Abstract
Although fractures had high mortality and morbidity, many studies proved that fracture risk might be decreased by pharmacological therapy, although a low treatment adherence rate is observed. The aim of this study was to identify factors associated with osteoporosis treatment in postmenopausal women.
A cross-sectional study was carried out from March to August 2013 at the primary care setting. Postmenopausal women were recruited. A standardized questionnaire was applied. Women who were using at least one of the following drugs at the moment of the survey were considered as current treatment: bisphosphonates, raloxifene, estrogen, calcitonin, teriparatide, or strontium ranelate. Women who had used any of the mentioned medications before the study were considered as past treatment.
Of the 1025 women included in the study, 8% were on current treatment, 5.7% had past treatment, and 86.3% had not received treatment. Treated women (either current or past) had a higher rate of osteoarthritis, had more falls, had higher education level, presented a higher rate of private health insurance, and received more information about osteoporosis. They also had more dual-energy x-ray absorptiometry (DXA) scans and were more frequently diagnosed with osteoporosis by these DXA scans. The factors independently associated with treatment in the regression analysis were the DXA scan itself, the diagnosis of osteoporosis by DXA, and information about osteoporosis.
Current and past treatments of osteoporosis were associated with DXA and information. These results suggest that some measures to inform women about osteoporosis and or even the popularization of DXA scans could improve the treatment.
Keywords: bone densitometry, information, osteoporosis, post-menopause, treatment
1. Introduction
Osteoporosis is a systemic disorder characterized by decreased bone mass and microarchitectural deterioration of bone tissue. Its consequences include increased bone fragility and high fracture risk.[1] Osteoporosis incidence increases with aging, affecting about 30% of postmenopausal women.[2,3] Many studies show that fracture risk in osteoporotic patients may be reduced by 70% with bone protective therapy.[4,5] Even with this favorable result, osteoporosis treatment faces a problem concerning low adherence, the same way that many other silent chronic diseases.[6] Studies show that adherence has not grown satisfactorily despite the increase in treatment prescription.[7,8] According to the Global Longitudinal Study of Osteoporosis in Women (GLOW), <40% of women with high risk of fracture use anti-osteoporosis drugs.[9] The top medication use rates described in patients with previous osteoporotic fractures were 75.0% after 1 year and 45.3% after 5 years.[10]
The reasons for these low adherence rates seem to be diverse. The high cost of some drugs used in osteoporosis treatment could, in part, influence it.[11] The drug posology may be another reason for treatment noncompliance.[12] The adherence rate increases by 5-fold when the corresponding medication is used once weekly instead of once daily. Furthermore, it could achieve an 8-fold increase when the drug is taken once monthly.[12] The comorbidities and the polypharmacy also may be involved in low compliance to osteoporosis treatment.[13] Moreover, age could be a major factor. Elderly patients—≥80 years—may present visual, hearing, and/or cognitive impairments that could further explain low treatment compliance.[10] Interestingly, patients with low bone mass in bone densitometry with greater awareness of their fracture risk appears to be more adherent to therapy.[14]
Low adherence to preventive treatment results in a higher risk of fractures, increased costs, and an increment in hospitalization and mortality rates.[15] On the other hand, high adherence rate is associated with decreased fracture risk and lower hospitalization and mortality rates.[5,16]
Hence, understanding the causes of inadequate treatment is essential to create screening and therapy strategies, with the primary purpose of reducing fractures.[4,17] Considering that the role of each cause is not well-established, the objective of the present study was to identify factors associated with osteoporosis treatment in postmenopausal women.
2. Methods
A cross-sectional study was carried out in Santa Maria (parallel 29°, South Brazil), from March 1st to August 31st, 2013. Women aged ≥55 years who had at least 1 appointment at their respective primary care unit in the 24 months before the study were invited to participate.[18] Women who were still menstruating or had communication impairments were excluded.
The data were collected using the GLOW study questionnaire—authorized by The Center for Outcomes Research, University of Massachusetts Medical School.[19] It includes sociodemographic characteristics, lifestyle, previous fractures, family history of fractures, falls, the age of menopause, previous dual-energy x-ray absorptiometry (DXA) scans, medication use, and comorbidities.[19] All variables were self-reported. Bone fractures, excluding head, hands, and feet, that occurred after the age of 45 years were considered fragility fractures. Hip, humerus, wrist, and clinical vertebral fractures were considered major fractures.
The criteria for the use of drugs for primary and secondary prevention of fractures should follow the Brazilian Health Ministry's Protocol and Therapeutic Guidelines on Osteoporosis, published in 2002 and revised on March 26, 2014. These criteria were: primary prevention primary: osteoporosis in postmenopausal women and osteopenia in patients >70 years and ≥2 falls in the last 6 months in postmenopausal women. Secondary prevention: major fractures in postmenopausal women.
Women who were using at least one of the following medications at the moment of the interview were considered as “current treatment”: bisphosphonates, raloxifene, hormone replacement therapy, calcitonin, teriparatide, or strontium ranelate. These are the drugs approved by ANVISA (The Brazilian National Health Surveillance Agency) for primary and secondary fracture prevention. Women who had used the aforementioned medications at any moment before the interview but were no longer on therapy were considered as “past treatment.”
The study was approved by the Municipality of Santa Maria (Ofício 492/2012/SMS/NEPeS) and by the Ethics Committee of the Federal University of Santa Maria (CAAE 11166012.6.0000.5346). All study participants provided signed informed consent.
2.1. Statistical analysis
Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS), IBM Corporation, Armonk, New York, version 18.0. Data were reported as mean (standard deviation) and prevalence rate (%). The chi-squared test and analysis of variance (ANOVA), followed by post hoc analysis (Tukey test), were used for the following variables: current treatment, past treatment, and non-treatment. Results with P-value <.05 were considered statistically significant and included in the regression models. Generalized linear models with Poisson distribution were performed to assess the factors that influenced osteoporosis treatment. In these models, the categories “current treatment” and “past treatment” were unified. As the results of the DXA scan results could have an interaction, i.e., women with positive results should be treated and women with negative results should not be treated, a correction term multiplying the variables “bone densitometry scan” and “diagnosis of osteoporosis” was included in the models. The best model was chosen by Bayesian Information Criterion.
3. Results
Initially, 1301 women were invited to participate. Of those, 239 refused to participate, 1 was not from Santa Maria, 3 were still menstruating, and 1 was younger than 55 years, resulting in a sample of 1057 participating women. Of whom, 1025 completed the study questionnaire and composed the study sample. Overall, 82 (8.0%) women were on current treatment, 59 (5.7%) had past treatment, and 884 (86.3%) had never been treated.
There was no age difference among the 3 groups. Also, there was no difference when only patients aged ≥80 years (n = 70) were considered. Treated women—both current or past treatment—referred the diagnosis of osteoarthritis and diabetes more often when compared with non-treated women. They also presented a higher number of falls in the year prior the study when compared with the group without treatment (Table 1).
Table 1.
Characteristics of studied women, according to treatment.
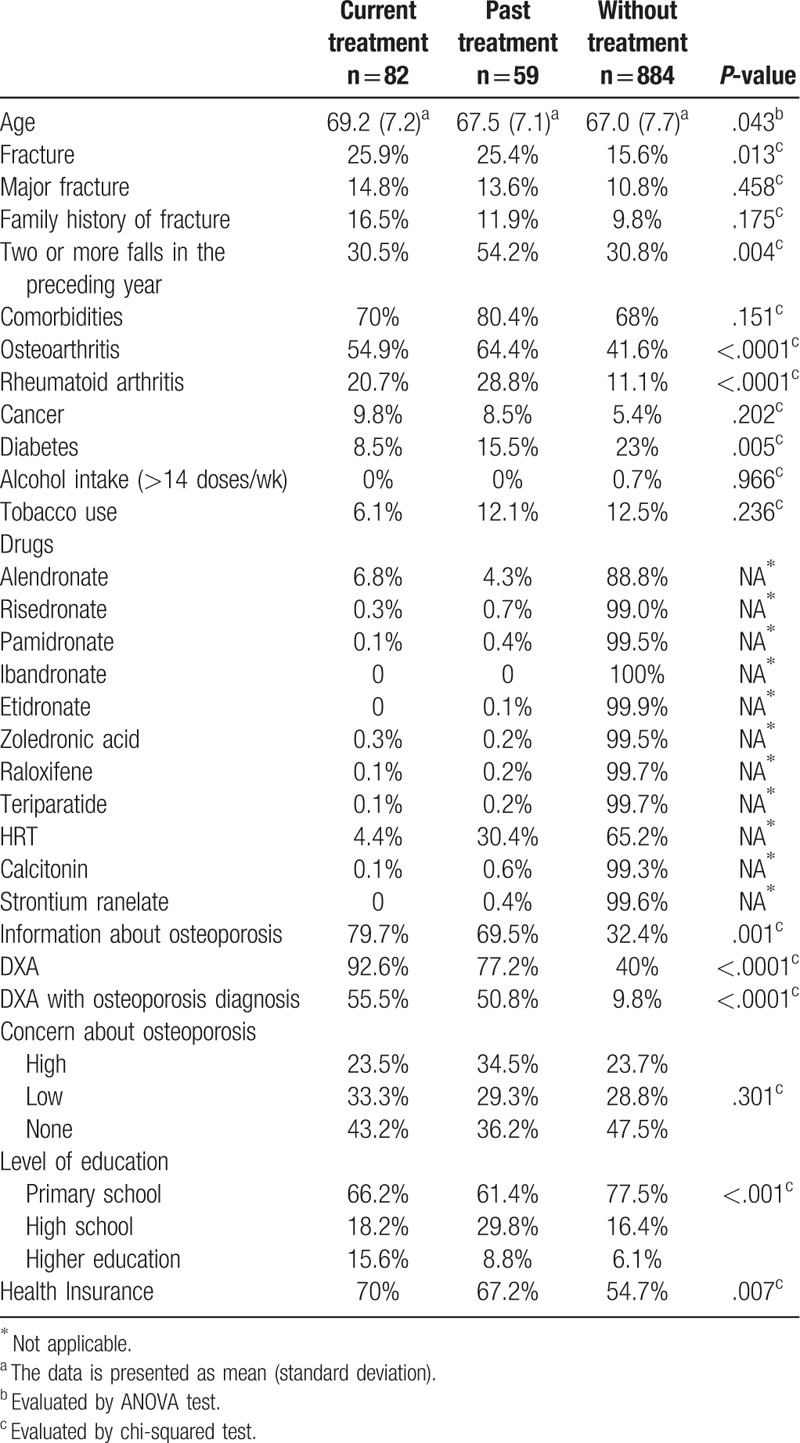
The following factors also were more frequent in women with current and past treatment: the DXA scan execution, osteoporosis diagnosis by DXA, higher level of education, private health insurance, and information about osteoporosis. Treated women did not present a greater degree of concern regarding the disease when compared with women without treatment (Table 1).
Interestingly, the ownership of a private health insurance was associated with a higher number of performed DXA scans: while 57.7% of women with health insurance completed DXA scans, only 30.9% of women without health insurance did so (data not shown). Moreover, from the 465 women who were submitted to a DXA scan, only 60.6% obtained the DXA results, whereas 39.4% did not receive such information.
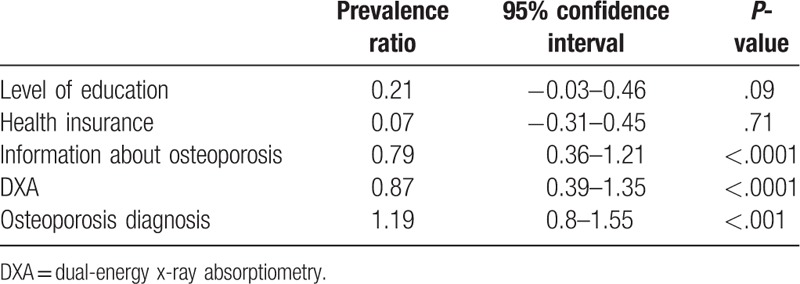
Only DXA scan execution, receiving information about osteoporosis, and having an osteoporosis diagnosis after the DXA execution (Table 2) remained independently associated with treatment in the generalized linear model. These results did not change after the inclusion of the correction term in the model (data not shown).
Table 2.
Factors that influence treatment in post-menopausal women in the Generalized Linear model with Poison distribution.
4. Discussion
Of the possible factors associated with the treatment of osteoporosis included in the study, our results show that 3 of them—DXA scans, osteoporosis diagnosis by DXA, and the fact of receiving information on osteoporosis—have a positive impact on osteoporosis treatment.
Currently, DXA scan is considered by WHO as the gold standard for distinction among normal bone mineral density, osteopenia, and osteoporosis. According to clinical guidelines, such test helps to select patients who need treatment, besides of monitoring bone mass over time.[20] Therefore, it was predictable that DXA would present a strong association with treatment, since its execution improves the awareness of osteoporosis risk, mainly when the disease is diagnosed.[21] Zhang et al[14] also found that patients with low bone mass evidenced by DXA were more adherent to therapy. In the present study, patients who owned private health insurance were submitted to DXA scan in a higher proportion (57.7%) than those who attended the public health care (30.9%) exclusively. However, the fact of possessing health insurance, by itself, did not affect treatment. The same finding was reported by Jacob et al[22] in Germany, who observed that patients with private health insurance were not more persistent to treatment. Likewise, Xu et al[23] found similar rates of treatment discontinuity in the United States between patients who purchased the medications and patients who earned them free of charge (40% after 12 months and 50% after 36 months).
Our study also highlights the power of information about osteoporosis and its consequences on treatment. Of those participants who were submitted to a DXA scan, 60.6% received information on the test results. Nevertheless, there may be communication gaps in medical appointments. Almost 40% of patients leave the office without being properly informed about their test results, even when there is no abnormal finding. The medical appointment is the ideal time to elucidation about the disease and its treatment, impacting on patient adherence. Thus, enhancing the communication process between practitioners and patients is essential. Many studies were carried out aiming at improving patient information about the disease and consequently increasing fidelity to therapy, with conflicting results.[11,24,25] In a study conducted by Nielsen et al[24] in Denmark, patients attended 1 meeting per month for 4 months in which they received information on osteoporosis. After 24 months, therapy adherence rate was significantly higher in the group that attended the meetings (92%) compared with the control group (80%). On the other hand, Bianchi et al[12] assessed the efficacy of 2 different methods of improving patient adherence in a multicenter study in Italy. The control group was composed of patients whose medical prescription was complemented by explanations and recommendations about the disease, with another medical appointment 1 year later. The second group received flyers with information about osteoporosis and the importance of treatment adherence, besides of additional reminders on when to take the medications. The third group received the same material and also calls every 3 months and invitations to regular meetings. No difference was found in the groups regarding adherence to treatment.[12] Alarcon et al[25] assessed the persistence in treatment with vitamin D in patients with hip fracture in Spain. The control group received prescriptions and explanations, whereas the second group received the same recommendations but also a phone call 3 months later. As in Bianchi et al[12,25] study, the reminder call did not significantly improve persistence to treatment in 1 year. In light of our results, these data suggest that the outpatient approach of information may be the determinant condition. An approach with multiple information may not be necessary.
In our sample, only 59 (41.8%) of the 141 women who started therapy were still on treatment. Similarly, studies from many countries systematically described low rates of treatment continuity. In Sweden, Landfeldt et al[8] showed that approximately 50% of patients who received bisphosphonate prescriptions maintained the drug therapy 1 year later. It is well established that the best results obtained in osteoporosis approach depend on an appropriate perseverance on treatment, among other factors. Hence, the investigation of different ways of encouraging patients in the maintenance of a long-term treatment becomes imperative.[4,5]
Many authors also report that older age (≥80 years) has a strong association with low treatment rates. Reynolds et al[26] reported that many patients did not even initiate the prescribed treatment, what was more common among elderly patients. Zarowitz et al[27] studied the prevalence of osteoporosis in institutionalized elderly and observed that 13.5% of them had a documented diagnosis of osteoporosis. Curiously, only one-third of them was receiving pharmacological treatment. The patients presented a mean age of 82.5 years, wherein 85.1% were women. In the sample studied by Zarowitz, two-third of the subjects with osteoporosis diagnosis presented moderate to severe cognitive impairment and had lower chances of receiving treatment.[27] In our study, no association was noticed between age and treatment. Possibly it was due to the small number of women aged ≥80 years among the participants. Another possible reason is the fact that our sample comprised only women attended in primary care units, that is to say, no bed bound or cognitive impaired patients were included.
Regarding the impact of previous fractures on the risk of discontinuing treatment, some authors have already assessed such association. In 2006, Rossini et al[28] carried out a study in Italy that included 9851 postmenopausal women and demonstrated that adherence to treatment was significantly higher in patients with previous vertebral fractures. The authors assumed that previous fractures would improve motivation to use prescribed medications.[28] Another study that comprised 533 women with osteoporosis in England also revealed that individuals with previous fractures had higher persistence rates when compared with patients with no history of fractures.[29] Moreover, another study including 1500 postmenopausal women with osteoporosis in 4 European countries concluded that family history of hip fracture is also associated with an increase in persistence to treatment with denosumab.[30] Nonetheless, in our study, the presence of family or personal history of fractures was not a motivating factor to treatment. Likewise, Zarowitz et al[27] reported that individuals with osteoporosis and history of hip fracture had treatment rates (31.7%) similar to patients with osteoporosis but no hip fracture (32%). Jacob et al[22] also compared women with and without fractures to assess their influence on treatment and found that adherence was not increased by fracture experience. According to these authors, the presence of persistent pain after fracture was the determinant factor in decreasing the risk of treatment discontinuity.
The present study did not show any association between the presence of comorbidities (and the consequent need of polypharmacy) and adherence to osteoporosis treatment in the multivariate analysis. Sitjar Martinez de Sas et al[31] performed a prospective study to assess persistence to treatment with risedronate monthly in patients with osteoporosis. They observed that patients who had a higher number of concomitant medications at the beginning of treatment were significantly more persistent. On the other hand, de Castro Gomes et al[32] carried out a study in Campinas and noticed that more than 60% of postmenopausal women with low bone mass used calcium and vitamin D supplements inappropriately. The only associated factor was concomitant use of other medications, what probably would confuse patients about the right posology of each drug. Perhaps the low level of education of the participants, of whom 40% presented reading difficulties or were illiterate, could also explain the finding. The present study did not identify the impact of level of education on treatment adherence. Women with a low degree of education predominated in all groups, what may have decreased the power of multivariate analysis on this variable.[32]
A major strength of this study is the sample selection, well representing the primary care of Brazilian population, which is multicultural and multiethnic.[33]
The main limitations of our investigation are inherent to the study setting. A cross-sectional study does not follow up patients over time. Also, the information was self-reported, but there is no reason to believe that there was any information bias among the groups. Women without osteoporosis should not be treated, and this fact should have an opposite effect on our results. However, the term to correct the interaction between the DXA results and the diagnosis of osteoporosis did not change our results.
In conclusion, receiving information about osteoporosis and fractures, DXA scan execution and osteoporosis diagnosis by DXA were factors associated with the use of medication to primary and secondary prevention of fractures in postmenopausal women in the primary care. This finding suggests that simple but extremely relevant measures, such as providing appropriate information to patients regarding their health problems during the medical appointment, could improve adherence to treatment. Moreover, DXA popularization could also have a positive impact in this context. Notwithstanding, more studies are necessary to corroborate these hypotheses.
Author contributions
Conceptualization: Lia Rossi, Rafaela Copes, Clovis Flores, Fabio Comim, Melissa Premaor.
Data curation: Fabio Comim, Melissa Premaor.
Formal analysis: Lia Rossi, Rafaela Copes, Melissa Premaor.
Funding acquisition: Melissa Premaor.
Investigation: Lia Rossi, Rafaela Copes, Leo Dal Osto, Fabio Comim, Melissa Premaor.
Methodology: Lia Rossi, Rafaela Copes, Leo Dal Osto, Clovis Flores, Fabio Comim, Melissa Premaor.
Project administration: Rafaela Copes, Melissa Premaor.
Resources: Melissa Premaor.
Supervision: Clovis Flores, Fabio Comim, Melissa Premaor.
Validation: Rafaela Copes, Fabio Comim, Melissa Premaor.
Visualization: Rafaela Copes, Melissa Premaor.
Writing – original draft: Lia Rossi, Leo Dal Osto, Fabio Comim, Melissa Premaor.
Writing – review and editing: Lia Rossi, Rafaela Copes, Leo Dal Osto, Clovis Flores, Fabio Comim, Melissa Premaor.
Footnotes
Abbreviations: ANOVA = analysis of variance, ANVISA = The Brazilian National Health Surveillance Agency, DXA = dual-energy x-ray absorptiometry, GLOW = Global Longitudinal Study of Osteoporosis in Women.
This study has grants from the Federal University of Santa Maria (edital FIPE/CCS 2013) and from the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico – CNPq (472211/2013-7 and 307057/2013-5). The funding sources had no role in the design, conduct, or analysis of our study or in the decision to submit this manuscript for publication.
Ethics approval: Approval for this study was obtained from the Federal University of Santa Maria Ethic Committee [Comitê de Ética em Pesquisa da UFSM (CEP – UFSM); CAAE 04320312.2.0000.5346] and from the Municipality of Santa Maria City [Núcleo de Educação Permanente em Saúde (Ofício 492/2012/SMS/NEPeS) da Secretaria de Saúde da prefeitura de Santa Maria].
Lia Mara Rossi, Rafaela Copes, Leo Dal Osto, Clovis Flores, Fábio Comim, and Melissa Premaor declare that they have no conflict of interest.
References
- [1].Kanis JA, Melton LJ, 3rd, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res 1994;9:1137–41. [DOI] [PubMed] [Google Scholar]
- [2].Dawson-Hughes B, Looker AC, Tosteson AN, et al. The potential impact of the National Osteoporosis Foundation guidance on treatment eligibility in the USA: an update in NHANES 2005–2008. Osteoporos Int 2012;23:811–20. [DOI] [PubMed] [Google Scholar]
- [3].Morrison A, Fan T, Sen SS, et al. Epidemiology of falls and osteoporotic fractures: a systematic review. Clinicoecon Outcomes Res 2013;5:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Eastell R, Walsh JS, Watts NB, et al. Bisphosphonates for postmenopausal osteoporosis. Bone 2011;49:82–8. [DOI] [PubMed] [Google Scholar]
- [5].Lakatos P, Toth E, Szekeres L, et al. Comparative statistical analysis of osteoporosis treatment based on Hungarian claims data and interpretation of the results in respect to cost-effectiveness. Osteoporos Int 2014;25:2077–87. [DOI] [PubMed] [Google Scholar]
- [6].Sheehy O, Kindundu C, Barbeau M, et al. Adherence to weekly oral bisphosphonate therapy: cost of wasted drugs and fractures. Osteoporos Int 2009;20:1583–94. [DOI] [PubMed] [Google Scholar]
- [7].Imaz I, Zegarra P, Gonzalez-Enriquez J, et al. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int 2010;21:1943–51. [DOI] [PubMed] [Google Scholar]
- [8].Landfeldt E, Strom O, Robbins S, et al. Adherence to treatment of primary osteoporosis and its association to fractures–the Swedish Adherence Register Analysis (SARA). Osteoporos Int 2012;23:433–43. [DOI] [PubMed] [Google Scholar]
- [9].Barcenilla-Wong AL, Chen JS, March LM. Concern and risk perception of osteoporosis and fracture among post-menopausal Australian women: results from the Global Longitudinal Study of Osteoporosis in Women (GLOW) cohort. Arch Osteoporos 2013;8:155. [DOI] [PubMed] [Google Scholar]
- [10].Klop C, Welsing PM, Elders PJ, et al. Long-term persistence with anti-osteoporosis drugs after fracture. Osteoporos Int 2015;26:1831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Qu B, Ma Y, Yan M, et al. The economic burden of fracture patients with osteoporosis in western China. Osteoporos Int 2014;25:1853–60. [DOI] [PubMed] [Google Scholar]
- [12].Bianchi ML, Duca P, Vai S, et al. Improving adherence to and persistence with oral therapy of osteoporosis. Osteoporos Int 2015;26:1629–38. [DOI] [PubMed] [Google Scholar]
- [13].Diez-Perez A, Adachi JD, Adami S, et al. Risk factors for treatment failure with antiosteoporosis medication: the global longitudinal study of osteoporosis in women (GLOW). J Bone Miner Res 2014;29:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang J, Delzell E, Curtis JR, et al. Use of pharmacologic agents for the primary prevention of osteoporosis among older women with low bone mass. Osteoporos Int 2014;25:317–24. [DOI] [PubMed] [Google Scholar]
- [15].Halpern R, Becker L, Iqbal SU, et al. The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and all-cause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. J Manag Care Pharm 2011;17:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Center JR, Bliuc D, Nguyen ND, et al. Osteoporosis medication and reduced mortality risk in elderly women and men. J Clin Endocrinol Metab 2011;96:1006–14. [DOI] [PubMed] [Google Scholar]
- [17].Camargo MB, Cendoroglo MS, Ramos LR, et al. Bone mineral density and osteoporosis among a predominantly Caucasian elderly population in the city of Sao Paulo, Brazil. Osteoporos Int 2005;16:1451–60. [DOI] [PubMed] [Google Scholar]
- [18].Copes RM, Comim FV, Langer FW, et al. Obesity and fractures in postmenopausal women: a primary-care cross-sectional study at Santa Maria, Brazil. J Clin Densitom 2015;18:165–71. [DOI] [PubMed] [Google Scholar]
- [19].Hooven FH, Adachi JD, Adami S, et al. The Global Longitudinal Study of Osteoporosis in Women (GLOW): rationale and study design. Osteoporos Int 2009;20:1107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lewiecki EM, Gordon CM, Baim S, et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone 2008;43:1115–21. [DOI] [PubMed] [Google Scholar]
- [21].Sato M, Vietri J, Flynn JA, et al. Bone fractures and feeling at risk for osteoporosis among women in Japan: patient characteristics and outcomes in the National Health and Wellness Survey. Arch Osteoporos 2014;9:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jacob L, Dreher M, Kostev K, et al. Increased treatment persistence and its determinants in women with osteoporosis with prior fracture compared to those without fracture. Osteoporos Int 2016;27:963–9. [DOI] [PubMed] [Google Scholar]
- [23].Xu Y, Viswanathan HN, Ward MA, et al. Patterns of osteoporosis treatment change and treatment discontinuation among commercial and Medicare Advantage Prescription Drug members in a national health plan. J Eval Clin Pract 2013;19:50–9. [DOI] [PubMed] [Google Scholar]
- [24].Nielsen D, Ryg J, Nielsen W, et al. Patient education in groups increases knowledge of osteoporosis and adherence to treatment: a two-year randomized controlled trial. Patient Educ Couns 2010;81:155–60. [DOI] [PubMed] [Google Scholar]
- [25].Alarcon T, Gonzalez-Montalvo JI, Martin-Vega A, et al. Improving persistence and adherence to osteoporosis treatment: a challenge to solve. Osteoporos Int 2016;27:1275–6. [DOI] [PubMed] [Google Scholar]
- [26].Reynolds K, Muntner P, Cheetham TC, et al. Primary non-adherence to bisphosphonates in an integrated healthcare setting. Osteoporos Int 2013;24:2509–17. [DOI] [PubMed] [Google Scholar]
- [27].Zarowitz BJ, Cheng LI, Allen C, et al. Osteoporosis prevalence and characteristics of treated and untreated nursing home residents with osteoporosis. J Am Med Dir Assoc 2015;16:341–8. [DOI] [PubMed] [Google Scholar]
- [28].Rossini M, Bianchi G, Di Munno O, et al. Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int 2006;17:914–21. [DOI] [PubMed] [Google Scholar]
- [29].Carr AJ, Thompson PW, Cooper C. Factors associated with adherence and persistence to bisphosphonate therapy in osteoporosis: a cross-sectional survey. Osteoporos Int 2006;17:1638–44. [DOI] [PubMed] [Google Scholar]
- [30].Hadji P, Papaioannou N, Gielen E, et al. Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int 2015;26:2479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sitjar Martinez de Sas S, Aguilera de la Fuente MT, Combalia Romera J, et al. [PERSIRIS study: observational study, postmarketing, prospective, to evaluate the persistence to treatment with monthly risedronate in women with osteoporosis]. Aten Primaria 2016;48:316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].de Castro Gomes DA, Valadares AL, Pinto-Neto AM, et al. Ability to follow drug treatment with calcium and vitamin D in postmenopausal women with reduced bone mass. Menopause 2012;19:989–94. [DOI] [PubMed] [Google Scholar]
- [33].IBGE. IBdGeE-. Census 2010. Distribution of population by sex, race and ethnicity, according to age groups. Available at: http://www.censo2010.ibge.gov.br. [Google Scholar]


