Abstract
Background:
Lung cancer is the leading cause of cancer mortality worldwide. It is often diagnosed at an advanced stage when treatment is no longer possible. Early population-based screening may provide an opportunity for early diagnosis and reduce mortality rates.
Methods:
Study characteristics were collected and outcome data (lung cancer diagnosis and mortality) were extracted and used for meta-analysis. Statistical analyses were performed using OpenMetaAnalyst-0.1503 software. The odds ratio (OR) and 95% confidence interval (CI) were used to assess LDCT compared to other screening methods under the random-effects model. The I2 statistic was used to assess heterogeneity.
Results:
Pooling data from 4 studies (64,129 patients) showed a higher incidence of diagnosed lung cancer with LDCT screening (OR = 1.86, 95% CI: 1.02–3.37), compared to other screening tools. However, no significant difference (OR = 1.13, 95% CI: 0.78–1.64) was found in lung cancer mortality between both groups.
Conclusions:
Although no significant difference was found between LDCT and other control groups in terms of lung cancer mortality, this meta-analysis suggests an increased diagnosis of lung cancer with LDCT as compared with other screening modalities. This meta-analysis displays the potential but also the limitations of LDCT for early lung cancer detection.
Keywords: low-dose computed tomography, lung cancer, meta-analysis, screening
1. Introduction
Lung cancer accounts for 26.8% of all cancer deaths making it the leading cause of cancer mortality worldwide.[1] Due to the asymptomatic nature of lung neoplasms, they are often diagnosed at an advanced stage when cure with the currently available therapies is unlikely. The survival rate from lung cancer has only slightly increased over the last 40 years, yet patients with early stage, localized nodules have a much higher 5-year survival rate as compared to those with later stage regional and metastasized cases (54.8% vs 27.4% vs 4.2%, respectively).[1] Therefore, early, effective population-based screening is a public health priority for increasing survival rates.
Conventional screening for lung neoplasms includes chest X-ray (CXR), sputum cytology, or no particular intervention or screening modality (standard care). Previous trials have shown that conventional screening methods are ineffective in detecting early lung cancer.[2–6] Low-dose computed tomography (LDCT) has emerged as a promising mass screening method for the early diagnosis of lung neoplasms. Several observational and randomized control trials (RCTs) since the 1990s have confirmed a high sensitivity for LDCT in early stage lung neoplasm detection.[7–13] Research suggests that LDCT results in overdiagnosis of lung cancer (a high false-positive rate),[14,15] resulting in excess follow-up testing, invasive procedures, and patient anxiety.[16–18]
Meta-analysis of clinical data is an increasingly common tool with the primary goal of obtaining a precise estimate of the overall effect of an intervention/screening technology. Early systematic reviews of LDCT[19–21] included single-arm prospective cohort studies and did not present the baseline findings of newer RCTs. Recent reviews[22–24] did not include meta-analysis of pooled data. The only found meta-analysis comparing LDCT to other screenings[25] reviewed the baseline data of 5 trials published at that time. In this article, we update the known meta-analysis with new published data from RCTs. Combining data from multiple studies allows us to compare its efficacy to conventional screening methods. Moreover, pooling the mortality data gives us a more precise estimate of LDCT screening efficacy in reducing death from lung cancer among high-risk tobacco-exposed subjects.
2. Methods
This meta-analysis was reported according to the PRISMA guidelines. The ethnic review was approved by the Affiliated Hospital of Bengbu Medical College.
2.1. Search strategy
We performed a literature search of Medline database (via PubMed) in November 31, 2017. The following search terms were used: “lung neoplasm” AND “mass screening” OR “early diagnosis” OR “computed tomography” OR “X-ray.” We also hand-searched the reference lists from the eligible studies for additional relevant records. Duplicates were manually removed. The remaining studies underwent title and abstract screening and were classified as included, more information needed, or excluded based on our predefined exclusion criteria. Studies classified as included or more information required were subjected to full-text screening. Screening was performed independently by 2 authors (HL and YS). Differences in classification between reviewers were resolved by consensus.
2.2. Selection criteria
Studies were included if they: were RCTs providing lung cancer diagnosis and mortality rates, compared LDCT to any other type of screening, reported the outcomes for both intervention and control arms. All age groups were eligible for inclusion. In trials with multiple endpoints, mortality outcomes after the longest follow-up period were used. The following studies were excluded: conference abstracts/summaries, case reports/series, reviews, and commentaries/editorials. Non-English articles were also excluded.
2.3. Data extraction
Two authors (HL and YS) independently completed data extraction using the criteria above. Disagreements were resolved by consensus. The following data were extracted from each study: author, publication year, country, study size and duration, patient characteristics, screening modality, follow-up duration, and outcomes.
2.4. Outcomes
The assessed outcomes included lung cancer diagnosis and mortality rates. These outcomes were used to assess the benefits of LDCT compared to other screening methods. Validity and risk of bias assessments within the study and across studies were performed separately by 2 reviewers (HL and YS), with conflicts resolved by consensus. The assessment evaluated generalizability, sample size, and follow-up duration.
2.5. Statistical analysis
Statistical analyses were performed using OpenMetaAnalyst—0.1503 software (open-source), funded by Agency of Health care Research and Quality (AHRQ). A P-value of less than .05 was deemed statistically significant. Meta-analysis was performed to compare the outcomes of patients screened with LDCT versus other screening methods (CXR, sputum cytology, usual care with no screening). Results were reported as odds ratio (OR) and associated 95% confidence intervals (CIs). Heterogeneity was measured using the Q test and the I2 statistic (with values of <25%, 25–50%, and 50–75% representing low, medium, and high heterogeneity). The DerSimonian–Laird random-effects model was used if there was high heterogeneity between studies.
3. Results
3.1. Literature search results
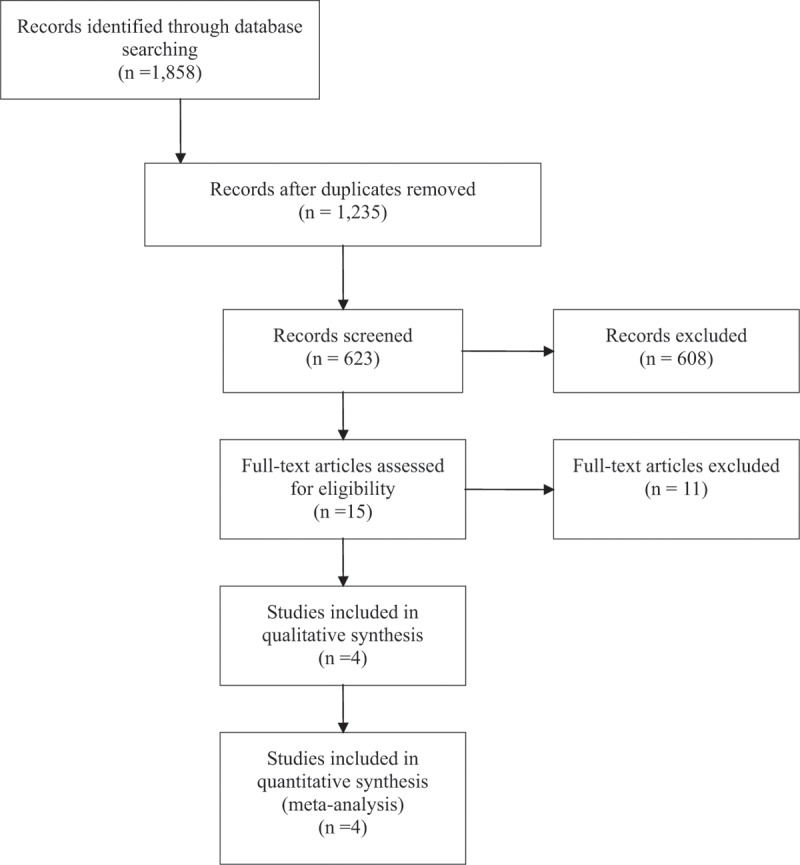
The initial literature search retrieved 1858 records. After elimination of nonrelevant records, 15 full-text articles were reviewed. After application of all inclusion and exclusion criteria, 4 studies were finally included in this meta-analysis including 64,129 participants in total (Table 1).
Table 1.
PRISMA: LDCT versus other screening methods: meta-analysis flow diagram.
3.2. Characteristics of included studies
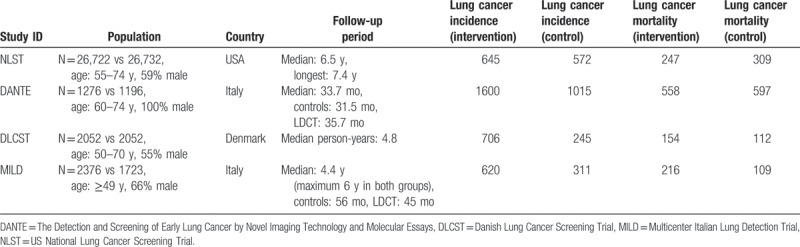
The U.S. National Lung Cancer Screening Trial (NLST)[15,26] randomized 53,454 male and female participants to compare three annual LDCT screenings to three annual CXRs. It was conducted in 33 urban, academic tertiary care centers. Participants were former (less than 15 years since quitting) or current smokers with more than 30 pack-years and were followed for a median of 6.5 years. Overall, the NLST cohort was younger, better educated and more frequently former smokers than the general eligible US population, introducing the risk of the healthy-volunteer effect.
The Detection and Screening of Early Lung Cancer by Novel Imaging Technology and Molecular Essays (DANTE) trial[27,28] randomized 2472 male smokers (with a minimum of 20 pack-year smoking history) to receive 5 years of annual LDCT screening or usual care. All participants received a baseline physical examination and clinical interview, as well as a CXR and sputum cytology. This Italian trial was conducted in 2 community hospitals with an adherence rate of 95% over 5 rounds of screening. Generizability was inhibited by inclusion of only male subjects and the relatively small ample size.
The Danish Lung Cancer Screening Trial (DLCST)[29,30] randomized 4104 men and woman who were current or former smokers (quit after the age of 50 years and within 10 years before trial enrollment) with ≥20 pack-years and were able to walk up 36 stairs without stopping. The DLCST was a single center trial comparing LDCT to no lung cancer screening over 5 years. All participants had initial and annual follow-up pulmonary function tests and completed health questionnaires. However, this was a smaller trial with limited statistical power.
The Multicentric Italian Lung Detection trial (MILD)[31] randomized 4099 men and women to compare annual (n = 1190) or biennial (1186) LDCT to no lung cancer screening (n = 1723). This single-center trial enrolled participants who were current or former (quit within 10 years of study enrollment) smokers. This study had a short median follow-up time of 4.4 years (at time of last published data) and more importantly differing follow-up periods among study arms (Table 2).
Table 2.
Characteristics of included studies.
3.3. Meta-analysis outcomes
The random-effects model was the best fit for this analysis because the I2 statistic was greater than 75%. The frequentist pairwise random effects meta-analysis on lung cancer incidence showed an OR of 1.86 (95% CI = 1.02–3.37), indicating people who underwent LDCT screening had a higher incidence of diagnosed lung cancer. The baseline 95% CIs demonstrated statistically significant differences between the screening modalities. As the MILD trial had different follow-up periods among the LDCT and control groups, we also performed the analysis excluding the MILD trial. The results of the pooled data, excluding the MILD trial, displayed an OR of 1.95 (95% CI = 0.78–4.90). This indicates no significant difference between the screening modalities due to the larger CI including the null hypothesis value (Figs. 1 and 2).
Figure 1.
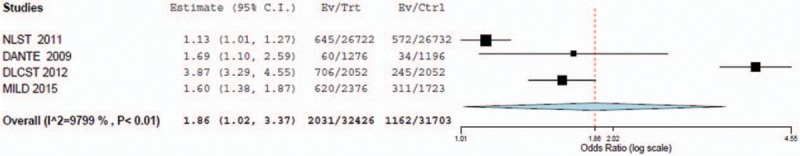
Forest plot for detection of lung cancer incidence with LDCT versus control (with MILD included).
Figure 2.
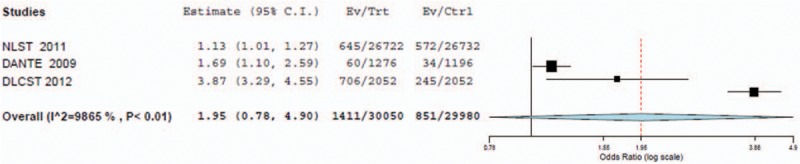
Forest plot for detection of lung cancer incidence with LDCT versus control (without MILD included).
The random effects meta-analysis on lung cancer mortality showed an OR of 1.13 (95% CI = 0.78–1.64). The OR after the exclusion of the MILD trial was OR = 1.02 (95% CI = 0.66–1.58). In both cases, the CIs render the ORs insignificant and demonstrate mortality due to lung cancer is approximately the same in both LDCT and control groups (Figs. 3 and 4).
Figure 3.
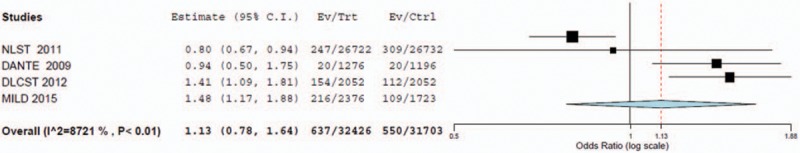
Forest plot for detection of lung cancer mortality with LDCT versus control (with MILD included).
Figure 4.

Forest plot for detection of lung cancer mortality with LDCT versus control (without MILD included).
4. Discussion
The increase in lung cancer detection among LDCT study arms as compared to other screening methods indicates LDCT may be an effective mass screening tool for high risk individuals. This may be especially important when considering diagnosed stage I cancers have a higher survival rate than later lung cancers stages. A decease in lung cancer mortality with LDCT screening continues to be unfounded in the literature. This may indicate that while the LDCT screening is more effective than other screening modalities for early lung cancer diagnosis, it does not translate into a meaningful reduction in mortality at the population level. This may be partially attributed to biases, common to screening programs, such as lead time, length time, and overdiagnosis bias. Moreover, the 2 smaller trials (DLCST and NLST) may have canceled out the positive screening effects of the NLST study. These smaller studies included a younger population with less pack-years than the higher-risk NLST participants.
While the NLST study may be more applicable to the US medical context and was the only study large enough to detect a difference among study arms, the efficacy of a population-based screening program will depend greatly on implementation factors. The NLST was conducted among a population with a higher risk than the European trials and the general eligible US population. Moreover, it was largely conducted at academic centers with specialty cancer centers and therefore, calls into question the generalizability to a greater population in the community care setting. The NLST was a head-to-head trial comparing LDCT against CXR where the European trials include a placebo-like group (community care) perhaps biasing the overdiagnosis rate in the NLST study leading to increased cost, risk and anxiety. Population-based screening among patients who would have been eligible, according to NLST inclusion criteria, is unlikely to be cost-effective with such a small decrease in mortality compared to the standard care. The inclusion criteria for a mass-screening program will have to be determined and if restricted to those only at the highest risk, may be perceived as inequitable among health consumers at risk but ineligible due to the current risk standards.
4.1. Limitations
This meta-analysis included a small number of studies and the trials included for analysis were under-powered at an individual level. The high level of heterogeneity between studies is a limitation to the ability to extrapolate results from this meta-analysis to the general population. Further, the trials varied in their enrollment criteria especially in the age of participants and level of tobacco exposure. They also varied in size; the largest study randomized more than 50,000 participants while the other three combined randomized fewer than 10,000. Also the trials differed in their follow-up periods even among study arms as in the MILD trial. In addition, the smaller trials demonstrated heterogeneous study methods, selection criteria, and screening modalities. The study outcomes may also vary due to location, participant demographics, and differences in healthcare systems between the international locations of the trials. At the review-level, there is the risk of incomplete retrieval of unidentified research or reporting bias.
4.2. Implications of key findings
Due to the lack of a significant difference in mortality after LDCT screening in individuals at high risk for lung cancer, patients and clinicians must weigh the individual benefits versus the overall risks of LDCT testing including radiation exposure, high false-positive rates, and costs of follow-up testing and treatments.[32] Policy makers will need further research, as well as developments in the computed tomography technology[33–35] to develop recommendations on the implementation of mass screening by LDCT both in higher-risk and lower-risk individuals.
Author contributions
Data curation: Hongli Liu.
Formal analysis: Hongli Liu.
Methodology: Yuanbing Shen.
Project administration: Wei Li, Yuqing Chen.
Resources: Yuanbing Shen.
Software: Xiaojing Wang, Yuanbing Shen.
Supervision: Wei Li, Yuqing Chen.
Validation: Wei Li, Yuqing Chen.
Writing – original draft: Xiaojing Wang.
Writing – review & editing: Hongtao Wang, Yuqing Chen.
Footnotes
Abbreviations: CI = confidence interval, CXR = chest X-ray, DANTE = The Detection and Screening of Early Lung Cancer by Novel Imaging Technology and Molecular Essays, DLCST = Danish Lung Cancer Screening Trial, LDCT = low-dose computed tomography, MILD = Multicenter Italian Lung Detection Trial, NLST = US National Lung Cancer Screening Trial, OR = odds ratio, RCT = randomized controlled trial.
Funding: This work was supported by Key Program of Natural Science Research of Higher Education of Anhui Province (Grant KJ2017A241, KJ2016A472), Key program for excellent young talents in college and university of Anhui province (Grant gxyqZD2016168), Science and Technology Program of Anhui province (Key Laboratories project: 2017070503B037, 2016080503B035), the National Natural Science Foundation of China (grant no. 81772493).
The authors have no conflicts of interest to disclose.
References
- [1].Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review (CSR) 1975-2012 [Internet]. 2014; National Cancer Institute, Bethesda, MD: [cited 2017 Dec 19, HL and YS]. Available from: http://seer.cancer.gov/csr/1975_2012/.
- [2].Berlin NI, Buncher CR, Fontana RS, et al. The National Cancer Institute Cooperative Early Lung Cancer Detection Program: results of the initial screen (prevalence). Am Rev Respir Dis 1984;130:545–9. [DOI] [PubMed] [Google Scholar]
- [3].Frost JK, Ball WC, Levin ML, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis 1984;130:549–54. [DOI] [PubMed] [Google Scholar]
- [4].Kubik A, Parkin DM, Khlat M, et al. Lack of benefit from semi-annual screening for cancer of the lung: follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. Int J Cancer 1990;45:26–33. [DOI] [PubMed] [Google Scholar]
- [5].Melamed MR. Lung cancer screening results in the National Cancer Institute New York study. Cancer 2000;89(11 suppl):2356–62. [DOI] [PubMed] [Google Scholar]
- [6].Marcus PM, Bergstralh EJ, Zweig MH, et al. Extended lung cancer incidence follow-up in the Mayo Lung Project and overdiagnosis. J Natl Cancer Inst, 2006; 98: 748–756. [DOI] [PubMed] [Google Scholar]
- [7].Sone S, Li F, Yang ZG, et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br J Cancer 2001;84:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tiitola M, Kivisaari L, Huuskonen MS, et al. Computed tomography screening for lung cancer in asbestos-exposed workers. Lung Cancer Amst Neth 2002;35:17–22. [DOI] [PubMed] [Google Scholar]
- [9].Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol 2002;20:911–20. [DOI] [PubMed] [Google Scholar]
- [10].Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165:508–13. [DOI] [PubMed] [Google Scholar]
- [11].Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology 2002;222:773–81. [DOI] [PubMed] [Google Scholar]
- [12].Nawa T, Nakagawa T, Kusano S, et al. Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest 2002;122:15–20. [DOI] [PubMed] [Google Scholar]
- [13].Henschke CI, Yankelevitz DF, et al. International Early Lung Cancer Action Program Investigators. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763–71. [DOI] [PubMed] [Google Scholar]
- [14].Bach PB, Jett JR, Pastorino U, et al. Computed tomography screening and lung cancer outcomes. JAMA 2007;297:953–61. [DOI] [PubMed] [Google Scholar]
- [15].Aberle DR, Adams AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].van den Bergh KAM, Essink-Bot M-L, Bunge EM, et al. Impact of computed tomography screening for lung cancer on participants in a randomized controlled trial (NELSON trial). Cancer 2008;113:396–404. [DOI] [PubMed] [Google Scholar]
- [17].van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J 2011;38:154–61. [DOI] [PubMed] [Google Scholar]
- [18].Gareen IF, Duan F, Greco EM, et al. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer 2014;120:3401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bach PB, Kelley MJ, Tate RC, et al. Screening for lung cancer: a review of the current literature. Chest 2003;123((1 suppl)):72S–82S. [DOI] [PubMed] [Google Scholar]
- [20].Black C, de Verteuil R, Walker S, et al. Population screening for lung cancer using computed tomography, is there evidence of clinical effectiveness? A systematic review of the literature. Thorax 2007;62:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yau G, Lock M, Rodrigues G. Systematic review of baseline low-dose CT lung cancer screening. Lung Cancer 2007;58:161–70. [DOI] [PubMed] [Google Scholar]
- [22].Boiselle PM. Computed tomography screening for lung cancer. JAMA 2013;309:1163–70. [DOI] [PubMed] [Google Scholar]
- [23].Manser R, Lethaby A, Irving LB, et al. Screening for Lung Cancer. In: Cochrane Database of Systematic Reviews [Internet]. John Wiley & Sons, Ltd; 2013 [cited 2017 Dec 19, HL and YS]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001991.pub3/Abstract.
- [24].Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the U.S. Preventive Services Task Force Recommendation. Ann Intern Med 2013;159:411–20. [DOI] [PubMed] [Google Scholar]
- [25].Gopal M, Abdullah SE, Grady JJ, et al. Screening for lung cancer with low-dose computed tomography: a systematic review and meta-analysis of the baseline findings of randomized controlled trials. J Thorac Oncol 2010;5:1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening trial. N Engl J Med 2013;369:920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Infante M, Lutman FR, Cavuto S, et al. Lung cancer screening with spiral CT: baseline results of the randomized DANTE trial. Lung Cancer 2008;59:355–63. [DOI] [PubMed] [Google Scholar]
- [28].Infante M, Cavuto S, Lutman FR, et al. A randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med 2009;180:445–53. [DOI] [PubMed] [Google Scholar]
- [29].Pedersen JH, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial—overall design and results of the prevalence round. J Thorac Oncol 2009;4:608–14. [DOI] [PubMed] [Google Scholar]
- [30].Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67:296–301. [DOI] [PubMed] [Google Scholar]
- [31].Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308–15. [DOI] [PubMed] [Google Scholar]
- [32].Abushouk AI, Taheri MS, Pooransari P, et al. Pregnancy screening before diagnostic radiography in emergency department; an educational review. Emergency 2017;5:e60. [PMC free article] [PubMed] [Google Scholar]
- [33].Guo W, Liu X, Gao Z, et al. Quantification of three-dimensional computed tomography angiography for evaluating coronary luminal stenosis using digital subtraction angiography as the standard of reference. Biomed Eng Online 2015;14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang T, Nakamoto K, Zhang H, et al. Reweighted anisotropic total variation minimization for limited-angle CT reconstruction. IEEE Trans Nucl Sci 2017;64:2742–60. [Google Scholar]
- [35].Zhang H, Gao Z, Xu L, et al. A Meshfree representation for cardiac medical image computing. IEEE J Transl Eng Health Med 2018;6:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]


