Abstract
Rationale:
Benign metastasizing leiomyoma (BML) is rare condition involving distant metastases secondary to benign uterine leiomyoma, and it is most commonly found in the lungs. It rarely metastasizes to the spine to cause osteolytic damage and spinal canal compression.
Patient concerns:
A 51-year-old woman with low back and bilateral leg pain and paresthesia was admitted to our ward. She has a previous medical history of uterine leiomyomas. Magnetic resonance imaging of the lumbar spine revealed vertebral body osteolytic destruction and soft tissue mass in the L4/5 with a secondary lumbar spinal stenosis. Positron emission tomography computed tomography showed moderately intense accumulation of 18F-fluorodeoxyglucose in the L4/5 mass, as well as multiple nodules with increased metabolic activity in both lungs.
Diagnoses:
Pulmonary and spinal BML.
Interventions:
The patient underwent a computed tomography-guided percutaneous needle biopsy of the lung nodule and lumbar corpectomy, tumor excision, and vertebroplasty in the L4/5.
Outcomes:
Pathologically, both pulmonary nodule and vertebral mass were diagnosed as leiomyomas without any malignant evidence. Estrogen and progesterone receptors were both positive in the metastatic tumors. The patient's symptoms completely disappeared after the surgery. The patient is currently receiving outpatient anti-estrogen tamoxifen treatment for a BML.
Lessons:
Through this case, we suggest that BML should be regarded as part of differential diagnosis in female patients with a previous medical history of uterine leiomyomas presenting with multiple nodules in any parts of the body.
Keywords: benign metastasizing leiomyoma, lung, metastasis, spine, uterine leiomyoma
1. Introduction
Benign metastasizing leiomyoma (BML) is a rare condition that mainly occurs in women of reproductive age with a history of uterine leiomyoma and/or myomectomy.[1] The clinical course of BML is closely associated with sex hormone levels. The lungs are the most common sites of involvement, which present with multiple asymptomatic nodules. Aside from the lungs, other sites of involvement have also been reported, such as the lymph nodes, omentum, pelvis, abdomen, mediastinum, vertebra, nervous system, bone, skeletal muscle, skin, inferior vena cava, right atrium, breast, trachea, esophagus, scars, liver, and adrenal glands.[2–4] Meanwhile, leiomyomas of the spine, particularly the lumbar spine, are extremely rare. The exact molecular mechanisms and the therapeutic strategies for leiomyomas are still unclear to date. Herein, we report a rare case of concurrent BML in the lung and lumbar spine that we encountered for the first time and present a review of the literature to deepen our understanding of such cases.
2. Case presentation
A 51-year-old woman presented with a progressive low back and bilateral leg pain and paresthesia for 2 months and was admitted to our hospital. Magnetic resonance imaging (MRI) of the lumbar spine revealed vertebral body osteolytic destruction as well as soft tissue mass in the L4/5 with a secondary lumbar spinal stenosis (Fig. 1). To rule out any possibility of a primary or metastatic tumor, a positron-emission tomography computed tomography (PET-CT) with 18F-fluorodeoxyglucose (FDG) of whole body was then performed. The imaging findings showed moderately intense accumulation of 18F-FDG in the L4/5 mass (standardized uptake value maximum [SUVmax], 5.8) (Fig. 2A, B). Aside from lesions in the lumbar spine, several nodules with increased metabolic activity were observed in both lungs (SUVmax, 5.5) (Fig. 2C–F). Thus, the patient was suspected to have lung cancer with lumbar metastasis and was consequently referred to the department of respiratory medicine for further investigation.
Figure 1.
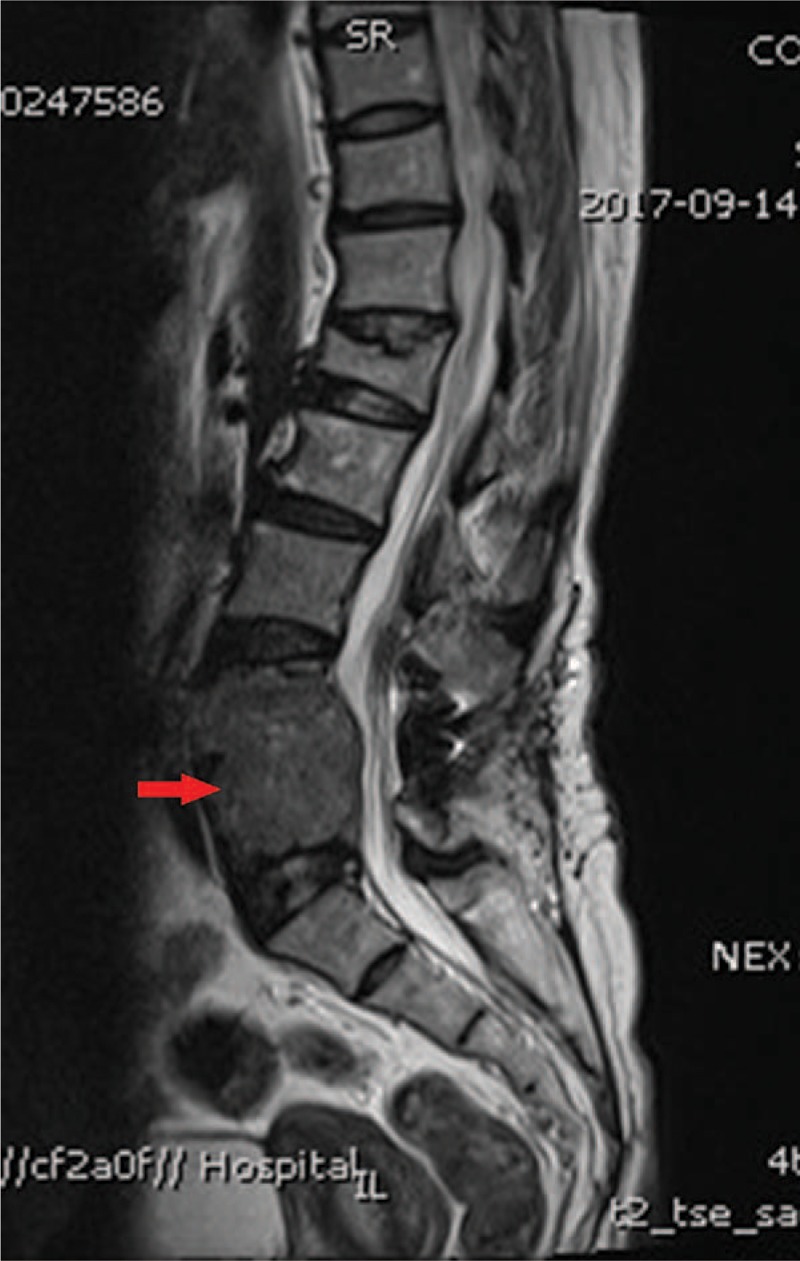
Magnetic resonance imaging of the lumbar spine of the patient. A vertebral metastatic mass was seen on the L4/5 that lead to osteolytic lesions and a secondary lumbar spinal stenosis.
Figure 2.
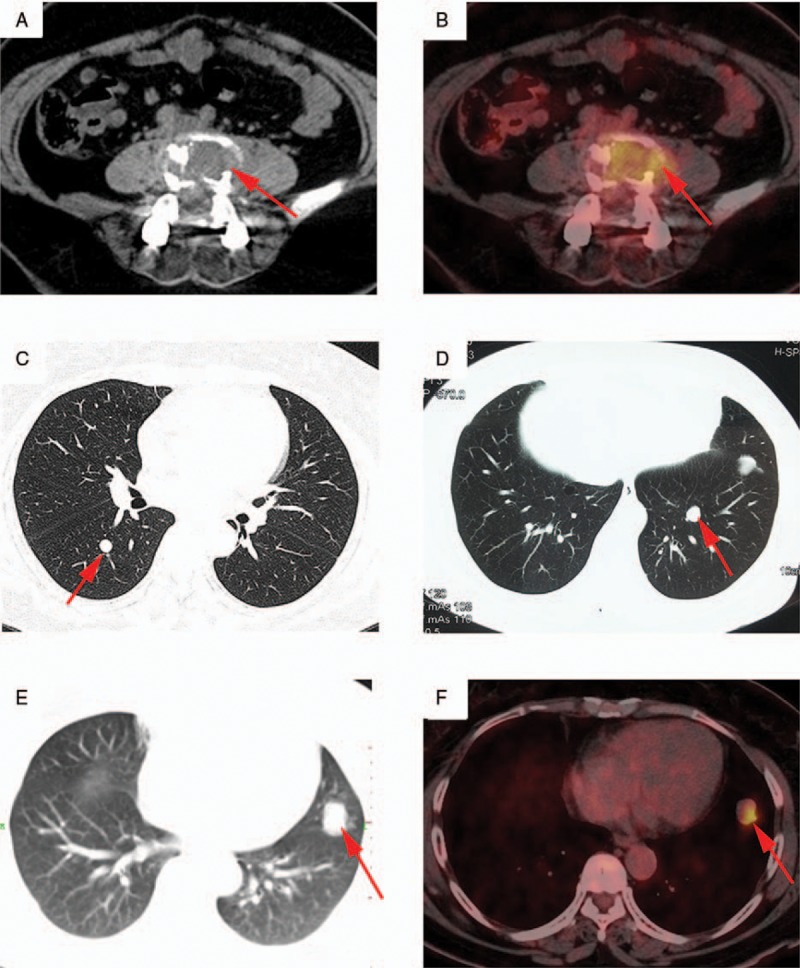
Positron emission tomography computed tomography (PET-CT) of the patient. (A, B) PET-CT scan of equivalent level of L4. Moderate 18F-FDG uptake (SUVmax, 5.8) was detected in the lesion. (C–F) Multiple nodules were found bilaterally in the lungs. Only 1 lesion in the lower lingular lobe of the left lung exhibited a positive accumulation of 18F-FDG (SUVmax, 5.5). FDG = fluorodeoxyglucose, SUV = standardized uptake value.
The patient has a medical history of L4-5 degenerative spondylolisthesis and underwent open reduction, internal fixation, and posterolateral fusion in 2010. She was previously diagnosed with a benign uterine leiomyoma and underwent a myomectomy in 2011.
No weakness or sensory loss of the upper and lower extremities was noted on physical examination. Her general condition was satisfactory except for the complaint of pain, and routine blood chemical tests showed no abnormalities. Serum concentrations of cancer markers such as CA-125, CA-199, CA-153, CA-242, carcinoembryonic antigen, and neuron-specific enolase were all within normal ranges. A CT-guided percutaneous needle biopsy of the nodule in the upper left lung was performed. The histopathology revealed a diagnosis of multiple leiomyomas of the lung. The nodule was composed of well-differentiated spindle-shaped cells strongly positive for α-smooth muscle actin, estrogen receptor (ER), and progesterone receptor (PR), but negative for CD34, S100, and HMB45. The Ki-67 ratio was <20%. The size and shape of the nucleoli and cells did not substantially differ. No evident nuclear atypia, mitotic figures, or necrosis was identified. Although the initial interpretation of the histopathology findings was leiomyomatous hamartoma with no evidence of malignancy or atypia (Fig. 3A, B), the possibility of malignant tumor could not be completely ruled out because the PET-CT showed increased metabolic activity of the multiple lesions in the lumbar spine and both lungs. Thus, an additional CT-guided needle biopsy of the tumor of the lumbar spine was performed. The pathological results also showed benign leiomyomas (Fig. 3C). Subsequently, the tumor on the L4/5 was totally removed with the L4/5 vertebral body under general anesthesia, and vertebroplasty was performed with polymethacrylate. The pathological results of the surgical specimens confirmed the pathological diagnosis from the first biopsy of the nodule in the lung and the second biopsy of vertebral mass (Fig. 3D–F). Given that the patient previously underwent hysterectomy for uterine leiomyoma, a definitive diagnosis of BML was established. The patient's complaints of low back and bilateral leg pain were soon relieved after surgery. The patient was given an outpatient treatment of anti-estrogen tamoxifen for BML. After 3 months of follow-up, the general condition of the patient was satisfactory. No recurrence of the lumbar lesion was noted on MRI (Fig. 4), and a shrinkage of the metastatic lung tumors as a result of treatment was noted on chest computed tomography (CT) (Fig. 5). The patient has been followed up for 5 months and will continue to be followed up.
Figure 3.
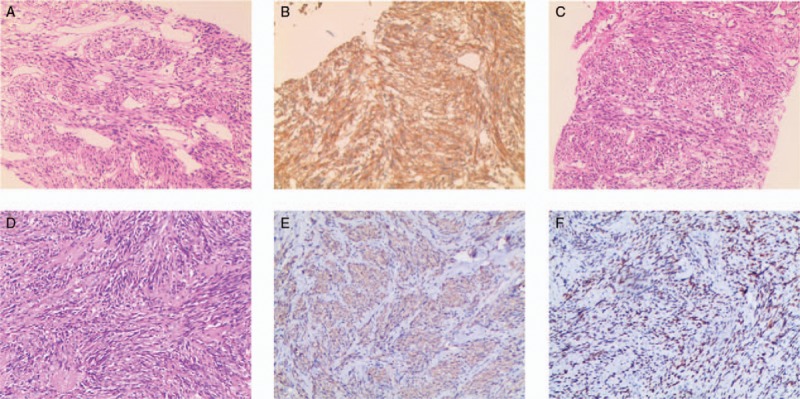
Histological findings of the specimen. (A, B) The histological findings of the biopsy specimen from the lung. (C) The histological findings of the biopsy specimen from the lumbar vertebra. (D–F) The histological findings of the surgical specimen from the lumbar vertebra. (A, C, D) Results of H&E staining. The tissue samples exhibited a similar appearance consisting of interlaced bundles of spindle-shaped tumor cells. (B, E) Immunohistochemical staining for α-SMA. The tumor cells positively stained for α-SMA. (F) Immunohistochemical staining for ER. The tumor cells stained positively for ER. ER = estrogen receptor, SMA = smooth muscle actin.
Figure 4.
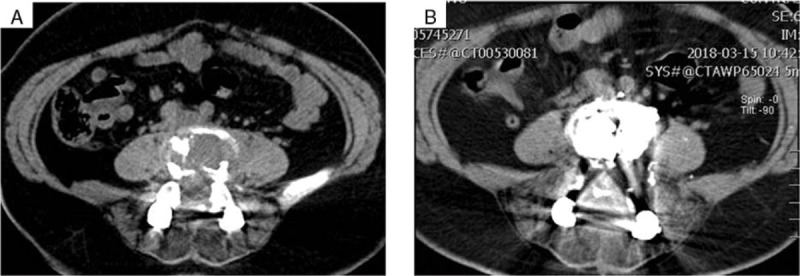
Imaging of the lumbar spine shows stable lesion. (A) Pre-treatment PET-CT. (B) MRI performed 3 months after the start of anti-estrogen treatment. MRI = magnetic resonance imaging, PET-CT = positron-emission tomography computed tomography.
Figure 5.
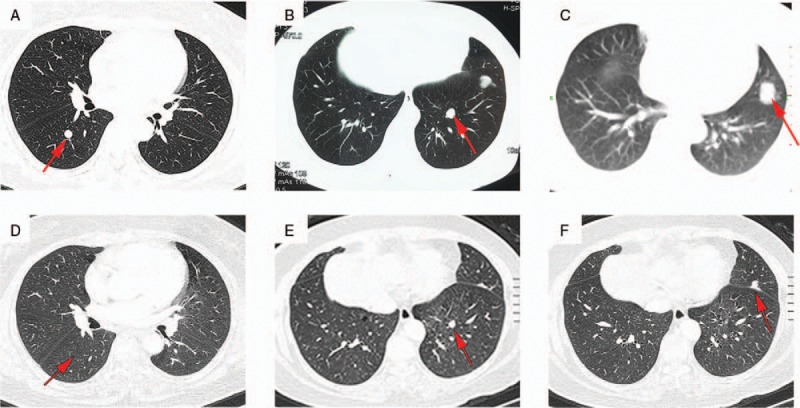
Chest CT shows shrinkage of lung nodules. (A–C) Pre-treatment imaging. (D–F) CT performed 3 months after the start of anti-estrogen treatment. Arrows indicate representative lung nodules. CT = computed tomography.
This case report was approved by the ethics committee of The Second Xiangya Hospital, Central South University, Changsha, China, and written informed consent to publish was obtained.
3. Discussion
Uterine leiomyoma are monoclonal tumors of the myometrium and are the most common pelvic nonmalignant tumor in women of reproductive age. BML is a very rare condition and commonly occurs in persons with a history of hysterectomy or operation for uterine leiomyomas 1 month to 20 years previously.[5] It is characterized by numerous well-differentiated smooth muscle tissue and vascular collagenous tissue at sites distant from the uterus.
The pathogenesis of BML remains unclear. The most accepted hypothesis is the vascular dissemination of benign uterine leiomyoma cells.[6] Canzonieri et al[7] reviewed 3 uterine leiomyomas with vascular invasion, 2 of which were associated with pulmonary BML (PBML), and 1 with intravenous leiomyomatosis (IVL), indicating microscopic vascular invasion as the common metastatic mechanism of BML and IVL. IVL is a rare hormone-dependent benign smooth muscle cell tumor that grows within venous channels from the intrauterine venules to the systemic veins. The paradoxical characteristic of the benign histopathology and the malignant biological behavior of IVL are similar to those of BML. Lee et al[8] investigated a patient with coexisting BML and IVL using comparative genomic hybridization and found that the genomic changes in BML and IVL are highly correlated to each other. These results suggested that the primary uterine leiomyoma may be capable of vascular invasion before the myomectomy. With the increase in genetic abnormalities, the tumor cells may spread over the whole body through blood flow and develop into BML. Moreover, sex hormones may also play a role in the pathogenesis of BML as indicated by the following: the growth of the tumor is cyclic premenstrual; the expression of PR and ER in the tumor is high; surgical oophorectomy or medical hormone therapy results in tumor regression and suppression of tumor recurrence.[9]
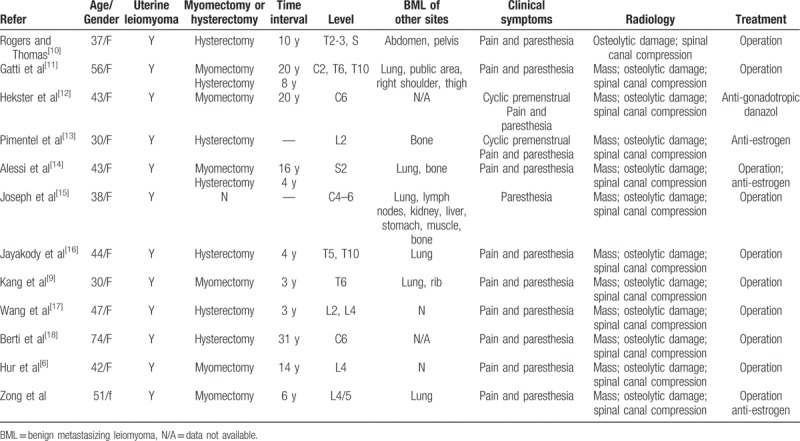
The most common site of BML metastases is the lung, while metastasis to the spine is extremely rare. We searched PubMed and found 11 reported cases of vertebral body involvement. We summarized the clinical characteristics of the current 12 cases of spinal BML in Table 1. All of the patients are women with ages ranged from 30 to 74 years. All patients have uterine leiomyoma, and 10 in 12 patients had definite history of myomectomy/hysterectomy. The metastatic foci of leiomyoma have been discovered 3 to 31 years after myomectomy/hysterectomy for benign leiomyoma of the uterus, and the average time interval of presentation is 12.7 years. Of these spinal BML cases, 4, 4, 4, and 2 occurred in the cervical spine, thoracic spine, lumbar spine, and sacrum, respectively, indicating an equal distribution of metastasis in the spine. Six patients were diagnosed with PBML, 6 had BMLs in other sites (bone, abdomen, pelvis, shoulder, thigh, lymph node, kidney, liver, stomach, muscle), and 2 had metastasis only to the spine, while 2 had no other lesions.
Table 1.
Characteristics of patients with spine BML.
Patients with PBML are generally asymptomatic, and the PBML is often discovered incidentally via imaging examinations. Some studies reported that PBML patients can also show some non-specific symptoms, such as cough, wheezing, chest pain, and recurrent pneumothorax.[19] If the lesions are located in the spine, patients may complain of pain and paresthesia due to the osteolytic damage and spinal canal compression by the tumor (Table 1), as seen in our patient. Notably, the main clinical symptoms of BML vary depending on the organs involved.
The pulmonary lesions typically manifest as multiple well-circumscribed solid pulmonary nodules of different sizes, ranging from a few millimeters to several centimeters. The nodules may be distributed in bilaterally or unilaterally in the lungs and are rarely calcified. Thin-walled or thick-walled cystic or cavitary lesions have also been reported.[19,20] We also reviewed the imaging findings of spinal BML. The MRI examination of the spine generally shows masses in the vertebral bodies, which may lead to osteolytic damage and spinal canal compression. Currently, 18F-FDG PET-CT is used for the staging, surveillance, and detection of various malignancies. A few reports have indicated that 18F-FDG PET-CT is helpful for distinguishing BML from leiomyosarcoma. Non-avid or mild uptake in 18F-FDG PET-CT without mediastinal and hilar lymph node enlargement or appearance of malignant signs in other parts of the body are thought to be characteristic of BML,[21] while leiomyosarcoma is a more glycolytically active tumor. However, not every patient has the above characteristics. Sawai et al[22] demonstrated that approximately 8.3% of patients with BML exhibit high 18F-FDG uptake, but the 18F-FDG in the tumors and the malignant level is not significantly correlated. In the present case, the patient showed moderately intense accumulation of 18F-FDG, which was consistent with previous reports.[21]
Histologically, BML lesions consist of well-differentiated spindle-shaped cells without cytological atypia or tumor necrosis. The low proliferative state and low Ki-67 index indicate the benignity of the disease, which is different from leiomyosarcoma.[5] The positive markers of desmin, muscle-specific actin, and vimentin support the mesenchymal derivation of these tumors with smooth muscle differentiation.[23] The high expression of ER and PR suggests that sex hormones may play an important role in the growth and development of this malignancy, which may be a basis for treatment.
No standard treatment guideline for BML has been established. Due to the long-term stability in terms of size and clinical symptoms and slow progression, some patient selects careful observation. If the lesions increase in size or suppress surrounding tissue, surgical resection of the mass is recommended to prevent potentially fatal complications. In addition, the presence of ER and PR suggests that BML is hormone sensitive; thus, treatment is based on hormonal manipulation, and surgical or medical castration may be an option.[3] Hormonal therapy has been suggested as the optimal treatment for the patients who refuse surgery, have unresectable metastatic disease, or those who progress despite surgery. The rationale for hormonal therapy is based on the presence of ERs and PRs in both primary tumors as well as in the metastatic tumors.[5] Recently, progestin, aromatase inhibitors, tamoxifen, raloxifene, and medical castration with gonadotropin-releasing hormone analogues have been used to treat BML.[24] Although some patients experienced disease control or regression with hormonal manipulation, hormone therapy does not work for all patients, and the side effects should be considered, such as flushes, fatigue, and nausea. The treatment should be individualized for each patient depending on the metastasis sites of the disease and the hormone receptor status. A combination of surgical and medical treatment would exert a synergistic effect and should be considered in the management of progressive and symptomatic lesions as in our patient. Overall, the prognosis of BML is favorable. Our patient underwent surgical resection of the spine lesion and received hormone treatment to antagonize the estrogen. The patient is under our follow-up schedule to observe prognosis.
4. Conclusions
In this report, we reviewed a rare case of BML developing in the lumber spine and lung. BML usually affects women of childbearing age with a history of uterine leiomyoma. 18F-FDG PET-CT showed 18F-FDG non-avid or mild uptake without mediastinal or hilar lymph node enlargement. The diagnosis of BML is based on the medical history and histopathologic and immunohistochemical findings of metastatic lesions. No standard treatment has been established. Because the clinical course of BML varies due to the metastasis sites and clinical symptoms, an individualized approach should be considered.
Author contributions
Conceptualization: Dandan Zong, Ping Chen, Ruoyun Ouyang.
Data curation: Dandan Zong, Wenlong He.
Writing – original draft: Dandan Zong.
Writing – review and editing: Dandan Zong, Wenlong He, Jinhua Li, Hong Peng, Ping Chen, Ruoyun Ouyang.
Footnotes
Abbreviations: BML = benign metastasizing leiomyoma, ER = estrogen receptor, FDG = fluorodeoxyglucose, IVL = intravenous leiomyomatosis, MRI = magnetic resonance imaging, PBML = pulmonary benign metastasizing leiomyoma, PET-CT = positron-emission tomography computed tomography, PR = progesterone receptor, SMA = smooth muscle actin, SUV = standardized uptake value.
The authors declare no conflict of interest, financial, or otherwise.
References
- [1].Cai A, Li L, Tan H, et al. Benign metastasizing leiomyoma. Case report and review of the literature. Herz 2014;39:867–70. [DOI] [PubMed] [Google Scholar]
- [2].Ağaçkiran Y, Findik G, Ustün LN, et al. Pulmonary benign metastasizing leiomyoma: an extremely rare case. Turk Patoloji Derg 2016;32:193–5. [DOI] [PubMed] [Google Scholar]
- [3].Ki EY, Hwang SJ, Lee KH, et al. Benign metastasizing leiomyoma of the lung. World J Surg Oncol 2013;11:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen S, Liu RM, Li T. Pulmonary benign metastasizing leiomyoma: a case report and literature review. J Thorac Dis 2014;6:E92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mahmoud MS, Desai K, Nezhat FR. Leiomyomas beyond the uterus; benign metastasizing leiomyomatosis with paraaortic metastasizing endometriosis and intravenous leiomyomatosis: a case series and review of the literature. Arch Gynecol Obstet 2015;291:223–30. [DOI] [PubMed] [Google Scholar]
- [6].Hur JW, Lee S, Lee JB, et al. What are MRI findings of spine benign metastasizing leiomyoma? Case report with literature review. Eur Spine J 2015;24:S600–5. [DOI] [PubMed] [Google Scholar]
- [7].Canzonieri V, D’Amore ES, Bartoloni G, et al. Leiomyomatosis with vascular invasion. A unified pathogenesis regarding leiomyoma with vascular microinvasion, benign metastasizing leiomyoma and intravenous leiomyomatosis. Virchows Arch 1994;425:541–5. [DOI] [PubMed] [Google Scholar]
- [8].Lee HJ, Choi J, Kim KR. Pulmonary benign metastasizing leiomyoma associated with intravenous leiomyomatosis of the uterus: clinical behavior and genomic changes supporting a transportation theory. Int J Gynecol Pathol 2008;27:340–5. [DOI] [PubMed] [Google Scholar]
- [9].Kang MW, Kang SK, Yu JH, et al. Benign metastasizing leiomyoma: metastasis to rib and vertebra. Ann Thorac Surg 2011;91:924–6. [DOI] [PubMed] [Google Scholar]
- [10].Rogers L, Thomas L. Paraplegia caused by extraspinal metastasis from a uterine fibroid. J Neurol Neurosurg Psychiatry 1959;22:141–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gatti JM, Morvan G, Henin D, et al. Leiomyomatosis metastasizing to the spine. A case report. J Bone Joint Surg Am 1983;65:1163–5. [PubMed] [Google Scholar]
- [12].Hekster RE, Lambooy N, van Hall EV, et al. Hormone-dependent spinal leiomyoma. Surg Neurol 1994;41:330–3. [DOI] [PubMed] [Google Scholar]
- [13].Pimentel JR, de Almeida AL, Aymoré IL, et al. Metastatic skeletal leiomyomatosis (leiomyomatosis ossea). Skeletal Radiol 2002;31:30–4. [DOI] [PubMed] [Google Scholar]
- [14].Alessi G, Lemmerling M, Vereecken L, et al. Benign metastasizing leiomyoma to skull base and spine: a report of two cases. Clin Neurol Neurosurg 2003;105:170–4. [DOI] [PubMed] [Google Scholar]
- [15].Joseph V, Chacko G, Raghuram L, et al. Benign metastasizing leiomyoma causing spinal cord compression. Surg Neurol 2003;60:575–7. discussion 577-8. [DOI] [PubMed] [Google Scholar]
- [16].Jayakody S, Young K, Young B, et al. Serial spread of benign metastasizing leiomyoma to the thoracic spine. J Clin Neurosci 2011;18:1135–7. [DOI] [PubMed] [Google Scholar]
- [17].Wang LX, Lv FZ, Ma X, et al. Multifocal osteolytic lesions within lumbar spine in a middle-aged Chinese woman: a benign metastasizing leiomyoma? Spine (Phila Pa 1976) 2012;37:E259–63. [DOI] [PubMed] [Google Scholar]
- [18].Berti AF, Santillan A, Velasquez LA. Benign metastasizing leiomyoma of the cervical spine 31 years after uterine leiomyomaresection. J Clin Neurosci 2015;22:1491–2. [DOI] [PubMed] [Google Scholar]
- [19].Okabe R, Shoji T, Huang CL. Benign metastasizing leiomyoma of the lung with spontaneous pneumothorax. Thorac Cardiovasc Surg Rep 2013;2:26–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Choe YH, Jeon SY, Lee YC, et al. Benign metastasizing leiomyoma presenting as multiple cystic pulmonary nodules: a case report. BMC Womens Health 2017;17:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lin X, Fan W, Lang P, et al. Benign metastasizing leiomyoma identified using 18F-FDG PET/CT. Int J Gynaecol Obstet 2010;110:154–6. [DOI] [PubMed] [Google Scholar]
- [22].Sawai Y, Shimizu T, Yamanaka Y, et al. Benign metastasizing leiomyoma and 18-FDG-PET/CT: a case report and literature review. Oncol Lett 2017;14:3641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wei WT, Chen PC. Benign metastasizing leiomyoma of the lung: a case report and literature review. Oncol Lett 2015;10:307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Loukeri AA, Pantazopoulos IN, Tringidou R, et al. Benign metastasizing leiomyoma presenting as cavitating lung nodules. Respir Care 2014;59:e94–7. [DOI] [PubMed] [Google Scholar]


