Supplemental Digital Content is available in the text
Keywords: allogeneic hematopoietic stem cell transplantation, chemotherapy, drug therapy, hematopoietic stem cell transplantation, precursor T-cell lymphoblastic leukemia-lymphoma, prognosis, risk factor
Abstract
To study the prognostic factors of adult patients with T-lymphoblastic lymphoma (T-LBL) and to evaluate therapeutic effects of acute lymphoblastic leukemia (ALL)-type chemotherapy in combination with allogeneic hematopoietic stem cell transplantation (allo-HSCT) in patients who achieved overall response (OR) with first line ALL-type chemotherapy.
This was a retrospective study of 59 adult patients with T-LBL treated with hyper-fractionated administration of cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate (hyper-CVAD/MA) chemotherapy alone or in combination with allo-HSCT between June 2008 and October 2015. Complete response (CR) and OR rates were evaluated after the initial chemotherapy. Clinical characteristics and the risk factors associated with prognosis and overall survival (OS) were analyzed in all patients and the effects of allo-HSCT on OS were evaluated in patients who had achieved OR after initial chemotherapy.
Forty-eight patients (81.4%) achieved OR by hyper-CVAD chemotherapy, among which, 22 patients (45.8%) further received allo-HSCT. The median follow-up was 31.5 months, ranging from 11 to 97 months. The 3-year OS and progression-free survival (PFS) were 45.7% and 45.0% for patients who achieved OR after chemotherapy and both 0 for patients who did not achieve OR (both P < .001). Three year OS and PFS were higher in patients who received chemotherapy + allo-HSCT than in patients who received chemotherapy alone (3-year OS: 72.8% vs 17.5%, P = .008; PFS: 65.1% vs 27.8%, P = 0.007). Shorter survival was independently associated with elevated lactic dehydrogenase (LDH), Ki-67≥75%, pleural effusion and no OR (all P < .05) in all patients. But shorter survival was only associated with elevated LDH level, leukocytosis (>10 G/L), and chemotherapy alone in patients who achieved OR (all P < .05).
The mid-term outcomes of adult patients with T-LBL are associated with response to chemotherapy (in all patients) and performance of allo-HSCT (in patients who achieved OR). Allo-HSCT could be a feasible and effective consolidation therapy for adult T-LBL.
1. Introduction
T-lymphoblastic lymphoma (T-LBL) is a rare, highly aggressive non-Hodgkin lymphoma (NHL), most commonly seen in children and adolescents.[1] T-LBL commonly manifests with large anterior mediastinal mass, pleural effusion, superior vena cava syndrome, airway obstruction and pericardial effusion, accompanied by B symptoms, and elevated serum lactate dehydrogenase (LDH), with less involvement of liver and spleen.[2] T-LBL and T-cell acute lymphoblastic leukemia (T-ALL), are morphologically and immunophenotypically the same and defined as precursor T-LBL/ALL in the WHO (2008) tumor classification criteria for the lymphoid hematopoietic system, but T-LBL has minimal marrow involvement and presents with an enlarged thymic/mediastinal mass in 90% of cases.[1] Cytogenetics shows T-LBL is quite different from T-ALL.[3] Translocations involving chromosome region 9q34 are significantly more common in T-LBL than in T-ALL and translocation t (9;17) (q34; q22-23) only occurred in T-LBL.[4] Animal models also suggest that these 2 diseases stem from different biological base[5,6]
ALL-type chemotherapy involves intensive multidrug chemotherapy, including central nervous system (CNS) prophylaxis, with or without mediastinal radiation therapy (MRT) and significantly improves complete response (CR) rate and disease free survival (DFS).[1,6,7] The hyper-CVAD (hyper-fractionated administration of cyclophosphamide, vincristine, doxorubicin and dexamethasone) regime is currently the basic ALL-type chemotherapy regime for T-LBL.[8]
Autologous or allogeneic hematopoietic stem cell transplantation (allo-HSCT) after consolidation therapy is also provided and may improve OS.[9,10] But the effect of HSCT in the treatment of T-LBL and patient populations that may benefit from HSCT are still controversial. Factors related to T-LBL prognosis, such as elevated LDH, CNS involvement, high international prognostic index (IPI) scor,e and Ann Arbor stage IV have not been clearly defined.[8,11]
Therefore, we analyzed the therapeutic effect of ALL-type chemotherapy combined with allo-HSCT and studied prognostic factors of adult patients with T-LBL.
2. Methods
2.1. Patients
Fifty-nine adult patients with T-LBL admitted to our center, at the first Affiliated Hospital of Medical School of Zhejiang University and the Second Affiliated Hospital of Zhejiang Chinese Medicine University, from June 2008 to October 2015 were included in this study. The inclusion criteria were diagnosed with T-LBL; no previous malignancy; no previous treatment for lymphoma; and received their complete therapy in 2 hospitals. The exclusion criteria were as follows: incomplete medical records and serious co-morbidities.
The patients all had a pathological diagnosis of T-LBL, which was conducted according to REAL classification or WHO (2008) tumor classification criteria for lymphoid hematopoietic system combined with immunohistochemistry results.[12] The patients underwent bone marrow morphological examination and bone marrow biopsy. Clinical staging was evaluated according to Ann Arbor International Staging System based on computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET).[13] A proportion of lymphoblasts/prolymphocytes in bone marrow < 25% was defined as lymphoma bone marrow involvement (stage IV). All patients had a mass which was confirmed to be T-LBL by biopsy and had the proportion of lymphoblasts/prolymphocytes in bone marrow < 25% at diagnosis.
The study was approved by the medical Ethics Committee of the First Affiliated Hospital of Medical School of Zhejiang University and the Second Affiliated Hospital of Zhejiang Chinese Medicine University.
2.2. Treatment grouping
All patients were treated with hyper-CVAD/MA chemotherapy. After 4 courses of hyper-CVAD/MA chemotherapy, the patients were evaluated for response and grouped according to whether they achieved overall response (OR). In patients who achieved OR (i.e., eligible for allo-HSCT health wise), the patients were further grouped according to whether they received allo-HSCT into chemotherapy alone (n = 26) or chemotherapy + allo-HSCT groups (n = 22). The details of their treatments are provided in the supplementary material
2.2.1. Treatment effect evaluation
All patients underwent CT, MRI, or PET for treatment effect evaluation. Flow cytometry was conducted for patients with bone marrow involvement. According to the revised efficacy evaluation criteria by International Working Group (IWG) of Malignant Lymphoma, the treatment response was evaluated as complete response (CR), partial response (PR), stable disease (SD), recurrence, or progressive disease (PD). CR was defined as the disappearance of all clinical evidence of disease, normalization of all laboratory abnormalities related to the lymphoma, and normalization of radiographic images and biopsies that had been abnormal before therapy. PR was defined as regression of the tumor burden by ≥ 50%. PD was defined as appearance of new lesions or the diameter of the old lesion increased by ≥50%. SD was defined as the remaining patients not defined as CR, PR, or PD. Overall response (OR) was defined as CR + PR.[14,15] Relapse was defined as recurrent or persistent disease, as previously described.[10] Overall survival (OS) was defined as the time from the day of diagnosis to the day of death due to any reason or the day of last follow-up. Progression free survival (PFS) was defined the time from the day of diagnosis to the day of disease progression or death due to any reason or the day of last follow-up. Acute graft-versus-host disease (aGVHD) and chronic GVHD (cGVHD) were diagnosed and graded according to the Seattle criteria.
2.2.2. Follow-up
Responses to treatment were evaluated by the department of hematology after each cycle of chemotherapy. After completion of the treatment, the patients were followed-up as outpatients until September 31, 2016. The median follow-up time for surviving patients was 31.5 months (range: 11–97 months). Patients were re-evaluated every 3 months for the first 2 years after completion of the therapy, every 6 months from year 3 to year 5, and once every year after year 5. The examined items included blood cell count, blood biochemistry test, including serum LDH level, bone marrow examination, whole body CT scan or PET-CT exam.
2.2.3. Statistical analysis
Statistical software SPSS 21.0 (IBM, Chicago, IL) was used for data analysis. The measurement data underwent a normality test and were analyzed using the Mann–Whitney U test or independent t test as appropriate, and the enumeration data were analyzed using χ2 test. Survival was assessed by the Kaplan–Meier method and log rank test. Prognostic factors for survival were analyzed by the multivariate COX regression model. A 2-sided P < .05 indicated a significant difference.
3. Results
3.1. Clinical characteristics
The main clinical features of all patients and those patients who achieved CR + PR are presented in Table 1. Among the 59 patients, there were 48 males and 11 females (male to female ratio: 4.36:1) with median age of 25 years (range: 18–61 years), including 31 patients (52.5%) aged ≤25 years and 28 patients (47.5%) aged >25 years. According to the Ann Arbor staging, 50 (84.7%) patients were in stage III/IV. Seventeen patients (28.8%) had B symptoms. Mediastinal mass was in 52.5% of patients. 50.8% of patients are bone marrow (BM) involvement. LDH levels were usually elevated, accounting for 49.2% of all patients. According to the IPI score, more than half of patients had low or low-intermediate risk disease. Seven patients (11.9%) had chromosomal abnormalities. The genetic abnormalities identified in the population were the following mutations: 47,XY,+2[2]/46,XY[8];55,XY,+16,+19,+20,+6mar[10]; 46,XX,-2,8,+3mar[2]/46,XX[3];46,XY,der(13)(q34)+mar[2]/46,XY[8];46,XY,t(11,17)(p15,p11)[4]/46,idem,del(1)(p35)[2]/46,XY[4];47,XX,add(6)(p24),+8,der(16)[10];46,XY,der(6)(q15)[5]/46,XY[5]. Patients who achieved OR (n = 48) and patients without OR (n = 11) have different clinical features. Different clinical features of 2 group were mainly in KI67≥ 75 and elevated LDH (P < .05), and the rest of the clinical features were similar (P > .05). In patients with OR, the basic characteristics of patients with chemotherapy and chemotherapy + allogeneic hematopoietic stem cell transplantation are shown in Table 1, and were similar between the 2 groups (P > .05).
Table 1.
Clinical characteristics of all patients and patient achieved CR + PR with T-LBL.

3.2. Survival
All treatment regimens included at least 4 courses of hyper-CVAD/MA chemotherapy. The treatment response is summarized as follows: 61% of T-LBL patients achieved CR, 20.4% achieved PR, and the overall response (OR) rate was 81.4%. Within a median follow-up time of 31.5 months (range: 11–97 months), the 3-year OS and PFS rates for all patients were 37.7% and 37.3%, respectively. The 3-year OS and PFS were 45.7% and 45.0% for patients who achieved CR + PR after chemotherapy and both 0 for patients who did not achieve OR (both P < .001). The OS and PFS of patients who achieved CR and PR after chemotherapy are similar (P = .718, P = .986). Patients who achieved CR and PR had significantly improved PFS and OS than those without OR (Fig. 1A and B).
Figure 1.

A, B Kaplan–Meier analysis comparing OS and PFS between patients who achieved complete remission (CR), partial remission (PR) and those who did not overall remission (NOR) after 4 cycles of chemotherapy. C, D Kaplan–Meier analysis of OS and PFS in the chemotherapy alone group and the chemotherapy + allo-HSCT group.
Patients who achieved OR received a strong consolidation treatment regimen, including chemotherapy alone (26 cases) and chemotherapy + allo-HSCT (22 cases). In the chemotherapy alone group, the median OS was 21 months (range: 10–62 months) with the 2-year and 3-year OS rates being 26.2% and 17.5%, respectively. The median PFS was 15 months (range: 6–62 months) with the 2-year and 3-year PFS rates being 37.2% and 27.8%, respectively. In the chemotherapy + allo-HSCT group, the median OS was 48 months (range: 11–64 months), with the 2-year and 3-year OS rates being 79.4% and 72.8%, respectively. The median PFS was 43 months (range: 7–64 months) with the 2-year and 3-year PFS rates being 79.5% and 65.1%, respectively. Significant differences were observed in OS and PFS between the 2 groups (P = .008; P = .007) (Fig. 1C and D). There were 3 cases of relapse in the chemotherapy + allo-HSCT group, and 12 cases of relapse in the chemotherapy alone group.
Until last follow-up, a total of 7 (31.8%) cases in the chemotherapy + allo-HSCT group died. Four cases (18.2%) were transplantation related mortality (TRM), with 2 developing grade 4 aGVHD. Three (13.6%) cases were mortality caused by disease relapse and progression. In the chemotherapy alone group, 2 (7.7%) mortalities were treatment related and 13 (50.0%) mortalities were disease relapse and progression. No significant difference was observed for treatment related mortality for the 2 groups (P = .502).
Cumulative incidence of aGVHD and grade 2 to 4 aGVHD were 36.4% (8/22) and 18.2% (4/22), respectively. Incidence of cGVHD was 22.7% (5/22) with 2 cases who had previously developed aGVHD. The main symptoms were mild dry mouth, dry throat, rough skin, and desquamation.
3.3. Prognostic factors
Single factor analysis showed that B symptoms, Ki-67≥75%, pleural effusion, leukocytosis and elevated LDH had effects on OS and were adverse prognostic factors in all patients and patients who achieved OR (P < .05). However, the difference was that no OR after chemotherapy was a poor prognostic factor for all patients, and chemotherapy alone was a poor prognostic factor for patients with OR after chemotherapy (P < .05) (Table 2).
Table 2.
Effect of clinical factors on the prognosis of patients with T-LBL (%).

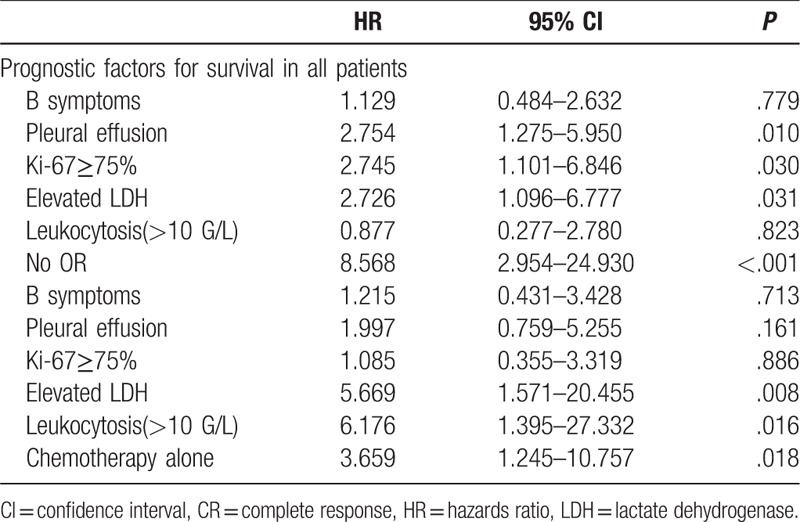
Multi-factor analysis showed that Ki-67≥75% (HR = 2.745, 95% CI: 1.101–6.846, P = .030), elevated LDH (HR = 2.726, 95% CI: 1.096–6.777, P = .031), pleural effusion (HR = 2.754, 95% CI: 1.275–5.950, P = .010) and no OR after chemotherapy (HR = 8.568, 95% CI: 2.954–24.930, P < .001) were independent prognostic factors in all patients (Table 3). But independent prognostic factors were elevated LDH (HR = 5.669, 95% CI: 1.571–20.455,P = .008),leukocytosis (>10G/L) (HR = 6.176, 95% CI: 1.395–27.332, P = .016) and chemotherapy alone (HR = 3.659, 95%CI: 1.245–10.757, P = .018) in patients who achieved OR.
Table 3.
Multiple-factor analysis of prognostic factors affecting the prognosis of patients with T-lymphoblastic lymphoma in all patients and patients who achieved CR + PR.
4. Discussion
The characteristics of the T-LBL cases in our center, such as occurring predominantly in males, mediastinal mass, mostly stage III/IV, with pleural effusion, and bone marrow invasion were consistent with previous reports.[16] The proportion of patients with mediastinal mass, bone marrow involvement was 52.5% and 50.8%, respectively, different with that previously reported, of 91% and 31%[7] and 70% and 15%.[8] The proportion of stage III-IV T-LBL was higher than that in the above 2 studies (84.7% vs 73.3% and 69.7%). The difference may be related to the small number of patients in this study group or regional disparities.
The strongest evidence of the high efficacy of ALL-type chemotherapy comes from a report of 105 children with T-LBL.[17] However, in this study we considered adult patients the CR, OR, 3-year OS, and PFS rates achieved by hyper-CVAD chemotherapy was 61.0%, 81.4%, 37.7%, and 37.3%, respectively, lower than those reported previously.[7,18,19] In this study, the reasons for these lower rates may be: patients in this group were mainly high-risk with advanced cancer stage, bone marrow involvement and elevated LDH. Racial differences, which is supported by this data being similar to other domestic data.[20] Many studies investigate LBL, and B-LBL has a better prognosis than T-LBL. Insufficient number of samples.
Auto- and allo-HSCT have both been used in consolidation therapy of high-risk T-LBL.[10,19,21] The difference between autologous and allogeneic HSCT is post-transplant disease recurrence and TRM. In our hospitals, 5 patients underwent autologous stem cell transplantation, and all relapsed at the end. Future studies should be conducted with a large number of auto-HSCT patients. In the study, considering the poor prognosis of patients who had not achieved OR, the patients were subdivided into either chemotherapy alone or chemotherapy + allo-HSCT in only patients who had achieved OR. The 3-year OS rate and PFS rate were significantly higher in the chemotherapy + allo-HSCT than chemotherapy alone group. There are several other advantages of combination treatment with allo-HSCT: allo-HSCT was also effective for patients who achieved PR. Among 22 patients, 5 patients who achieved PR after chemotherapy underwent allo-HSCT and achieved long-term survival; it shortened the course of chemotherapy; it significantly reduced the relapse rate compared with the chemotherapy alone group. In summary, allo-HSCT significantly improved survival.
All patients here received a preventative treatment regimen to avoid GVHD, but nevertheless the incidence of aGVHD was 36% and 2 cases with grade 4 aGVHD died; cGVHD was 23%. These rates are similar to other studies.[22,23] GVHD is of concern because even low grades may lead to worse prognosis or high grades may lead to TRM.[10] However, it has also been suggested that cGVHD may be related to a lower incidence of relapse.[24]
Patients with T-LBL recurrence have a very poor prognosis. A few studies have investigated T-LBL relapse in adults, and the current standard treatment regimen for recurrent T-LBL has not been determined. The analysis showed that the relapse rate of the chemotherapy alone group was 65.4%, while the chemotherapy + allo-HSCT group relapse rate was 13.6%, there was a significant difference between the 2 groups (P < .001). Four relapsed patients in the chemotherapy alone group received Nelarabine, but the efficacy was still disappointing. In the chemotherapy + allo-HSCT group, high OS and PFS rates and low relapse rate were achieved. Further studies with large sample size are required to determine if allo-HSCT can be used as the standard consolidation therapy of adult T-LBL.
Mediastinal radiotherapy in T-LBL is still controversial, it may cause adverse events, such as heart disease, radiation pneumonitis, and secondary malignancies.[6] The mediastinal recurrence rate was 5% to 10% without prevention or consolidation radiotherapy.[16] Dabaja et al[25] believed that the mediastinal recurrence may be reduced by mediastinal radiotherapy, but OS and PFS were not improved. Cortelazzo et al[26] reported that the CR rate of mediastinal mass was 62% after chemotherapy, and the proportion of mediastinal residual disease requiring radiotherapy was 28.5%. Our data showed that 25 (80.6%) achieved CR, while 6 (19.4%) required additional irradiation. The mediastinal recurrence rate was 5 (16.1%). Early central nervous system (CNS) invasion by T-LBL is rarely seen (3%–9%); however, intrathecal injection of chemotherapeutic agents should be regularly conducted to prevent CNS invasion, and prophylactic cranial irradiation is not recommended. Katz at al[27] used an NHL-BFM95 regimen to treat 37 patients with T-LBL who were not given cranial irradiation except 1 patient with confirmed CNS invasion at diagnosis. The results showed that only 1 patient had CNS recurrence. In this study, CNS invasion was observed in no patients at diagnosis and in 2 patients (3.4%) after regular intrathecal injection during chemotherapy. We believe that high-dose chemotherapy combined with prophylactic intrathecal injection may reduce the risk of CNS recurrence.
A variety of T-LBL prognostic factors have been reported, including older age (> 30–40 years), elevated LDH, bone marrow involvement, stage IV, B symptoms, or early CNS invasion.[28,29] However, these need to be clarified. The GMALL[30] group believes that the only prognostic factor of T-LBL is 1.5 times elevated LDH. Multi-factor analysis revealed that Ki-67≥75%, elevated LDH, pleural effusion and no OR after chemotherapy were independent prognostic factors affecting OS in all patients. But independent prognostic factors were only elevated LDH, leukocytosis, and chemotherapy alone in patients who achieved OR. In the prognosis of T-LBL, Ki-67 and pleural effusion needs to be studied further in a larger sample of patients. Due to the small sample size, further studies are required to confirm whether leukocytosis as an independent prognostic factor for a good prognosis.
In summary, the present study showed that allo-HSCT, as consolidation therapy in patients achieved OR, reduced the recurrence of T-LBL and improved survival. Allo-HSCT is likely to be a more appropriate option with patients of T-LBL, especially in patients with overall OR.
4.1. Limitations
This study has some limitations. This article was retrospective. The patients were not randomly selected to receive the different treatment regimens. There is a certain bias in chemotherapy alone group. The sample size was relatively small.
Author contributions
Conceptualization: Meiwei Hu, Huafeng Wang, Lei Wang.
Data curation: Meiwei Hu, Min Yang, Yinjun Lou, Jie Jin.
Formal analysis: Meiwei Hu, Huafeng Wang.
Investigation: Huafeng Wang, Lei Wang, Yinjun Lou, Jie Jin.
Methodology: Lei Wang, Min Yang.
Software: Huafeng Wang, Jie Jin.
Supervision: Min Yang.
Validation: Meiwei Hu, Yinjun Lou.
Visualization: Lei Wang.
Writing – original draft: Meiwei Hu, Huafeng Wang, Lei Wang, Min Yang.
Writing – review & editing: Huafeng Wang, Yinjun Lou, Jie Jin.
Supplementary Material
Footnotes
Abbreviations: ALL = acute lymphoblastic leukemia, allo-HSCT = allogeneic hematopoietic stem cell transplantation, BM = bone marrow, CNS = central nervous system, CR = complete response, CT = computed tomography, cytarabine IPI = high international prognostic index, DFS = disease free survival, GVHD = graft-versus-host disease, hyper-CVAD = hyper-fractionated administration of cyclophosphamide, vincristine, doxorubicin and dexamethasone, IWG = International Working Group, LDH = lactic dehydrogenase, MA= methotrexate, MRI = magnetic resonance imaging, MRT = mediastinal radiation therapy, NHL = non-Hodgkin lymphoma, OR = overall response, OS = overall survival, PD = recurrence or progressive disease, PET = positron emission tomography, PFS = progression-free survival, PR = partial response, SD = stable disease, T-ALL = T-cell acute lymphoblastic leukemia, T-LBL = T-lymphoblastic lymphoma, TRM = transplantation related mortality.
Supplemental Digital Content is available for this article.
The authors declare no conflicts of interest.
References
- [1].Bassan R, Maino E, Cortelazzo S. Lymphoblastic lymphoma: an updated review on biology, diagnosis, and treatment. Eur J Haematol 2016;96:447–60. [DOI] [PubMed] [Google Scholar]
- [2].Borthakur G, O’Brien SM. Younes A, Coiffier B. Lymphoblastic Lymphoma. Lymphoma: Diagnosis and Treatment.. Totowa, NJ: Humana Press; 2013. 330–43. [Google Scholar]
- [3].Raetz EA, Perkins SL, Bhojwani D, et al. Gene expression profiling reveals intrinsic differences between T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma. Pediatr Blood Cancer 2006;47:130–40. [DOI] [PubMed] [Google Scholar]
- [4].Sekimizu M, Sunami S, Nakazawa A, et al. Chromosome abnormalities in advanced stage T-cell lymphoblastic lymphoma of children and adolescents: a report from Japanese Pediatric Leukaemia/Lymphoma Study Group (JPLSG) and review of the literature. Br J Haematol 2011;154:612–7. [DOI] [PubMed] [Google Scholar]
- [5].Feng H, Stachura DL, White RM, et al. T-lymphoblastic lymphoma cells express high levels of BCL2, S1P1, and ICAM1, leading to a blockade of tumor cell intravasation. Cancer Cell 2010;18:353–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Portell CA, Sweetenham JW. Adult lymphoblastic lymphoma. Cancer J 2012;18:432–8. [DOI] [PubMed] [Google Scholar]
- [7].Hoelzer D, Gokbuget N, Digel W, et al. Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood 2002;99:4379–85. [DOI] [PubMed] [Google Scholar]
- [8].Thomas DA, O’Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood 2004;104:1624–30. [DOI] [PubMed] [Google Scholar]
- [9].Song KW, Barnett MJ, Gascoyne RD, et al. Primary therapy for adults with T-cell lymphoblastic lymphoma with hematopoietic stem-cell transplantation results in favorable outcomes. Ann Oncol 2007;18:535–40. [DOI] [PubMed] [Google Scholar]
- [10].Levine JE, Harris RE, Loberiza FR, Jr, et al. A comparison of allogeneic and autologous bone marrow transplantation for lymphoblastic lymphoma. Blood 2003;101:2476–82. [DOI] [PubMed] [Google Scholar]
- [11].Cortelazzo S, Ponzoni M, Ferreri AJ, et al. Lymphoblastic lymphoma. Crit Rev Oncol Hematol 2011;79:330–43. [DOI] [PubMed] [Google Scholar]
- [12].Sabattini E, Bacci F, Sagramoso C, et al. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica 2010;102:83–7. [PubMed] [Google Scholar]
- [13].Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 1971;31:1860–1. [PubMed] [Google Scholar]
- [14].Wang L, Wang JH, Bi XW, et al. Interim PET-CT may predict PFS and OS in T-ALL/LBL adult patients. Oncotarget 2017;8:99104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–86. [DOI] [PubMed] [Google Scholar]
- [16].Engelhard M, Brittinger G, Gerhartz HH, et al. Lymphoblastic lymphoma: Results of a prospective treatment trial (BMFT study). Br J Haematol 1996;93:1391a. [Google Scholar]
- [17].Reiter A, Schrappe M, Ludwig WD, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood 2000;95:416–21. [PubMed] [Google Scholar]
- [18].Ellin F, Jerkeman M, Hagberg H, et al. Treatment outcome in T-cell lymphoblastic lymphoma in adults: a population-based study from the Swedish Lymphoma Registry. Acta Oncol 2014;53:927–34. [DOI] [PubMed] [Google Scholar]
- [19].Fukushima T, Miyazaki Y, Honda S, et al. Allogeneic hematopoietic stem cell transplantation provides sustained long-term survival for patients with adult T-cell leukemia/lymphoma. Leukemia 2005;19:829–34. [DOI] [PubMed] [Google Scholar]
- [20].Shi Y, Zhou S, He X, et al. Autologous hematopoietic stem cell transplantation in chemotherapy-sensitive lymphoblastic lymphoma: treatment outcome and prognostic factor analysis. Chin J Cancer Res 2015;27:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fortune A, O’Leary H, Gilmore R, et al. T-lymphoblastic leukemia/lymphoma: a single center retrospective study of outcome. Leuk Lymphoma 2010;51:1035–9. [DOI] [PubMed] [Google Scholar]
- [22].Mohty M, Bay JO, Faucher C, et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood 2003;102:470–6. [DOI] [PubMed] [Google Scholar]
- [23].Stein AS, Palmer JM, O’Donnell MR, et al. Reduced-intensity conditioning followed by peripheral blood stem cell transplantation for adult patients with high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2009;15:1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Luo Y, Xiao H, Lai X, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood 2014;124:2735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dabaja BS, Ha CS, Thomas DA, et al. The role of local radiation therapy for mediastinal disease in adults with T-cell lymphoblastic lymphoma. Cancer 2002;94:2738–44. [DOI] [PubMed] [Google Scholar]
- [26].Cortelazzo S, Intermesoli T, Oldani E, et al. Results of a lymphoblastic leukemia-like chemotherapy program with risk-adapted mediastinal irradiation and stem cell transplantation for adult patients with lymphoblastic lymphoma. Ann Hematol 2012;91:73–82. [DOI] [PubMed] [Google Scholar]
- [27].Katz OB, Ben Barak A, Abrahami G, et al. Treatment of T cell lymphoblastic lymphoma in children and adolescents: Israel Society of Pediatric Hematology Oncology retrospective study. Isr Med Assoc J 2011;13:161–5. [PubMed] [Google Scholar]
- [28].Morel P, Lepage E, Brice P, et al. Prognosis and treatment of lymphoblastic lymphoma in adults: a report on 80 patients. J Clin Oncol 1992;10:1078–85. [DOI] [PubMed] [Google Scholar]
- [29].Zinzani PL, Bendandi M, Visani G, et al. Adult lymphoblastic lymphoma: clinical features and prognostic factors in 53 patients. Leuk Lymphoma 1996;23:577–82. [DOI] [PubMed] [Google Scholar]
- [30].Lepretre S, Touzart A, Vermeulin T, et al. Pediatric-like acute lymphoblastic leukemia therapy in adults with lymphoblastic lymphoma: the GRAALL-LYSA LL03 Study. J Clin Oncol 2016;34:572–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


