Abstract
Background:
Glioblastoma multiforme (GBM) is a rare and deadly disease, with a reported average incidence rate of 3.19 cases per 100,000 inhabitants. Fotemustine, a third-generation nitrosourea with an alanine phosphor carrier that facilitates cellular penetration, has been extensively investigated in the setting of recurrent/progressive disease after initial treatment. Fotemustine is usually administered following a schedule consisting of 3 doses every week, followed by maintenance doses administered every 3 weeks.
Methods:
In this phase I/II trial, we aimed to assess whether the use of a biweekly regimen allowed administration of higher dose than the standard 100 mg/m2 dose approved per label indication in a population of patients with recurrent GBM. In this phase I/II trial, fotemustine was administered intravenously over 1 hour every 2 weeks at either 120 or 140 mg/m2 doses for up to 1 year, until disease progression, unacceptable toxicity, or patient's request to withdraw from the study. The phase I part of the trial was conducted following the classic 3+3 study design. The phase II part of the trial was a single-arm study. The primary efficacy endpoint was the percentage of patients who had not progressed after 24 weeks (PFS-24).
Results:
Thirty-seven patients were enrolled in this phase I/II trial from August 2006 to November 2011. Treatment was well tolerated in the overall population. Main severe toxicity was grades 3 and 4 thrombocytopenia, which occurred in 4 of 6 patients treated at the 140 mg/m2 dose level and in 3 of 31 patients treated at 120 mg/m2. Median PFS and overall survival were 12.1 (1–40.2) weeks and 19.7 (1–102) weeks, respectively.
Conclusion:
We conclude that fotemustine can be safely administered at 120 mg/m2 biweekly. The efficacy of such modified schedule and doses should be compared to the biweekly schedule at 80 mg2 and the standard weekly schedule at 80 to 100 mg/m2.
Keywords: fotemustine, glioblastoma, MGMT
1. Introduction
Glioblastoma multiforme (GBM) is a rare and deadly disease, with a reported average incidence rate of 3.19 cases per 100,000 inhabitants.[1] The prognosis of patients with GBM is poor, with a median overall survival (OS) of only 15 months, despite surgery, radiotherapy, and chemotherapy.[2] Optimal management of GBM at diagnosis includes surgical tumor removal to the maximum possible extent, with or without the use of carmustine wafers, followed by alternating electrical field therapy and radiation therapy plus concurrent and adjuvant temozolomide-based systemic treatment.[3]
Unfortunately, virtually all patients recur after initial multimodality treatment, so systemic treatment may offer a possibility for symptom palliation and possibly survival prolongation, although no prospective randomized-controlled trials are available. Main available pharmacologic classes with potential therapeutic efficacy include chemotherapy, antiangiogenetic agents[4] and immunotherapy.[5] Among chemotherapy agents, fotemustine, a third-generation nitrosourea with an alanine phosphor carrier that facilitates cellular penetration, has been extensively investigated in the setting of recurrent/progressive disease after initial treatment.[6–19]
Fotemustine is usually administered following a schedule consisting of 3 doses every week, followed by maintenance doses administered every 3 weeks. On the grounds of pharmacokinetic data suggesting incomplete drug elimination after weekly intervals,[20,21] we decided to assess whether the use of a biweekly regimen allowed administration of higher dose than the standard 100 mg/m2 dose approved per label indication in a population of patients with recurrent GBM.
2. Patients and methods
2.1. Eligibility
Eligible patients were those who signed a written consent form for study participation and presented histologically confirmed GBM, with recurrent or progressive disease after receiving surgery and systemic treatment with temozolomide (TMZ) administered concomitantly with and/or adjuvant to radiotherapy. Progressive/recurrent disease must have been proven by 1 magnetic resonance imaging (MRI) scan performed ≥3 months after completing radiotherapy or undergoing surgery or by 2 consecutive MRI tests. Measurable disease (at least 1 unidimensionally measurable lesion of ≥2 cm in diameter) must be detected at study entry by the use of MRI with contrast enhancement within 2 weeks before treatment initiation. Furthermore, eligible patients are required to have been receiving stable or decreasing corticosteroid doses for ≥2 weeks before patient's inclusion in the study; to be older than 18 years; to have an Eastern Cooperative Oncology Group performance status ≥2; to have adequate bone marrow, renal, and liver function (absolute neutrophils count >1.5 × 109/L; platelets >100 × 109/L; hemoglobin >10 g/dL, serum creatinine <1.25 × upper limit of the normal range [ULN]; blood urea nitrogen <25 mg/dL; serum bilirubin ≤1.25 × ULN; AST and ALT ≤1.5 × ULN; alkaline phosphatase ≤2 × ULN). Main exclusion criteria included the presence of uncontrolled diseases (eg, hypertension, active infections, and psychiatric conditions), and a history of cancer, with the exception for resected nonmelanoma skin cancer and in situ carcinoma of the uterine cervix. The study was approved by the Institutional Ethics Committee of the participating center and was conducted according to the principles of the declaration of Helsinki and the rules of good clinical practice.
2.2. Treatment schedule
In this phase I/II trial, fotemustine was administered intravenously over 1 hour every 2 weeks at either 120 or 140 mg/m2 doses for up to 1 year, until disease progression, unacceptable toxicity, or patient's request to withdraw from the study. The phase I part of the trial was conducted following the classic 3+3 study design. Grade 4 leukopenia, grade 4 neutropenia, grades 3 and 4 anemia, and grades 3 and 4 thrombocytopenia were the dose-limiting toxicities (DLTs). Fotemustine was initially administered at 120 mg/m2 to the first 3 enrolled patients. If no patient experienced any DLT, additional 3 were enrolled at the 140 mg/m2. If 1 patient experienced any DLT, then additional 3 patients were enrolled at 120 mg/m2. If none of the additional 3 patients experienced any DLT, then the next dose level was explored. If at least one of the additional 3 patients experienced a DLT, then the protocol would be amended to study lower doses of fotemustine. This same pattern was also followed to evaluate the 140 mg/m2 dose level. The phase II study was conducted following a standard single-arm phase II study design. Throughout the entire study course, if treatment was suspended for more than 2 weeks beyond the next planned cycle of treatment planned, the patient was withdrawn from the study. Based on the most severe toxicity experienced since the last cycle, the subsequent dose was reduced to 75% in the presence of grade 3 or 4 platelet toxicity, grade 4 neutrophils or white blood cells or hemoglobin toxicity. In cases of nonhematologic toxicity, chemotherapy was delayed until recovery to grade 1, for a maximum of 2 weeks (after which the patient was withdrawn from the study). In case of grade 3 or 4 toxicity, the dose administered was reduced to 75% after recovery to grade 1 toxicity.
2.3. Efficacy measures and toxicity monitoring
Progression-free survival (PFS) was measured from the initiation of fotemustine to progression or death due to any cause or last follow-up assessment, whichever came first. OS was measured from the start of fotemustine to death for any reason, or last follow-up assessment. The analysis was performed as per intention to treat. Response was evaluated by using clinical and neurologic examinations and MRI or computed tomography neuroimaging according to MacDonald criteri. Patients were evaluated for tumor response every 8 weeks or earlier if indicated until disease progression. Neurologic status was assessed by considering signs and symptoms possibly related with progression, as compared to the previous examination; each variation in daily corticosteroids dosage was recorded. Responses were confirmed as complete response (CR), partial response (PR), and stable disease (SD), if they were constant at subsequent scans obtained at least 4 weeks apart from each other. All adverse events were recorded and graded according to the common toxicity criteria of the National Cancer Institute, version 4.0 (http://ctep.cancer.gov/forms/CTCAEv3.pdf).
2.4. Endpoints and statistical analysis
The primary efficacy endpoint of the study was evaluated in the entire patient cohort and was the percentage of patients who had not progressed after 24 weeks (PFS-24). Patients for whom at least 1 tumor evaluation was available and that had received at least 1 drug delivery were included in the response analysis. Treatment efficacy was evaluated following a 1-stage Fleming study design for determination of PFS rate at 24 weeks based on a single-treatment group. A sample size of 38 patients was estimated using exact binomial method and assuming: 1-tailed α equals to 0.05, 1-β equals to 0.9, and π < 0.05 (null hypothesis) versus π ≥ 0.20 (alternative hypothesis), where π was the observed 24-week progression-free rate. If 5 or more patients were evaluated as progression free after 24 weeks, it was assumed that the drug would be worthy of further investigation. Secondary objectives were the rate of best observed response, defined as the best response during the treatment and evaluated with MacDonald criteria,[22] OS, and toxicity. All patients receiving the study drug were included in the safety analysis. Median time to progression (mTTP) and median survival were also estimated with associated 95% confidence interval (CI). PFS and OS were calculated using the Kaplan–Meier method.
3. Results
3.1. Study population
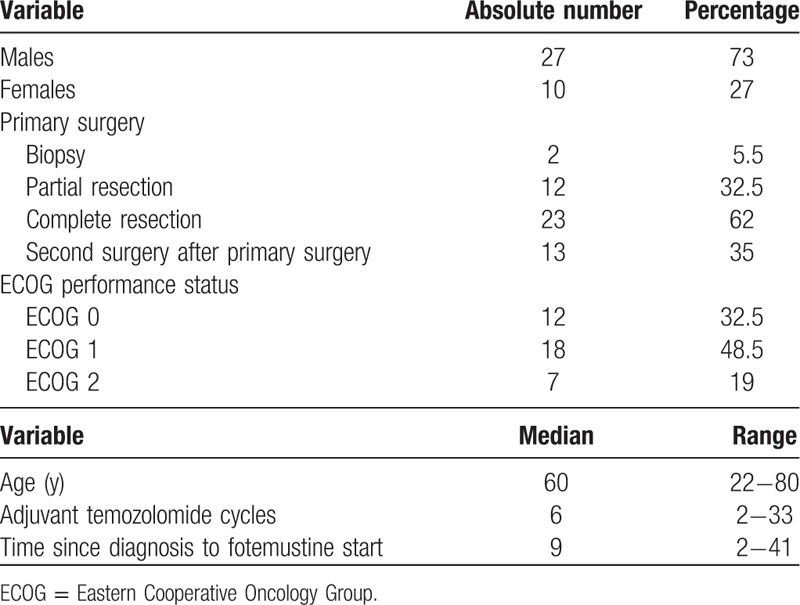
Thirty-seven patients were enrolled in this phase I/II trial from August 2006 to November 2011. Overall population characteristics are depicted in Table 1.
Table 1.
Patient population (n = 37).
3.2. Treatment
Of the 37 patients enrolled, 31 received the 120 mg/m2 dose and 6 patients received the 140 mg/m2 dose. After no DLT occurred in the first 3 patients treated at 120 mg/m2 dose level, one of 3 patients treated at the next dose level of 140 mg/m2 experienced grade 4 thrombocytopenia. Considering that all of the 3 additional patients treated at the 140 mg/m2 dose level experienced grade 4 thrombocytopenia, the dose level chosen for the phase II part of the trial was 120 mg/m2. A total of 135 cycles were administered, with a median number of 4 cycles administered to each patient.
3.3. Safety analysis
Treatment was well tolerated in the overall population. Main severe toxicity was grades 3 and 4 thrombocytopenia, which occurred in 4 of 6 patients treated at the 140 mg/m2 dose level and in 3 of 31 patients treated at 120 mg/m2. No other significant toxicities were reported in this study.
3.4. Efficacy analysis
The primary endpoint of the study was met, with 5 patients being progression free after 24 weeks of treatment. Median PFS and OS were 12.1 (1–40.2) weeks and 19.7 (1–102) weeks, respectively. Kaplan–Meier of PFS and OS are shown in Figures 1 and 2.
Figure 1.
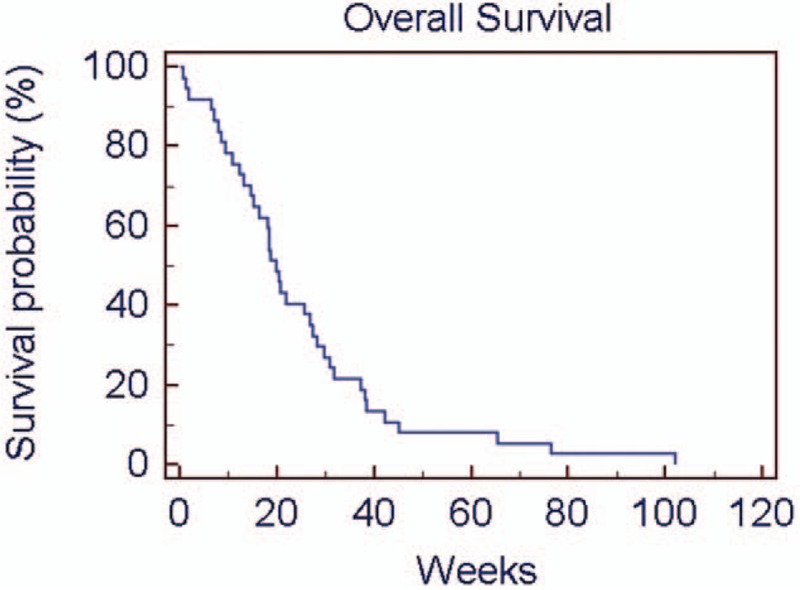
Overall survival rate using Kaplan–Meier method.
Figure 2.

Progression-free survival rate using Kaplan–Meier method.
4. Discussion
In the phase I trial conducted by Khayat et al,[20] which established the maximum tolerated dose of fotemustine to be 100 mg/m2 administered weekly for 3 to 4 weeks, thrombocytopenia was the only acute DLT and started on day 22, with a nadir on day 35. If the total induction dose of 300 mg/m2 is administered by day 15, following the registered schedule, it may not be possible to postpone fotemustine administration or to adjust the dose before thrombocytopenia occurs, hence the attractiveness of alternative fotemustine schedules.[7,9] In a monoinstitutional retrospective study conducted by Lombardi et al, 44 patients with GBM aged 65 years or older who had previously received radiation therapy with concomitant and adjuvant TMZ were treated with an alternative fotemustine schedule as second-line treatment, consisting of fotemustine administrated at 80 mg/m2 every 2 weeks for 5 consecutive administrations (induction phase), and then every 4 weeks at 80 mg/m2 as maintenance. The average age of the cohort was 70 years, with a median PFS and OS of 4.1 months (95% CI 3.1–5.2) and 7 months (95% CI 5.2–8.4), respectively (Fig. 3). Of note, grades 3 and 4 thrombocytopenia was reported only in 9% of such elderly population. Consistent results were obtained in a prospective phase II study by Addeo et al, who enrolled 40 patients with recurrent GBM treated with fotemustine after radiotherapy/temozolomide treatment. Fotemustine was given via intravenous infusion at a dose of 80 mg/m2 every 2 weeks for 5 consecutive administrations during the induction phase, followed by fotemustine administrations every 4 weeks at 80 mg/m2 as maintenance treatment. After a median of 8 administered cycles, a CR (2.5%, 95% CI 0–10%), 9 PRs (22.5%, 95% CI 15–37%), and 16 SDs (40%, 95% CI 32–51%) were reported, with an mTTP of 6.7 months (95% CI 3.9–9.1 months), and a median survival of 11.1 months. Importantly, grades 3 and 4 thrombocytopenia was reported in 5% of patients. Both of these studies show that fotemustine can be administered every 2 weeks with a favorable safety and efficacy profile. On the grounds of a complete radiologic response obtained in a case of GBM treated with fotemustine at 120 mg/m2, we hypothesized that the potential improved safety profile associated with biweekly administrations of fotemustine could allow to administer higher fotemustine doses. As originally speculated by Marinelli et al,[13] higher fotemustine dose could serve to reverse resistance to nitrosoureas mediated by the DNA repair enzyme O6-methylguanine methyltransferase (MGMT). In fact, in the retrospective study by Lombardi et al, patients with methylated versus unmethylated MGMT had a median PFS of 4.5 (3.1–5.9) versus 2.9 (2.4–3.5) months. The median PFS of 12 weeks obtained in our study appears to be consistent. Of note, we reported a 10% grades 3 and 4 thrombocytopenia rate in the cohort treated at 120 mg/m2, with no treatment-related deaths.
Figure 3.
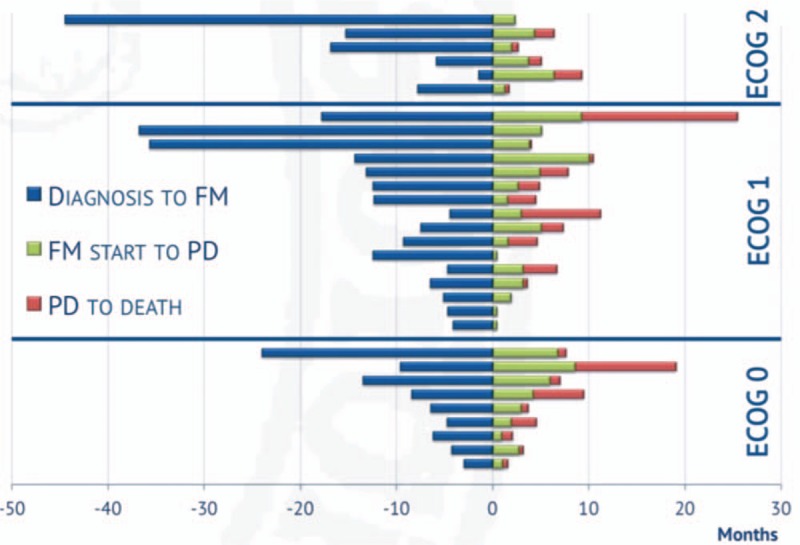
Time from diagnosis to fotemustine (FM) initiation, time from FM initiation to progressive disease (PD) and time from progressive disease (PD) to death according to Eastern Cooperative Oncology Group (ECOG) performance status.
We conclude that fotemustine can be safely administered at 120 mg/m2 biweekly. The efficacy of such modified schedule and doses should be compared to the biweekly schedule at 80 mg2 and the standard weekly schedule at 80 to 100 mg/m2.
Author contributions
Conceptualization: Alfredo Marinelli, Giuseppe Lamberti, Carlo Buonerba, Gianfranco Peluso, Giuseppe Di Lorenzo, Sabino De Placido.
Data curation: Alfredo Marinelli, Giuseppe Lamberti, Luigi Cerbone, Nadia Cordua.
Formal analysis: Alfredo Marinelli.
Investigation: Alfredo Marinelli.
Methodology: Alfredo Marinelli.
Project administration: Alfredo Marinelli.
Resources: Alfredo Marinelli.
Supervision: Alfredo Marinelli.
Validation: Alfredo Marinelli.
Visualization: Alfredo Marinelli.
Writing – original draft: Alfredo Marinelli.
Writing – review & editing: Alfredo Marinelli.
Footnotes
Abbreviations: BUN = blood urea nitrogen, CI = confidence interval, DLT = dose-limiting toxicity, ECOG = Eastern Cooperative Oncology Group, GBM = glioblastoma multiforme, MGMT = enzyme O6-methylguanine methyltransferase, mTTP = median time to progression, MRI = magnetic resonance imaging, OS = overall survival, PFS = progression-free survival, TMZ = temozolomide, ULN = upper limit of the normal range.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev 2014;23:1985–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- [3].Nabors LB, Portnow J, Ammirati M, et al. Central Nervous System Cancers, Version 1.2015. J Natl Compr Canc Netw 2015;13:1191–202. [DOI] [PubMed] [Google Scholar]
- [4].Wang Y, Xing D, Zhao M, et al. The role of a single angiogenesis inhibitor in the treatment of recurrent glioblastoma multiforme: a meta-analysis and systematic review. PLoS One 2016;11:e0152170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Malkki H. Trial watch: glioblastoma vaccine therapy disappointment in phase III trial. Nat Rev Neurol 2016;12:190. [DOI] [PubMed] [Google Scholar]
- [6].Khayat D, Giroux B, Berille J, et al. Fotemustine in the treatment of brain primary tumors and metastases. Cancer Invest 1994;12:414–20. [DOI] [PubMed] [Google Scholar]
- [7].Lombardi G, Bellu L, Pambuku A, et al. Clinical outcome of an alternative fotemustine schedule in elderly patients with recurrent glioblastoma: a mono-institutional retrospective study. J Neurooncol 2016;128:481–6. [DOI] [PubMed] [Google Scholar]
- [8].Perez-Segura P, Manneh R, Ceballos I, et al. GEINOFOTE: efficacy and safety of fotemustine in patients with high-grade recurrent gliomas and poor performance status. Clin Transl Oncol 2016;18:805–12. [DOI] [PubMed] [Google Scholar]
- [9].Addeo R, Caraglia M, De Santi MS, et al. A new schedule of fotemustine in temozolomide-pretreated patients with relapsing glioblastoma. J Neurooncol 2011;102:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Santoni M, Scoccianti S, Lolli I, et al. Efficacy and safety of second-line fotemustine in elderly patients with recurrent glioblastoma. J Neurooncol 2013;113:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brandes AA, Tosoni A, Franceschi E, et al. Fotemustine as second-line treatment for recurrent or progressive glioblastoma after concomitant and/or adjuvant temozolomide: a phase II trial of Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Cancer Chemother Pharmacol 2009;64:769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].De Felice F, Bulzonetti N, Musio D, et al. Low-dose fotemustine as second-line chemotherapy for recurrent glioblastoma multiforme. Anticancer Res 2013;33:4013–6. [PubMed] [Google Scholar]
- [13].Gallo C, Buonerba C, Di Lorenzo G, et al. Can high-dose fotemustine reverse MGMT resistance in glioblastoma multiforme? J Neurooncol 2010;100:311–9. [DOI] [PubMed] [Google Scholar]
- [14].Li L, Li S, Sun G, et al. Influence of the expression level of O6-alkylguanine-DNA alkyltransferase on the formation of DNA interstrand crosslinks induced by chloroethylnitrosoureas in cells: a quantitation using high-performance liquid chromatography-mass spectrometry. PLoS One 2015;10:e0121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bihan C, Foscolo S, Boone M, et al. Upfront bevacizumab and temozolomide or fotemustine before radiotherapy for patients with glioblastoma and severe neurological impairment at diagnosis. Case Rep Oncol 2012;5:530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Scoccianti S, Detti B, Sardaro A, et al. Second-line chemotherapy with fotemustine in temozolomide-pretreated patients with relapsing glioblastoma: a single institution experience. Anticancer Drugs 2008;19:613–20. [DOI] [PubMed] [Google Scholar]
- [17].Fabrini MG, Silvano G, Lolli I, et al. A multi-institutional phase II study on second-line fotemustine chemotherapy in recurrent glioblastoma. J Neurooncol 2009;92:79–86. [DOI] [PubMed] [Google Scholar]
- [18].Paccapelo A, Lolli I, Fabrini MG, et al. A retrospective pooled analysis of response patterns and risk factors in recurrent malignant glioma patients receiving a nitrosourea-based chemotherapy. J Transl Med 2012;10:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brandes AA, Finocchiaro G, Zagonel V, et al. AVAREG: a phase II, randomized, noncomparative study of fotemustine or bevacizumab for patients with recurrent glioblastoma. Neuro Oncol 2016;18:1304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Khayat D, Lokiec F, Bizzari JP, et al. Phase I clinical study of the new amino acid-linked nitrosourea, S 10036, administered on a weekly schedule. Cancer Res 1987;47:6782–5. [PubMed] [Google Scholar]
- [21].Ings RM, Gray AJ, Taylor AR, et al. Disposition, pharmacokinetics, and metabolism of 14C-fotemustine in cancer patients. Eur J Cancer 1990;26:838–42. [DOI] [PubMed] [Google Scholar]
- [22].Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277–80. [DOI] [PubMed] [Google Scholar]


