the best of our knowledge, this is the first population-based study to investigate the relationship between gastrectomy after any disease and pulmonary TB.
The baseline characteristics of our study population demonstrated that the gastrectomy group had a higher proportion of splenectomy than the non-gastrectomy group. A decrease in immunity may be the explanation for this condition. Recent evidence revealed that humoral immunity and B-cells may play an independent role for the immune response to different microorganisms, including mycobacterium TB.[42] In addition, the role of spleen as a protector of the human body against invading microorganisms is well established.[43] A recent Taiwan population-based study also demonstrated the association between pulmonary TB and splenectomy in 2014.[32] Gastrectomy causes a poor nutritional status leading to poor immunity, as demonstrated by a previous study.[44] Due to reasons stated above, the patients’ post-gastrectomy or post-splenectomy, disregarding resulted from any disease might be in lower resistance to mycobacterium TB. We also found that the groups with gastrectomy alone, splenectomy alone, and combination of gastrectomy and splenectomy all showed higher risk of pulmonary tuberculosis than the group without gastrectomy and without splenectomy in Table 4. Furthermore, synergistic effect over the risk of pulmonary TB could be expected and surmised when patients with gastrectomy and splenectomy at the same time from our study, therefore, should be applied in the medical field in the future.
In the present study, the incidence of pulmonary TB, as stratified by sex and age, was higher in the gastrectomy group than the non-gastrectomy group. Gastrectomy indicates that a certain portion of the stomach was surgically removed due to gastric cancer, peptic ulcer, or other diseases. Folic acid, iron, minerals zinc, copper, and vitamins A, C, E, and B are mostly absorbed from the stomach or a certain part of the duodenum. Thus, decreasing absorption of micronutrients post-gastrectomy may result in abnormal cellular function and lower the resistance against invading microorganisms.[45] An Indonesian study demonstrated that a lower concentration of micronutrients led to a decreased host defense to TB.[46] Another meta-analysis review study revealed that long-term malnutrition was an important risk factors in developing pulmonary TB.[47] In the sub-analysis of the present study, the partial gastrectomy group and the total gastrectomy group demonstrated higher incidence of pulmonary TB than the non-gastrectomy group (5.58 and 20.9 per 1000 person-years, respectively), compatible with the mechanism and explanation provided above.
In the current study, male sex and old age were the potential risk factors related with pulmonary TB, following adjustment for covariables. These conditions were compatible with previous literature.[2,8,10,34] A previous study demonstrated that the elderly are easily infected by pulmonary TB due to a decreased immune response.[3] As for the male sex being another risk factor for pulmonary TB, this may be due to the fact that men are more involved with alcohol drinking and tobacco smoking than females. In addition, previous studies demonstrated that smoking, drinking silicosis, and air pollution were also associated with a high incidence of pulmonary TB,[4,48] therefore further research is required to fully understand the reason of the male sex being a risk factor associating with pulmonary TB.
COPD was one of the factors that could be related to pulmonary TB noted in our study. Previous studies discussed the association between COPD and pulmonary TB directly. In one of the studies, cardiovascular, COPDs, and DM were the most frequent concomitant diseases after emergency surgery, including 19.5% gastrectomy (Billroth II resection) in perforated peptic ulcer patients.[49] Furthermore, 6 patients who developed a postoperative abscess had a previous history of COPD. A possible explanation may be that the reduced tissue oxygenation resulted in the damage of postsurgical wound healing process.
This possibility was also supported by other recent studies.[50] Physicians at local clinics should pay more attention to this the condition and prevent this comorbidity earlier.
5. Limitations
In the present study, various risk factors of pulmonary TB, such as alcohol consumption, tobacco smoking, socioeconomic status mentioned above[7] and albumin, were not enrolled due to our database inherent limitations. Furthermore, the current study did not differentiate for the reason the patients received gastrectomy, such as gastric cancer or other gastrointestinal malignancies, and this may have served an independent role in the development of pulmonary TB. Finally, the present study had an inherent limitation, as the diagnosis of pulmonary TB and gastrectomy confirmed by ICD-9 only. A further definite evaluation for pulmonary TB, such as chest x-ray or sputum culture could be added for the reduction of confounding effects in future investigations.
6. Strength
One of the strengths of the present study is that the set of ICD-9 codes used has been validated in previous published studies.[25,26,30–32] Under the normal physical mechanism and plausible hypothesis, the long-term observation period from 2000 to 2012 allowed for more credibility compared with other similar studies.
7. Conclusion
In conclusion, gastrectomy is associated with 1.97-fold increased risk of pulmonary TB in Taiwan compared with the non-gastrectomy group. Following adjustment for covariables, male sex, age, COPD, and splenectomy were other factors that could be associated with pulmonary TB. In light of this, regular and conventional surveillance for pulmonary TB should be emphasized and recommended for patients with gastrectomy. Future prospective and comprehensive studies are required to further support the results of the present study.
Author contributions
Kao-Chi Cheng and Kuan-Fu Liao initiated the draft of the article, revised the article, and contributed equally to the article.
Cheng-Li Lin conducted the data analysis and revised the article.
Shih-Wei Lai contributed to the conception of the article, initiated the draft of the article, and revised the article.
Conceptualization: Kao-Chi Cheng, Shih-Wei Lai.
Formal analysis: Cheng-Li Lin.
Investigation: Kuan-Fu Liao.
Supervision: Shih-Wei Lai.
Writing – original draft: Kao-Chi Cheng.
Writing – review & editing: Kao-Chi Cheng.
Footnotes
Abbreviations: CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, HIV = human immunodeficiency virus, ICD-9 code = International Classification of Diseases, 9th Revision, Clinical Modification, pulmonary, TB = pulmonary tuberculosis, WHO = World Health Organization.
This study was supported in part by the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212–123004), China Medical University Hospital (DMR-107-192), Academia Sinica Stroke Biosignature Project (BM10701010021), MOST Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005), Tseng-Lien Lin Foundation, Taichung, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. These funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors disclose no conflicts of interest.
References
- [1].Zumla A, George A, Sharma V, et al. The WHO 2014 global tuberculosis report—further to go. Lancet Glob Health 2015;3:e10–2. [DOI] [PubMed] [Google Scholar]
- [2].Huang SF, Li CP, Feng JY, et al. Increased risk of tuberculosis after gastrectomy and chemotherapy in gastric cancer: a 7-year cohort study. Gastric Cancer 2011;14:257–65. [DOI] [PubMed] [Google Scholar]
- [3].Zevallos M, Justman JE. Tuberculosis in the elderly. Clin Geriatr Med 2003;19:121–38. [DOI] [PubMed] [Google Scholar]
- [4].Corbett EL, Churchyard GJ, Clayton TC, et al. HIV infection and silicosis: the impact of two potent risk factors on the incidence of mycobacterial disease in South African miners. AIDS 2000;14:2759–68. [DOI] [PubMed] [Google Scholar]
- [5].Wu HP, Pan YH, Hua CC, et al. Pneumoconiosis and liver cirrhosis are not risk factors for tuberculosis in patients with pulmonary infection. Respirology 2007;12:416–9. [DOI] [PubMed] [Google Scholar]
- [6].Lee CH, Lee MC, Shu CC, et al. Risk factors for pulmonary tuberculosis in patients with chronic obstructive airway disease in Taiwan: a nationwide cohort study. BMC Infect Dis 2013;13:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gupta S, Shenoy VP, Mukhopadhyay C, et al. Role of risk factors and socio-economic status in pulmonary tuberculosis: a search for the root cause in patients in a tertiary care hospital, South India. Trop Med Int Health 2011;16:74–8. [DOI] [PubMed] [Google Scholar]
- [8].Yokoyama T, Sato R, Rikimaru T, et al. Tuberculosis associated with gastrectomy. J Infect Chemother 2004;10:299–302. [DOI] [PubMed] [Google Scholar]
- [9].Boman K. Tuberculosis occurring after gastrectomy. Acta Chir Scand 1956;110:451–7. [PubMed] [Google Scholar]
- [10].Thorn P, Brookes V, Waterhouse J. Peptic ulcer, partial gastrectomy, and pulmonary tuberculosis. Br Med J 1956;1:603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ministry of Health and Welfare T. 2016 Taiwan Health and Welfare Report. Available at: http://wwwmohwgovtw. [Cited on March 1, 2017, English version]. [Google Scholar]
- [12].Yang JS, Peng YR, Tsai SC, et al. The molecular mechanism of contrast-induced nephropathy (CIN) and its link to in vitro studies on iodinated contrast media (CM). Biomedicine 2018;8:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang X, Sheu JJ-C, Lai M-T, et al. RSF-1 overexpression determines cancer progression and drug resistance in cervical cancer. Biomedicine 2018;8:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang JS, Lu CC, Kuo SC, et al. Autophagy and its link to type II diabetes mellitus. Biomedicine (Taipei) 2017;7:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu MH, Lee TH, Lee HP, et al. Kuei-Lu-Er-Xian-Jiao extract enhances BMP-2 production in osteoblasts. Biomedicine (Taipei) 2017;7:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wen YJ, Yin MC. The anti-inflammatory and anti-glycative effects of rosmarinic acid in the livers of type 1 diabetic mice. Biomedicine 2017;7:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pan CC, Huang HL, Chen MC, et al. Lower risk of end stage renal disease in diabetic nurse. Biomedicine 2017;7:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu YL, Liu JH, Wang IK, et al. Association of inflammatory cytokines with mortality in peritoneal dialysis patients. Biomedicine (Taipei) 2017;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yu CC, Chien CT, Chang TC. M2 macrophage polarization modulates epithelial-mesenchymal transition in cisplatin-induced tubulointerstitial fibrosis. Biomedicine (Taipei)n 2016;6:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ooi H. Bedside pleuroscopy in Taiwan: a great vision for critically-ill patients and intensivists. Biomedicine (Taipei) 2016;6:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Maa MC, Leu TH. Src is required for migration, phagocytosis, and interferon beta production in Toll-like receptor-engaged macrophages. Biomedicine (Taipei) 2016;6:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chang LC, Yu YL. Dietary components as epigenetic-regulating agents against cancer. Biomedicine (Taipei) 2016;6:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chan CY, Lien CH, Lee MF, et al. Quercetin suppresses cellular migration and invasion in human head and neck squamous cell carcinoma (HNSCC). Biomedicine (Taipei) 2016;6:10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liao KF, Cheng KC, Lin CL, et al. Etodolac and the risk of acute pancreatitis. Biomedicine (Taipei) 2017;7:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cheng KC, Liao KF, Lin CL, et al. Correlation of proton pump inhibitors with pulmonary tuberculosis: a case-control study in Taiwan. Front Pharmacol 2017;8:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cheng KC, Liao KF, Lin CL, et al. Increased risk of pulmonary tuberculosis in patients with depression: a cohort study in Taiwan. Front Psychiatry 2017;8:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lai SW, Liao KF, Liao CC, et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine 2010;89:295–9. [DOI] [PubMed] [Google Scholar]
- [28].Liao KF, Huang PT, Lin CC, et al. Fluvastatin use and risk of acute pancreatitis:a population-based case-control study in Taiwan. Biomedicine (Taipei) 2017;7:24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shen ML, Liao KF, Tsai SM, et al. Herpes zoster correlates with pyogenic liver abscesses in Taiwan. Biomedicine (Taipei) 2016;6:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lai SW, Lin CL, Liao KF, et al. Increased risk of pulmonary tuberculosis among patients with appendectomy in Taiwan. Eur J Clin Microbiol Infect Dis 2014;33:1573–7. [DOI] [PubMed] [Google Scholar]
- [31].Lin HF, Liao KF, Chang CM, et al. Anti-diabetic medication reduces risk of pulmonary tuberculosis in diabetic patients: a population-based Cohort Study in Taiwan. Kuwait Med J 2017;49:22–8. [Google Scholar]
- [32].Lai SW, Wang IK, Lin CL, et al. Splenectomy correlates with increased risk of pulmonary tuberculosis: a case–control study in Taiwan. Clin Microbiol Infect 2014;20:764–7. [DOI] [PubMed] [Google Scholar]
- [33].Lai SW, Lin CL, Liao KF. Population-based cohort study examining the association between weight loss and pulmonary tuberculosis in adults. Biomedicine (Taipei) 2018;8:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pearson RB. Pulmonary tuberculosis following partial gastrectomy. Digestion 1954;81:91–7. [DOI] [PubMed] [Google Scholar]
- [35].Daley A. Report of the county Medical Officer of Health and School Medical Officer for the Year 1950 1950. [Google Scholar]
- [36].Kirste, Bruusgaard C. The Operative Treatment of Gastric and Duodenal Ulcer. A Clinical and Roentgenologic Study. 1946. [Google Scholar]
- [37].Johnsson S. Gasiric resection in gastric ulcer. Acta Med Scand 1951;140:12–24. [PubMed] [Google Scholar]
- [38].Hanngren Å, Reizenstein P. Studies in dumping syndrome. Am J Digest Dis 1969;14:700–10. [DOI] [PubMed] [Google Scholar]
- [39].Steiger Z, Nickel WO, Shannon GJ, et al. Pulmonary tuberculosis after gastric resection. Am J Surg 1976;131:668–71. [DOI] [PubMed] [Google Scholar]
- [40].Snider DE. Tuberculosis and gastrectomy. Chest J 1985;87:414–5. [DOI] [PubMed] [Google Scholar]
- [41].Association JA-T. Statistics of Tuberculosis (KEKKAKU NO TOUKEI). Tokyo: PNKekkaku Yobo kai (JATA); 2003. [Google Scholar]
- [42].Kozakiewicz L, Phuah J, Flynn J, et al. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection, The New Paradigm of Immunity to Tuberculosis. 2013;New York, NY: Springer, 225–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jirillo E, Mastronardi M, Altamura M, et al. The immunocompromised host: immune alterations in splenectomized patients and clinical implications. Curr Pharma Des 2003;9:1918–23. [DOI] [PubMed] [Google Scholar]
- [44].Papathakis P, Piwoz E. Nutrition and Tuberculosis: A review of the literature and considerations for TB control programs. United States Agency for International Development, Africa's Health 2010 Project 2008:1. [Google Scholar]
- [45].Chandra R. Nutritional deficiency and susceptibility to infection. Bull World Health Organ 1979;57:167–77. [PMC free article] [PubMed] [Google Scholar]
- [46].Karyadi E, Schultink W, Nelwan RH, et al. Poor micronutrient status of active pulmonary tuberculosis patients in Indonesia. J Nutr 2000;130:2953–8. [DOI] [PubMed] [Google Scholar]
- [47].Pérez-Guzmán C, Vargas MH, Torres-Cruz A, et al. Does aging modify pulmonary tuberculosis? A meta-analytical review. Chest J 1999;116:961–7. [DOI] [PubMed] [Google Scholar]
- [48].Nava-Aguilera E, Andersson N, Harris E, et al. Risk factors associated with recent transmission of tuberculosis: systematic review and meta-analysis [Review article]. Int J Tuberc Lung Dis 2009;13:17–26. [PubMed] [Google Scholar]
- [49].Testini M, Portincasa P, Piccinni G, et al. Significant factors associated with fatal outcome in emergency open surgery for perforated peptic ulcer. World J Gastroenterol 2003;9:2338–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Testini M, Piccinni G, Amoruso M, et al. Chronic obtructive pulmonary disease and failure of large bowel anastomosis. It J Coloproctol 2000;3:91–4. [Google Scholar]


