Abstract
Background:
To investigate the association between exposure to environmental tobacco smoke (EVT) and the incidence of lung cancer (LC) in nonsmoking adults.
Method:
PubMed, Cochrane, Embase, Wanfang, CNKI, and VIP database were searched by the index words to identify the qualified case-control studies, and relevant literature sources were also searched. The latest research was done in June 2017. Odds radio (OR) along with 95% confidence interval (95% CI) were used to analyze the main outcomes.
Result:
Twenty RCTs were involved in the meta-analysis with 13,004 adults in the case group and 11,199 adults in the control group. The results indicated that compared with the nonexposure population, the risk of LC incidence was significantly higher in EVT exposure (OR: 1.64, 95% CI: 1.34–2.01), EVT male exposure (OR: 1.62, 95% CI: 1.16–2.28), EVT female exposure (OR: 1.57, 95% CI: 1.43–1.72), EVT exposure at workplace (OR: 1.78, 95% CI: 1.29–2.44), EVT exposure at home (OR: 1.53, 95% CI: 1.01–2.33), and EVT female exposure at home (OR: 1.55, 95% CI: 1.34–1.79). However, there is still no significant difference among the risk of LC incidence in EVT male exposure at workplace (OR: 1.51, 95% CI: 0.74–3.06), EVT female exposure at workplace (OR: 1.23, 95% CI: 0.99–1.53), and EVT male exposure at home (OR: 1.24, 95% CI: 0.68–2.26).
Conclusion:
EVT exposure is prospectively associated with a significantly increased risk of LC incidence. More high quality studies are required to address the association between EVT exposure and LC incidence.
Keywords: environmental tobacco smoke, lung cancer, meta-analysis
1. Introduction
Lung cancer (LC) is one of the most prevalent and deadliest human cancers. There were about 1.8 million new LC cases globally in 2012 that accounted for 13.0% of all cancer cases, and about 1.59 million deaths from LC that accounted for 19.4% of all cancer deaths.[1] In China, LC ranked first in the incidence and mortality of all cancer. In the 1990s, the mortality of LC in China was 17.5/100,000, and the male mortality (24.3/100,000) was higher than female (10.7/100,000).[2] However, in 2009, the LC mortality increased to 45.57/100,000, with 61/100,000 of male mortality and 29.77/100,000 of female mortality.[3] Therefore, it's meaningful to emphasize the prevention of LC. In China, the morbidity number of LC accounts for 35.78% of all cancer cases worldwide; the mortality rate of LC accounts for 37.55% of all cancer deaths worldwide.[4] Smoking and second-hand smoke are the risk factors of LC, and there are 72.4% of nonsmokers exposed to second-hand smoke.[5–7]
Many studies suggest that smoking is the most important risk factor of LC.[8,9] However, the incidence of LC in nonsmoking population is still up, so it is essential to investigate the influence of ETS exposure on nonsmoking population. Because of the high incidence, poor prognosis, and serious infection to human health, it's important to explore the risk factor of LC in nonsmoking population and formulate the prevention and control strategies. Based on these considerations, the aim of this study was to perform a meta-analysis of all available literature to obtain updated evidence on the association between ETS exposure and LC in nonsmoking population.
2. Methods
2.1. Search strategy
The Cochrane, PubMed, Embase, CNKI (China National Knowledge Infrastructure), Wanfang, and Weipu (VIP) were searched for all the case-control studies regarding the association between the ETS exposure and LC risk of nonsmoker. Others related articles and reference materials were also searched. The latest research was performed on June 2017. Two investigators searched the literature independently; a third investigator was involved when a disagreement occurred. Ethics approval was waived because this study does not involve any human participants or animals.
2.2. Inclusion and exclusion criteria
A study was included if it was case-control study; the research objects in case group were nonsmoking population diagnosed with LC, the research objects in control group were cancer-free population that matched on age, gender, and ethnic background with case group; the data included OR and 95% CI; and only included English and Chinese articles.
A study was excluded if it was republished article, or the contents and results were same; data had obvious mistake or were uncomplete; and if it was case report, theoretical research, conference report, systematic review, meta-analysis, expert comment, or theoretical analysis.
All the studies were screened by 2 reviewers independently to determine whether they satisfied the criteria; discrepancies were resolved by third reviewer.
2.3. Data extraction and quality assessment
The analyses data were extracted from all the included studies and consisted of 2 parts: basic information and main outcomes. The first part was about the basic information: the author name, the sample size, the percentage of male, mean age. The second part was the clinical outcomes: the odds ratio of EVT exposure and LC incidence in different groups. All the above processes were done by 2 reviewers independently; disagreements between reviewers were resolved by discussion until a consensus was reached.
2.4. Statistical Analysis
All statistical analyses were performed in the STATA 10.0 (TX, USA). Chi-squared and I2 tests were used to test the heterogeneity of clinical trial results and decide the analysis model (fixed-effect model or random-effect model). When the Chi-squared test P value was ≤.05 and I2 tests value was >50%, it was defined as acceptable heterogeneity and assessed by random-effects model. When the Chi-squared test P value was >.05 and I2 tests value was ≤50%, it was defined as homogeneous data and assessed by fixed-effects model. The continuous variables are expressed as the mean ± standard deviation and analyzed by mean difference (MD). The categorical data are presented as percentages and analyzed by relative risk (RR) or odds ratio (OR). All the results were analyzed by OR and 95% CI.
3. Results
3.1. Characteristics of the included studies
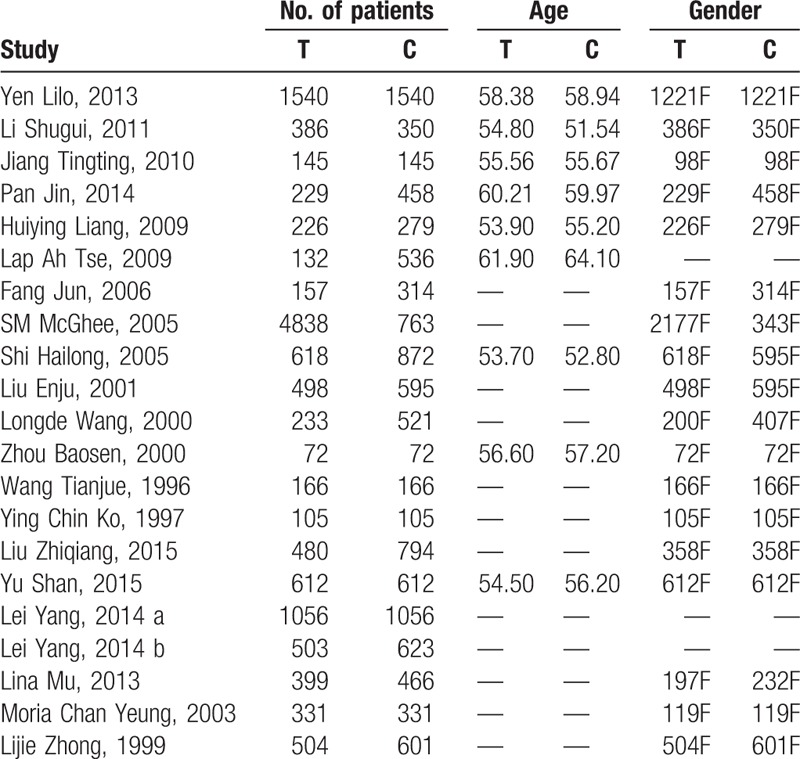
A total of 1261 articles were searched by the indexes, and 1193 articles were excluded by screening the title or abstract, leaving 68 articles for further evaluation. After obtaining and thorough reviewing the complete manuscript, 48 articles did not meet the inclusion criteria: republished (6), cooking fumes or gene analysis (5), no clinical outcomes (17), nonqualified grouping (11), theoretical research (9). At last 20 studies[10–29] were involved in the meta-analysis with 13,004 adults in the case group and 11,199 adults in the control group. The selection process is presented in Figure 1. The main characteristics of the included studies are summarized in Table 1. The basic information includes age and gender. Out of this, 11 studies investigated only females, 2 studies are unclear, and 7 studies investigated both males and females.
Figure 1.
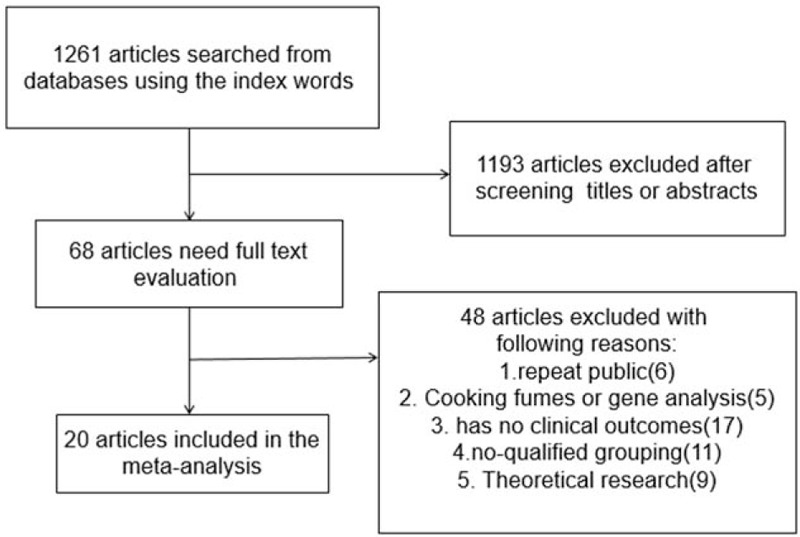
Flow diagram of the literature search and selection process.
Table 1.
The basic characteristics description of included studies.
3.2. EVT exposure and risk of lung cancer incidence
Nine studies with 13,520 adults (case group, 8156; control group, 5364) reported the association between EVT exposure and risk of LC incidence. Based on the Chi-squared test P value (P = .003) and I2 tests value (I2 = 66.3%), we chose random-effects model to analyze the association. The pooled results showed the risk of LC incidence was significantly higher (OR: 1.64, 95% CI: 1.34–2.01, Fig. 2) in exposure population than in nonexposure population.
Figure 2.
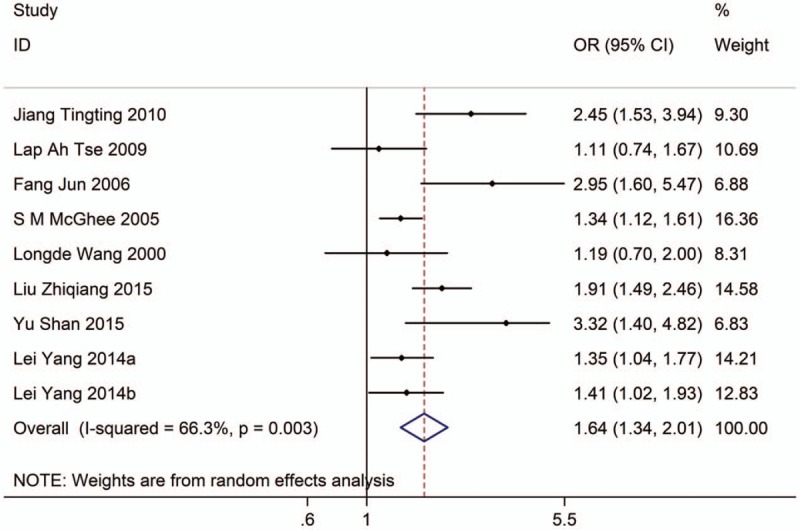
Forest plot showing the association between EVT exposure and lung cancer incidence.
In the subgroups analysis, we have investigated the association in subgroups of male and female.
Seven studies with 12,526 adults (case group, 7966; control group, 4560) reported the association between EVT exposure and risk of LC incidence in male population. Based on the Chi-squared test P value (P = .018) and I2 tests value (I2 = 60.6%), we chose random-effects model to analyze the association. The pooled results showed the risk of LC incidence was significantly higher (OR: 1.62, 95% CI: 1.16–2.28, Fig. 3) in exposure male population than in nonexposure male population.
Figure 3.
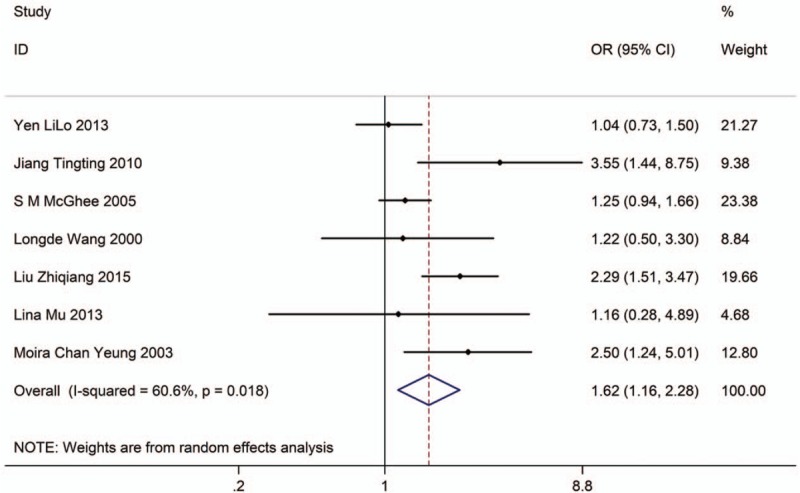
Forest plot showing the association between EVT exposure and lung cancer incidence of male.
Eleven studies with 15,718 adults (case group, 9199; control group, 6519) reported the association between EVT exposure and risk of LC incidence in female population. Based on the Chi-squared test P value (P = .364) and I2 tests value (I2 = 8.4%), we chose fixed-effects model to analyze the association. The pooled results showed the risk of LC incidence was significantly higher (OR: 1.57, 95% CI: 1.43–1.72, Fig. 4) in exposure female population than in nonexposure female population.
Figure 4.
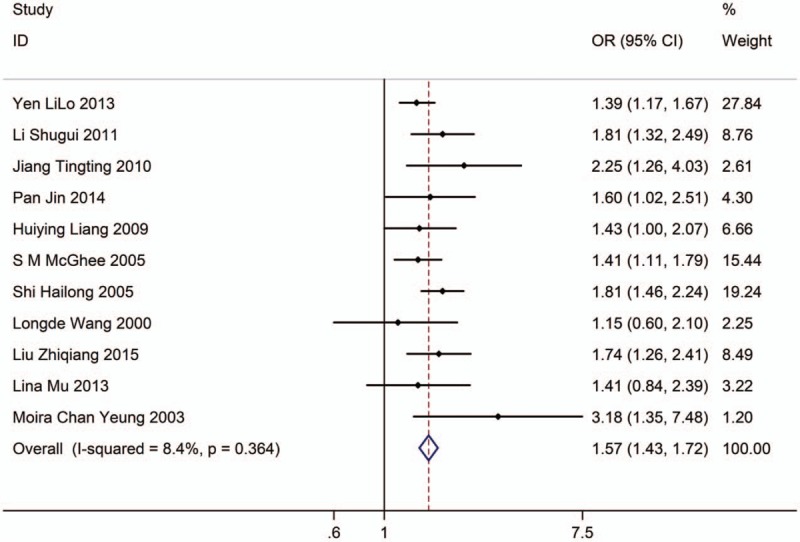
Forest plot showing the association between EVT exposure and lung cancer incidence of female.
3.3. EVT exposure at workplace and risk of lung cancer incidence
Three studies with 2024 adults (case group = 637, control group = 1387) reported the association between EVT exposure at workplace and risk of LC incidence. Based on the Chi-squared test P value (P = .134) and I2 tests value (I2 = 50.2%), we chose random-effects model to analyze the association. The pooled results showed the risk of LC incidence was significantly higher (OR: 1.78, 95% CI: 1.29–2.44, Fig. 5) in exposure population than in nonexposure population.
Figure 5.
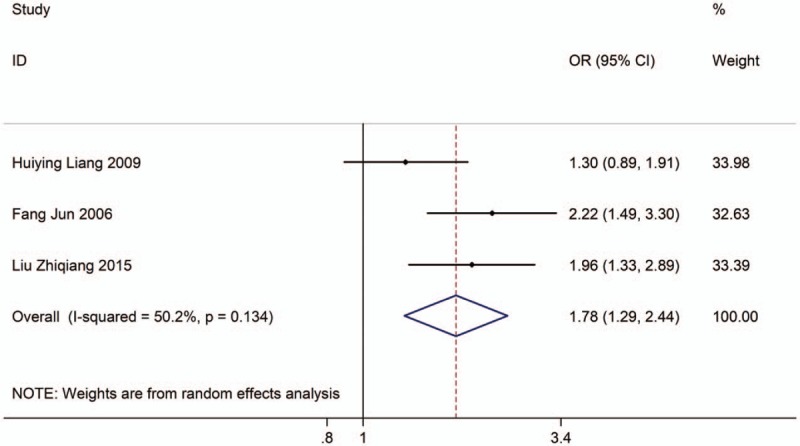
Forest plot showing the association between EVT exposure at workplace and lung cancer incidence.
In the subgroups analysis, we have investigated the association in subgroups of male and female.
Three studies with 5219 adults (case group, 2419; control group, 2800) reported the association between EVT exposure at workplace and risk of LC incidence in male population. Based on the Chi-squared test P value (P = .014) and I2 tests value (I2 = 76.5%), we chose random-effects model to analyze the association. The pooled results showed the risk of LC incidence has no significant difference (OR: 1.51, 95% CI: 0.74–3.06, Fig. 6) between exposure male population and nonexposure male population.
Figure 6.
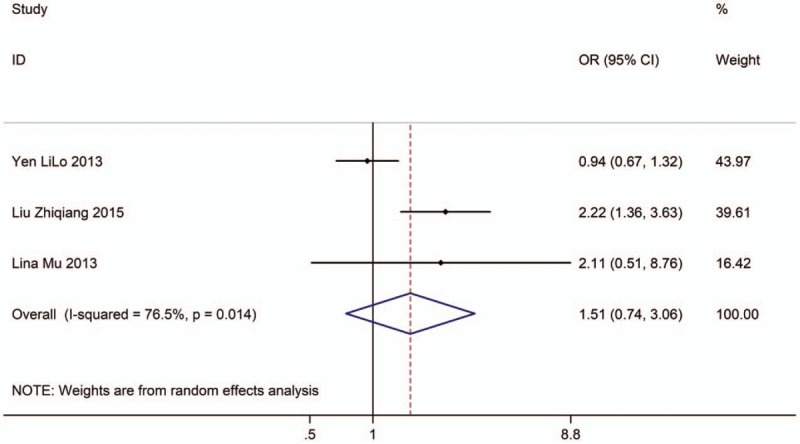
Forest plot showing the association between EVT exposure at workplace and lung cancer incidence of male.
Ten studies with 10,329 adults (case group, 4768; control group, 5561) reported the association between EVT exposure and risk of LC incidence in female population. Based on the Chi-squared test P value (P = .009) and I2 tests value (I2 = 58.8%), we chose random-effects model to analysis the association. The pooled results showed the risk of LC incidence has no significant difference (OR: 1.23, 95% CI: 0.99–1.53, Fig. 7) between exposure female population and nonexposure female population.
Figure 7.
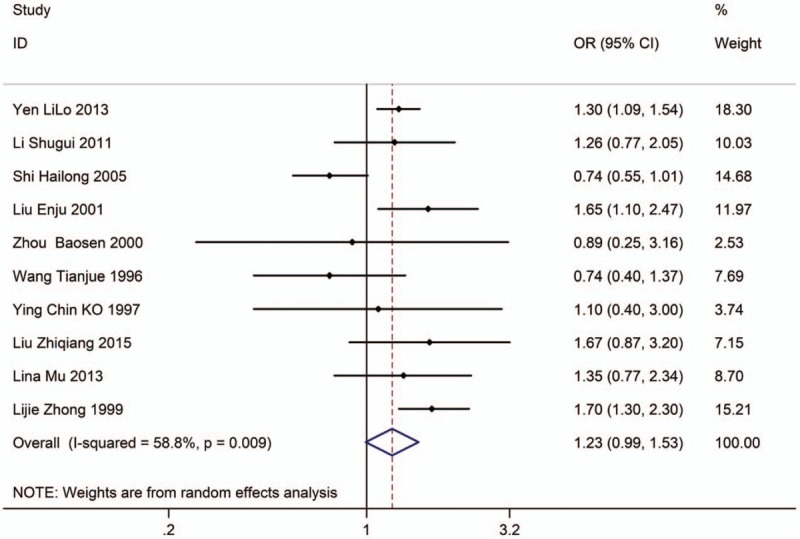
Forest plot showing the association between EVT exposure at workplace and lung cancer incidence of female.
3.4. EVT exposure at home and risk of lung cancer incidence
Three studies with 2413 adults (case group, 769; control group, 1644) reported the association between EVT exposure at home and risk of LC incidence. Based on the Chi-squared test P value (P = .021) and I2 tests value (I2 = 74.0%), we chose random-effects model to analyze the association. The pooled results showed the risk of LC incidence was significantly higher (OR: 1.53, 95% CI: 1.01–2.33, Fig. 8) in exposure population than in nonexposure population.
Figure 8.
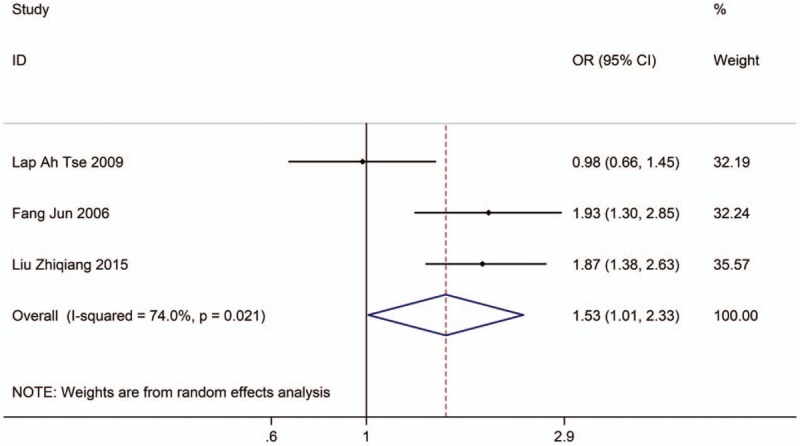
Forest plot showing the association between EVT exposure at home and lung cancer incidence.
In the subgroups analysis, we have investigated the association in subgroups of male and female.
Three studies with 5219 adults (case group, 2419; control group, 2800) reported the association between EVT exposure at home and risk of LC incidence in male population. Based on the Chi-squared test P value (P = .132) and I2 tests value (I2 = 50.7%), we chose random-effects model to analyze the association. The pooled results showed the risk of LC incidence has no significant difference (OR: 1.24, 95% CI: 0.68–2.26, Fig. 9) between exposure male population and nonexposure male population.
Figure 9.
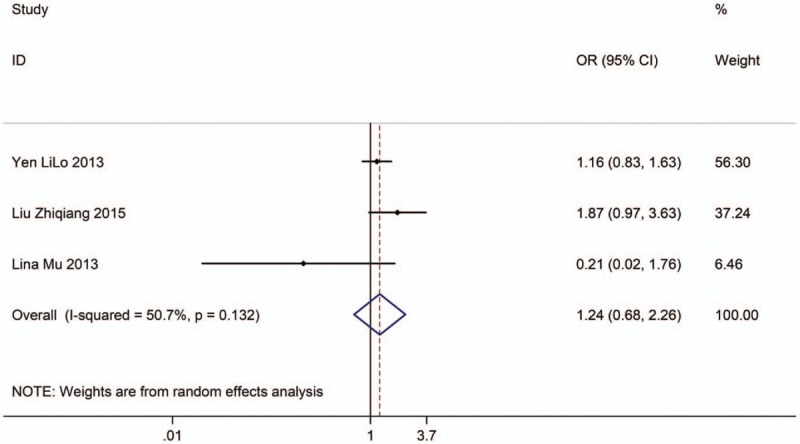
Forest plot showing the association between EVT exposure at home and lung cancer incidence of male.
Six studies with 7304 adults (case group, 3365; control group, 3939) reported the association between EVT exposure at home and risk of LC incidence in female population. Based on the Chi-squared test P value (P = .531) and I2 tests value (I2 = 0.0%), we chose fixed-effects model to analyze the association. The pooled results showed the risk of LC incidence was significantly higher (OR: 1.55, 95% CI: 1.34–1.79, Fig. 10) in exposure female population than in nonexposure female population.
Figure 10.
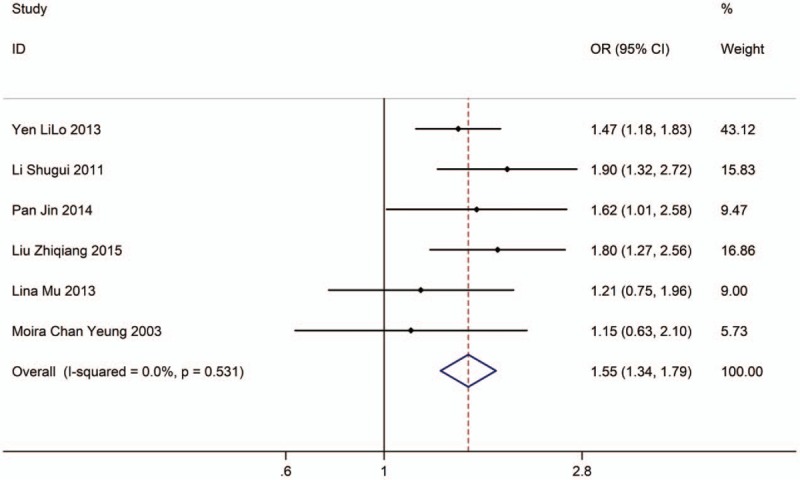
Forest plot showing the association between EVT exposure at home and lung cancer incidence of female.
4. Discussion
Several similar meta-analyses explored the relationship between EVT exposure and LC. Taylor et al[30] found that passive smoking by a spouse increased the risk of LC in nonsmoking women (OR: 1.17, 95% CI: 1.17–1.37). Zou et al[31] suggested that the relative risk of LC was associated with smoking in husband who had been exposed to ETS, and the relative risk of LC exposure to ETS was 1.16 (95% CI: 1.05–1.28). The relative risk for male nonsmokers was 1.48 (95% CI: 1.13–1.92). Jerrett et al[32] reported that the main risk factors for LC in nonsmoking women include lung disease, family history of cancer, and passive smoking. The LC relative risk for adult workplace ETS exposure was 1.47 (95% CI: 1.28–1.69). The LC relative risk of household ETS exposure in adulthood was 1.22 (95% CI: 1.09–1.36), and the LC relative risk of ETS exposure in all life stages was 1.52 (95% CI: 1.29–1.79). Zhao et al[33] found that there was statistical significance between passive smoking and LC (OR: 1.13, 95% CI: 1.05–1.21) and the relative risk of LC for ETS exposure in female was 1.50 (95% CI: 1.19–1.90), at workplace was 1.41(95% CI: 1.19–1.66). The comprehensive results of Fu et al[34] showed that ETS exposure may increase the risk of LC with combined OR (95% CI) 1.52 (1.42–1.64); ETS exposure was found to be significantly associated with increased LC risk in nonsmoking men and women, with combined OR (95% CI) 1.58 (1.42–1.75) and 1.34 (1.08–1.65), respectively; Exposure of ETS from home or work environment may increase LC risk, with combined OR (95% CI) 1.48 (1.20–1.82) and 1.38 (1.13–1.69), respectively.
The tobacco smoke includes more than 7000 kinds of chemical substance. There have been several hundred poisonous chemical, 69 kinds of which are known as carcinogen. The carcinogen would damage DNA and lead to the incidence of LC; the basic principle of ETS exposure leading to LC is similar to smoking. ETS or passive smoking is defined as nonsmoker exposed in smoke that comes from the smokers exhale. The more smokers in environment.
However, there are some limitations which should be paid attention to in this analysis. The limitations are as follows: only case-control studies were included; differences in the inclusion criteria and exclusion criteria for objects; LC patients with previous disease and treatments were unavailable; some of the included studies were old; all the included studies were from Chinese or English articles and this may be the source of bias; pooled date were used for analysis, and individual patients’ data were unavailable, so it limited more comprehensive analysis.
Author contributions
Writing – original draft: Lin Sheng, Jun-Wei Tu, Jiang-Hua Tian, Hui-Jun Chen, Chu-Li Pan, Ren-Zhi Zhou.
Writing – review & editing: Lin Sheng, Jun-Wei Tu, Jiang-Hua Tian, Hui-Jun Chen, Chu-Li Pan, Ren-Zhi Zhou.
Footnotes
Abbreviations: EVT = environmental tobacco smoke, LC = lung cancer, MD = mean difference, OR = odd radio, RR = relative risk.
All authors contributed equally to the article.
The author(s) have no conflicts of interest to disclose.
References
- [1].International Agency for Research on Cancer. GLOBOCAN2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. Available at: URL:http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. [Google Scholar]
- [2].Liandi L, et al. Analysis the variation tendency of malignant tumor mortality and predict recently. Chin J Oncol 1997;19:3–9. [Google Scholar]
- [3].Jie H, et al. Annual Tumor Registry Review of China in 2012. Beijing: Press of Military Medical Sciences; 2012. [Google Scholar]
- [4].IARC. Lung Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012 [EB/OL]. Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.2012. [Google Scholar]
- [5].Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen WQ, Zheng RS, Zhang SW, et al. Report of cancer incidence and mortality in China, 2013. Zhongguo Zhong Liu 2017;26:1–7. [DOI] [PubMed] [Google Scholar]
- [7].Chinese Center for Disease Control and Prevention. Global adult tobacco survey China 2010 country report. 1st ed. Beijing, China: Three Gorges Press; 2011:8–18. [Google Scholar]
- [8].Brownson RC, Alavanja MCR, Caporaso N, et al. Epidemiology and prevention of lung cancer in nonsmokers. Epidemiol Rev 1998;20:218–36. [DOI] [PubMed] [Google Scholar]
- [9].Peto R, Darby S, Deo H, et al. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 2000;321:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lo YL, Darby S, Deo H, et al. Risk factors for primary lung cancer among never smokers by gender in a matched case-control study. Cancer Causes Control 2013;24:567–76. [DOI] [PubMed] [Google Scholar]
- [11].Li S, et al. Analysis the risk factor of lung cancer in female. Chin J Lab Diagn 2011;10:1767–9. [Google Scholar]
- [12].Jiang T, Song H, Peng X, et al. A case-control study on non-smoking primary lung cancers in Sichuan, China. Zhongguo Fei Ai Za Zhi 2010;13:511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pan J, et al. A case-control study on risk factors of lung cancer among non-smoking women. Zhejiang Pre Med 2014;26:772–82. [Google Scholar]
- [14].Liang H, Guan P, Yin Z, et al. Risk of lung cancer following nonmalignant respiratory conditions among nonsmoking women living in Shenyang, Northeast China. J Women's Health 2009;18:1989–95. [DOI] [PubMed] [Google Scholar]
- [15].Tse LA, Yu IT, Au JS, et al. Environmental tobacco smoke and lung cancer among Chinese nonsmoking males: might adenocarcinoma be the culprit? Am J Epidemiol 2009;169:533–41. [DOI] [PubMed] [Google Scholar]
- [16].Fang J, Gan DK, Zheng SH, et al. A case-control study of the risk factors for lung cancer among Chinese women who have never smokes. Wei Shang Yan Jiu 2006;35:464–7. [PubMed] [Google Scholar]
- [17].McGhee SM, Ho SY, Schooling M, et al. Mortality associated with passive smoking in Hong Kong. BMJ 2005;330:287–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hailong S, et al. Study on risk factors of lung cancer in non-smoking women. Chin J Lung Cancer 2005;8:279–82. [DOI] [PubMed] [Google Scholar]
- [19].Enju L, et al. Risk factors for lung cancer among nonsmoking females in urban Shanghai: a population-based case-control study. Tumor 2001;21:421–5. [Google Scholar]
- [20].Wang L, Lubin JH, Zhang SR, et al. Lung cancer and environmental tobacco smoking in a non-industrial area of China. Int J Cancer 2000;88:139–45. [DOI] [PubMed] [Google Scholar]
- [21].Baosen Z, et al. The risk factors of female lung adenocarcinoma. China Public Heath 2000;16:536–9. [Google Scholar]
- [22].Wang T, et al. A case-control study of risk factor for nonsmoker lung cancer. Chin Academic J 1996;15:257–9. [Google Scholar]
- [23].Ko YC, Lee CH, Chen MJ, et al. Risk factors for primary lung cancer among non-smoking women in Taiwan. Int J Epidemiol 1997;26:24–31. [DOI] [PubMed] [Google Scholar]
- [24].Yang L, Lu X, Deng J, et al. Risk factors shared by COPD and lung cancer and mediation effect of COPD: two center case-control studies. Cancer Causes Control 2014;26:11–24. [DOI] [PubMed] [Google Scholar]
- [25].Mu L, Liu L, Niu R, et al. Indoor air pollution and risk of lung cancer among Chinese female non-smoker. Cancer Causes Control 2013;24:439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu Z, et al. A case-control study on smoking, passive smoking and the risk of lung cancer. Chin J of Disease Control 2015;19:145–9. [Google Scholar]
- [27].Yu B, et al. A case-control study of 612 nonsmoking lung cancer in Shenyang. Chin Practical J of Rural Doctor 2015;22:38–41. [Google Scholar]
- [28].Chan Yeung M, Koo LC, Ho JC, et al. Risk factors associated with lung cancer in Hong Kong. Lung Cancer 2003;40:131–40. [DOI] [PubMed] [Google Scholar]
- [29].Zhong L, Goldberg MS, Gao YT, et al. A case-control study of lung cancer and environmental tobacco smoke among nonsmoking women living in Shanghai, China. Cancer Cause and Control 1999;10:607–16. [DOI] [PubMed] [Google Scholar]
- [30].Taylor R, Nafaji F, Dobson A, et al. Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int J Epidemiol 2007;36:1048–59. [DOI] [PubMed] [Google Scholar]
- [31].Zou L, et al. A meta-analysis of passive smoking and lung cancer among non-smoking women. Occupation and health 2005;21:168–71. [Google Scholar]
- [32].Jerrett M, Burnett RT, Ma R, et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology 2005;16:727–36. [DOI] [PubMed] [Google Scholar]
- [33].Zhao H, Gu J, Xu H, et al. Meta-analysis of the relationship between passive smoking population in China and lung cancer. Zhongguo Fei Ai Za Zhi 2010;13:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fu X, Feng T, Wu M, et al. Relationship between environment tobacco and lung cancer risk among nonsmokers in China: a meta-analysis. Zhonghua Yu Fang Yi Xue Za Zhi 2015;49:644–8. [PubMed] [Google Scholar]


