Abstract
Rationale:
Acute liver failure (ALF) induced by amatoxin-containing mushrooms accounts for more than 90% of deaths in patients suffering from mushroom poisoning. However, due to the fact that most hospitals cannot identify the species of mushrooms involved, or detect amatoxins, the early diagnosis of amatoxin intoxication remains a significant challenge in clinical practice.
Patient concerns:
Two patients were had ingested wild mushrooms 15 hours before admission. Six hours prior to admission they experienced nausea, vomiting, weakness, abdominal pain and diarrhea. The species of mushrooms they had consumed could not be identified.
Diagnoses:
According to their delayed gastroenteritis, the two patients were clinically diagnosed with amatoxin poisoning. One week after the patients were discharged, the species of the mushrooms was identified as Amanita fuliginea and the diagnosis was confirmed.
Interventions:
The two patients were treated with silibinin, penicillin G and plasma exchange.
Outcomes:
Although the two patients progressed to ALF they fully recovered and were discharged on day 10 after admission.
Lessons:
Our case reports suggested that patients with unidentified wild mushroom intoxication with delayed gastroenteritis could be clinically diagnosed with amatoxin poisoning; in such cases, liver coagulation function should be frequently evaluated. Early diagnosis and treatment are crucial for survival in patients with ALF induced by amatoxin poisoning.
Keywords: acute liver failure, amatoxins, mushroom, poisoning, prognosis
1. Introduction
Acute liver failure (ALF) is a disease with high mortality, although the prognosis of patients with ALF depends largely on their etiology.[1] The most common cause of ALF in China is viral hepatitis. ALF caused by mushroom poisoning is relatively rare and is almost entirely induced by amatoxin-containing mushrooms.[2] ALF induced by amatoxin poisoning accounts for >90% of deaths in patients suffering from mushroom poisoning.[3] Patients with amatoxin-induced ALF have a much poorer prognosis and liver transplantation is the only lifesaving option.[4,5] However, due to a shortage of donors, liver transplantation is not readily available nor feasible for most patients with ALF in China. The effects of other treatment options, including various combinations of a number of drugs and supportive therapy and artificial liver support systems (ALSS) remain limited.[6] The early identification of patients with amatoxin poisoning and improvements in the efficacy of specific therapies will benefit most patients with amatoxin-induced ALF.
The early diagnosis of amatoxin poisoning depends on the rapid identification of the mushrooms causing the poisoning, or the detection of amatoxin in patients. However, the identification of mushroom species consumed by patients is difficult for clinical doctors and patients, and the detection of amatoxin is not available in most hospitals in China and most other countries. In a previous report, 82.7% of mushrooms causing poisoning could not be identified.[7] Therefore, the early diagnosis of amatoxin intoxication remains a significant clinical challenge. In this paper, we report 2 patients suffering from poisonous mushroom-induced ALF in which the species of mushroom consumed could not be identified until 1 week after the patients had been discharged. According to their delayed gastroenteritis, the 2 patients were suspected of amatoxin poisoning and were successfully treated with silibinin and penicillin G. We also reviewed the clinical characteristics of patients with amatoxin induced ALF.
2. Case reports
Our 2 patients (patient 1 and patient 2) were both women and were 41 and 48 years old, respectively. The 2 patients were relatives and lived in the same country (Shuiyang country, Guizhou Province, China). Patient 1 had collected the mushrooms in a forest and consumed the wild mushrooms with patient 2 by cooking at home approximately 15 hours prior to admission. No one else had eaten the mushrooms. Approximately, 6 hours before admission they experienced nausea, vomiting, and weakness. Approximately, 3 hours before admission, they experienced abdominal pain and diarrhea. They were admitted to the infectious department of our hospital in October 2012 and had not consumed alcohol with the mushrooms.
Upon admission, both patients were still suffering from abdominal pain, nausea, vomiting, diarrhea, and weakness. No fever was noted. Both patients had normal blood pressure. On physical examination, patient 1 had right upper quadrant tenderness to palpation. Neither had guarding or rebound. For both patients, the remainder of the physical examination was normal. To exclude other causes of ALF, additional investigations showed that the 2 patients were negative for IgM antibodies for Epstein-Barr virus (EBV), hepatitis A and E viruses. Tests for hepatitis B virus surface antigen and core antibody, and hepatitis C virus antibody were negative. Tests for antinuclear antibody, double-stranded DNA antibody, anti-liver-kidney microsomal antibody, anti-smooth muscle antibody, and anti-mitochondrial antibody M2 also were negative. Serum ceruloplasmin level was also normal and neither patient reported a history of liver disease.
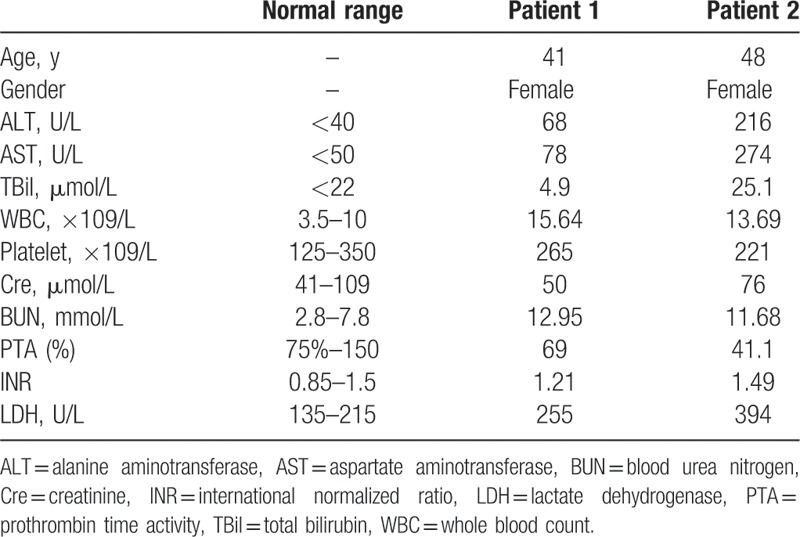
The background data and laboratory findings of the patients at the time of admission are shown in Table 1. The species of mushrooms consumed by the patients could not be identified by either the doctors or patients because the patients had no mushrooms left for examination. Furthermore, we were unable to detect amatoxins in our hospital because we did not have an appropriate assay kit. Considering both patients had ingested wild mushrooms and gastroenteritis symptoms appeared approximately 9 hours after ingestion, we clinically diagnosed the 2 patients with amatoxin poisoning and commenced treatment. Both patients received standard conservative therapy, including bed rest, nutritional and energy supplements, intravenous infusion of plasma, water-electrolytes, and acid–base equilibrium maintenance, along with the prevention and treatment of complications.
Table 1.
Background data and laboratory findings of 2 patients with amatoxin-induced acute liver failure at the time of admission.
Patient 1 weighed 55 kg; penicillin G was intravenously administered at a dose of 24,000,000 U/d (436,364 U/kg/d) and silybin was administered orally at a dose of 3 g/d (0.055 g/kg/d). The symptoms of gastroenteritis were alleviated during the first day after admission. However, her alanine aminotransferase (ALT) and total bilirubin (TBil) levels steadily increased to a peak of 3798 U/L (normal level: <40 U/L) and 50.5 μmol/L (normal level: <22 mmol/L) on the third and fourth day after admission, respectively. Prothrombin time activity (PTA) decreased to 30.7% (normal range, 75–150%) on the third day after admission (Fig. 1). The patient developed transient hepatic encephalopathy on the third day. Plasma exchange was performed with 2500 mL of frozen plasma. From the fifth day, her liver function improved steadily.
Figure 1.
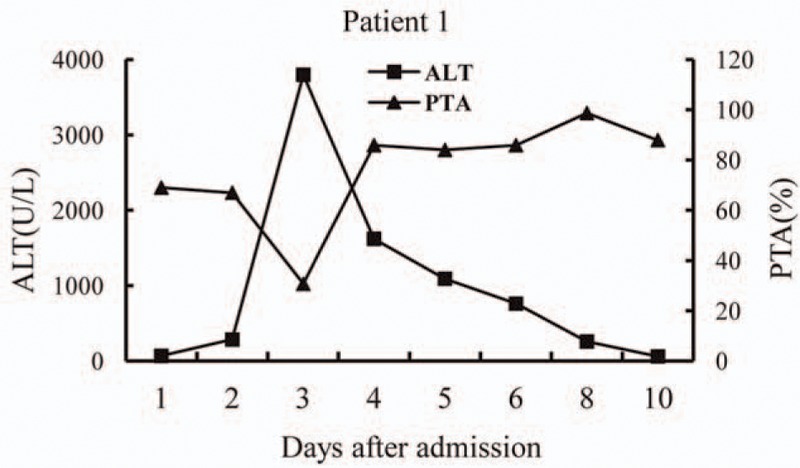
Alanine aminotransferase (ALT) levels and prothrombin time activity (PTA) over time (days) after admission in patient 1.
Patient 2 weighed 62 kg; penicillin G was intravenously administered at a dose of 32,000,000 U/d (516,129 U/kg/d) and silybin was administered orally at a dose of 4 g/d (0.065 g/kg/d). Her symptoms of gastroenteritis were also significantly alleviated during the first day after admission. The peak ALT for this patient was 2128 U/L (normal: <40 U/L) and TBil was 90.9 μmol/L (normal: <22 mmol/L), on the fourth and the fifth day, respectively, after admission. PTA fell to 22.0% (normal range, 75–150%) on the third day after admission (Fig. 2). She subsequently developed grade 2 hepatic encephalopathy on the third day. Plasma exchange was performed on 2 occasions on the third and fifth day after admission; 2000 to 2500 mL of frozen plasma was used each time. From the fourth day, her hepatic encephalopathy disappeared and liver function improved.
Figure 2.
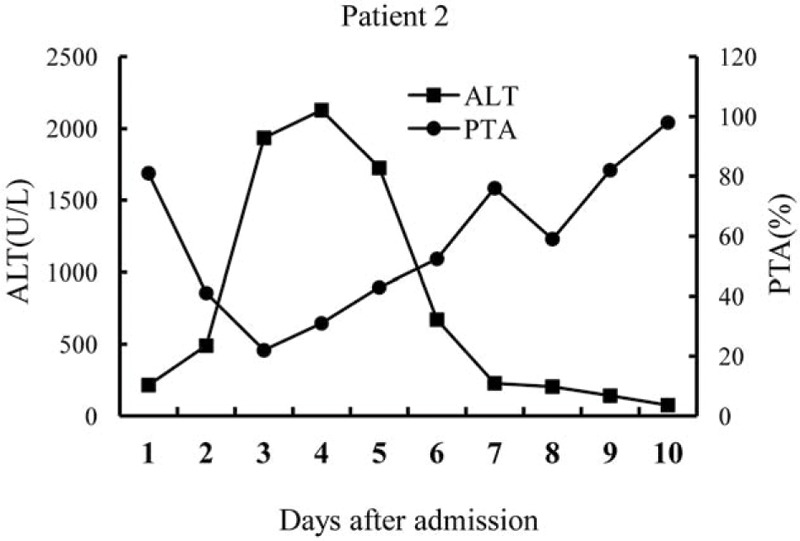
Alanine aminotransferase (ALT) levels and prothrombin time activity (PTA) over time (days) after admission in patient 2.
The 2 patients were discharged on day 10 after admission with normal aspartate aminotransferase (AST), ALT, and PTA levels. One week after the patients were discharged, the mushrooms they had consumed were brought to the hospital and the species was identified as Amanita fuliginea. Three months after discharge, the patients were asymptomatic, with no evidence of ongoing toxicity or sequelae.
The therapeutic protocol was approved by the Human Ethics Review Committee of Zunyi Medical College, Guizhou, China and informed consent was obtained from each patient.
3. Discussion
There are more than 3000 species of mushrooms in China. Approximately, 400 of these are toxic to humans.[8] Acute liver injury can be caused by the ingestion of amatoxin-containing mushrooms.[9] Amatoxins have been found in several species of wild mushrooms. In Southern China, 78.05% of patients suffering from wild mushroom poisoning, and 70.49% of deaths, are caused by species from the genus Amanita. In particular, Amanita fuliginea and Amanita exitialis cause approximately 40% of deaths due to wild mushroom intoxication.[8]
Two mechanisms have been suggested for the way in which amatoxin induces hepatocyte apoptosis.[10] Firstly, the amatoxins bind non-covalently to and inhibit RNA polymerase II and protein synthesis in hepatocytes. Since the liver is an organ with a high rate of protein synthesis and cell turnover, amatoxin poisoning induces the most serious injury in the liver compared with other organs.[11] The second mechanism is that amatoxin induces the production of cytokines such as tumor necrosis factor-α (TNF-α); amatoxin and TNF-α synergistically induce the death of hepatocytes.[12] Furthermore, it has also been suggested that free radical production induced by amatoxin also plays an important role in the pathogenesis of amatoxin-induced liver injury. It has been found that amatoxin can increase the production of superoxide dismutase and reduce catalase activity, causing hepatocellular dysfunction through a peroxidative pathway.[13]
Patients with amatoxin intoxication usually experience 4 clinical stages.[2] The first stage is known as the latent stage. During this stage, patients do not have any symptoms. This stage usually lasts 6 to 12 hours after the ingestion of mushrooms. After patients progress to the second stage, most of them experience gastroenteritis characterized by the acute onset of nausea and vomiting, diarrhea, abdominal pain or electrolyte abnormalities, and dehydration. This the second stage lasts 12 to 24 hours. After this stage, the patient might feel better for 12 to 24 hours, and the symptoms of gastroenteritis are alleviated or disappear. This third stage has also been described as the “pseudo-remission period” or false recovery stage. However, after the third stage, patients rapidly progress to the fourth stage, which is characterized by differing degrees of liver injury. Some patients go on to develop ALF and multi-organ failure. Death usually occurs within 5 to 8 days of ingestion.
The clinical characteristics of patients with amatoxin intoxication make this a challenge to diagnose early and treat promptly. During the first stage, most patients do not seek medical attention because they have no symptoms. Most patients are admitted to hospital after the symptoms of gastroenteritis appear. However, it is difficult for clinical doctors and patients to identify the species of mushrooms consumed and the assay kit needed to detect amatoxin is not readily available in most hospitals in China and other countries. Consequently, the differential diagnosis of patients with amatoxin poisoning from other poisoning caused by non-amatoxin-containing mushrooms is, therefore, very difficult.[14] Many patients with non-amatoxin containing mushroom poisoning also present with gastroenteritis.[15–17] In a previous report, 82.7% of cases involving mushroom intoxication were attributed to an unknown species of mushroom.[7] It has been suggested that delayed gastroenteritis symptoms after the ingestion of mushrooms is indicative of amatoxin poisoning.[9,16,18] Most studies have found that a latent phase lasting >6 hours could be indicative of amatoxin-induced mushroom poisoning. However, 1 previous study found that patients with fatal Amanita phalloides poisoning show an interval between ingestion and diarrhea of 7 ± 2 hours.[19] In another report, involving 4 patients with ALF caused by mushroom poisoning, 3 patients had a latent phase lasting 6 to 24 hours while 1 patient had a latent phase of <6 hours.[20] Therefore, although patients with amatoxin poisoning have a longer latent phase than other types of poisoning that do not involve amatoxin, the time point by which we need to distinguish patients with amatoxin poisoning still requires further studies.
The “pseudo-remission period,” or the third stage, is a key stage in the diagnosis and management of patients with amatoxin intoxication. In this stage, massive liver injury has not yet occurred. Patients with more benign mushroom poisoning will recover well during this stage; consequently, it is difficult to distinguish patients who have truly recovered from those showing false recovery, such as those with amatoxin intoxication. Frequent evaluation of liver and coagulation function must be emphasized in all patients. At the end of this stage the ALT and AST in most patients with amatoxin intoxication begins to increase; TBil then also increases and PT is prolonged.
During the fourth stage, in which liver injury occurs, the diagnosis of amatoxin-containing mushroom poisoning would become clear. However, due to the rapid progression of liver injury during this stage, the opportunities to successfully save the lives of patients would be lost. The most effective treatment method during this stage is liver transplantation. However, in patients with amatoxin-induced ALF, it is difficult to identify the right time-point for transplantation.[10,21] Therefore, the precise evaluation of the degree of liver injury, and the prognosis of the patient, is important during this stage.[22,23]
Several non-specific treatment methods have been used in patients with amatoxin-induced ALF, including gastric lavage and administering activated charcoal to reduce the absorbance of toxins by preventing and/or reducing enterohepatic circulation.[14] Penicillin G, silybin, and N-acetylcysteine (NAC) have been used most widely in patients with amatoxin poisoning as a specific treatment strategy. However, the mechanisms of these drugs in terms of hepatoprotection and as an antidote for amatoxin intoxication have not yet been fully elucidated. Penicillin G has been previously shown to inhibit the penetration of amatoxin into hepatocytes and directly compete with amatoxin for transmembrane transport.[9] Silybin is the component with the highest antioxidant activity. NAC is often combined with penicillin G and/or silibinin as free radical scavengers,[24] but in animal studies, NAC has not been shown to be effective in the management of amatoxin intoxication.[25] Although clinical studies have shown that these drugs, either alone or in combination, improved the survival of patients with amatoxin-induced ALF,[4,24] there is still a need for randomized controlled trials to confirm their effect.[26,27] Therefore, early diagnosis and treatment with non-specific and specific treatment modalities are crucial for the survival of patients with ALF induced by amatoxin poisoning.
4. Conclusions
Our 2 case reports suggest that patients suffering from unidentified wild mushroom intoxication with delayed gastroenteritis could be clinically diagnosed with amatoxin poisoning. In such patients, it is important that liver and coagulation function should be frequently evaluated. Early diagnosis and treatment are crucial for survival in patients with ALF induced by amatoxin poisoning.
Author contributions
Conceptualization: Shide Lin.
Data curation: Ling Yuan, Baimei Zeng.
Formal analysis: Baimei Zeng.
Funding acquisition: Shide Lin.
Investigation: Ying Li, Maoyuan Mu.
Methodology: Ling Yuan, Baimei Zeng.
Project administration: Shide Lin.
Resources: Maoyuan Mu, Ling Yuan.
Software: Baimei Zeng.
Supervision: Shide Lin.
Validation: Shide Lin.
Visualization: Shide Lin.
Writing – original draft: Maoyuan Mu, Ying Li.
Writing – review and editing: Ying Li, Shide Lin.
Footnotes
Abbreviations: ALF = acute liver failure, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BUN = blood urea nitrogen, Cre = creatinine, EBV = Epstein-Barr virus, HAV = hepatitis A virus, HBsAg = hepatitis B virus surface antigen, INR = international normalized ratio, LDH = lactate dehydrogenase, NAC = N-acetylcysteine, PT = prothrombin time, PTA = prothrombin time activity, PLT = platelet, TBil = total bilirubin, TNF-α = tumor necrosis factor-α, WBC = white blood cell.
YL and MM have contributed equally to this work.
This work was supported by the Funds of the Chinese National Natural Science Foundation Project (814600124/H0316, 81160067/H0318).
The authors have no conflicts of interest to disclose.
References
- [1].Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position paper on acute liver failure in 2011. Hepatology 2012;55:965–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Santi L, Maggioli C, Mastroroberto M, et al. Acute liver failure caused by amanita phalloides poisoning. Int J Hepatol 2012;2012:487480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Unluoglu I, Tayfur M. Mushroom poisoning: an analysis of the data between 1996 and 2000. Eur J Emerg Med 2003;10:23–6. [DOI] [PubMed] [Google Scholar]
- [4].Enjalbert F, Rapior S, Nouguier-Soule J, et al. Treatment of amatoxin poisoning: 20-year retrospective analysis. J Toxicol Clin Toxicol 2002;40:715–57. [DOI] [PubMed] [Google Scholar]
- [5].Panaro F, Andorno E, Morelli N, et al. Liver transplantation represents the optimal treatment for fulminant hepatic failure from Amanita phalloides poisoning. Transpl Int 2006;19:344–5. [DOI] [PubMed] [Google Scholar]
- [6].Shi Y, He J, Chen S, et al. MARS: optimistic therapy method in fulminant hepatic failure secondary to cytotoxic mushroom poisoning-a case report. Liver 2002;22(suppl):78–80. [DOI] [PubMed] [Google Scholar]
- [7].Mowry JB, Spyker DA, Cantilena LR, et al. 2012 annual report of the american association of poison control centers’ national poison data system (NPDS): 30th annual report. Clin Toxicol (Phila) 2013;51:949–1229. [DOI] [PubMed] [Google Scholar]
- [8].Chen Z, Zhang P, Zhang Z. Investigation and analysis of 102 mushroom poisoning cases in Southern China from 1994 to 2012. Fungal Divers 2014;64:123–31. [Google Scholar]
- [9].Diaz JH. Amatoxin-containing mushroom poisonings: species, toxidromes, treatments, and outcomes. Wilderness Environ Med 2018;29:111–8. [DOI] [PubMed] [Google Scholar]
- [10].Mas A. Mushrooms, amatoxins and the liver. J Hepatol 2005;42:166–9. [DOI] [PubMed] [Google Scholar]
- [11].Garcia J, Costa VM, Carvalho A, et al. Amanita phalloides poisoning: mechanisms of toxicity and treatment. Food Chem Toxicol 2015;86:41–55. [DOI] [PubMed] [Google Scholar]
- [12].Leist M, Gantner F, Naumann H, et al. Tumor necrosis factor-induced apoptosis during the poisoning of mice with hepatotoxins. Gastroenterology 1997;112:923–34. [DOI] [PubMed] [Google Scholar]
- [13].Zheleva A, Tolekova A, Zhelev M, et al. Free radical reactions might contribute to severe alpha amanitin hepatotoxicity--a hypothesis. Med Hypotheses 2007;69:361–7. [DOI] [PubMed] [Google Scholar]
- [14].Ward J, Kapadia K, Brush E, et al. Amatoxin poisoning: case reports and review of current therapies. J Emerg Med 2013;44:116–21. [DOI] [PubMed] [Google Scholar]
- [15].Lin S, Mu M, Yang F, et al. Russula subnigricans poisoning: from gastrointestinal symptoms to Rhabdomyolysis. Wilderness Environ Med 2015;26:380–3. [DOI] [PubMed] [Google Scholar]
- [16].Chan CK, Lam HC, Chiu SW, et al. Mushroom poisoning in Hong Kong: a ten-year review. Hong Kong Med J 2016;22:124–30. [DOI] [PubMed] [Google Scholar]
- [17].Broussard CN, Aggarwal A, Lacey SR, et al. Mushroom poisoning-from diarrhea to liver transplantation. Am J Gastroenterol 2001;96:3195–8. [DOI] [PubMed] [Google Scholar]
- [18].Kaufmann P. Mushroom poisonings: syndromic diagnosis and treatment. Wien Med Wochenschr 2007;157:493–502. [DOI] [PubMed] [Google Scholar]
- [19].Escudie L, Francoz C, Vinel JP, et al. Amanita phalloides poisoning: reassessment of prognostic factors and indications for emergency liver transplantation. J Hepatol 2007;46:466–73. [DOI] [PubMed] [Google Scholar]
- [20].Colak S, Kandis H, Afacan MA, et al. Assessment of patients who presented to the emergency department with mushroom poisoning. Hum Exp Toxicol 2015;34:725–31. [DOI] [PubMed] [Google Scholar]
- [21].Ganzert M, Felgenhauer N, Zilker T. Indication of liver transplantation following amatoxin intoxication. J Hepatol 2005;42:202–9. [DOI] [PubMed] [Google Scholar]
- [22].Alves A, Gouveia Ferreira M, Paulo J, et al. Mushroom poisoning with Amanita phalloides-a report of four cases. Eur J Intern Med 2001;12:64–6. [DOI] [PubMed] [Google Scholar]
- [23].Rengstorff DS, Osorio RW, Bonacini M. Recovery from severe hepatitis caused by mushroom poisoning without liver transplantation. Clin Gastroenterol Hepatol 2003;1:392–6. [DOI] [PubMed] [Google Scholar]
- [24].Montanini S, Sinardi D, Pratico C, et al. Use of acetylcysteine as the life-saving antidote in Amanita phalloides (death cap) poisoning. Case report on 11 patients. Arzneimittelforschung 1999;49:1044–7. [DOI] [PubMed] [Google Scholar]
- [25].Tong TC, Hernandez M, Richardson WH, et al. Comparative treatment of alpha-amanitin poisoning with N-acetylcysteine, benzylpenicillin, cimetidine, thioctic acid, and silybin in a murine model. Ann Emerg Med 2007;50:282–8. [DOI] [PubMed] [Google Scholar]
- [26].Lacombe G, St-Onge M. BET 1: Silibinin in suspected amatoxin-containing mushroom poisoning. Emerg Med J 2016;33:76–7. [DOI] [PubMed] [Google Scholar]
- [27].Erden A, Esmeray K, Karagöz H, et al. Acute liver failure caused by mushroom poisoning: a case report and review of the literature. Int Med Case Rep J 2013;6:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


