Abstract
Rationale:
The clinical application of robotic surgery in breast conserving surgery or volume replacement with robotic latissimus dorsi flap harvest (RLDFH) has been rarely reported. In this study, we report the preliminary experience and clinical outcome of robotic assisted quadrantectomy (RAQ) and immediate partial breast reconstruction (IPBR) with RLDFH.
Patient concern:
Decreasing and avoid back scar length after latissimus dorsi flap harvest.
Diagnoses:
One 28 years old female with left breast cancer underwent RAQ and IPBR with RLDFH. Initially, she was diagnosed with left breast infiltrating carcinoma that was clinical stage T3N1M0 and triple negative.
Interventions:
Neoadjuvant chemotherapy consisting of 4 cycles of epirubicin and cyclophosphamide followed by 4 cycles of docetaxel was performed. Breast magnetic resonance imaging showed residual breast cancer about 4.5 cm over the left upper outer quadrant of the breast. Sentinel lymph node biopsy showed no lymph node metastasis. RAQ, which took 82 minutes, was performed first, and the resected breast specimen's weight was 203 gm. She received IPBR with RLDFH, which took 97 minutes.
Outcomes:
The overall blood loss was 40 mL. The final pathology result was ypT2 (4.2 cm)N0 (sn0/3)M0 and stage IIA. The resection margin was free of tumors. The post-operative recovery was smooth except for seroma formation over the back, which was relieved after repeated aspiration at an outpatient clinic. The patient was satisfied with the post-operative scar and aesthetic outcome. No local recurrence, distant metastasis or case mortality was found during 5 months of follow-up.
Lessons:
RAQ and IPBR with RLDFH is a safe alternative for small-to-medium-breast-size women with breast cancer who desire breast conservation and are indicated for volume replacement with autologous latissimus dorsi flap.
Keywords: breast cancer, breast conserving surgery, robotic assisted quadrantectomy, robotic latissimus dorsi flap harvest, volume replacement
1. Introduction
Breast conserving surgery (BCS) has become the mainstream treatment for early breast cancer.[1,2] The concept of oncoplastic breast surgery also extended the indication of BCS.[3–5] When resected volume was less than 20% of breast tissue, the oncoplastic level I technique with volume displacement was sufficient to prevent breast shape deformity.[5] When resection volume was greater than 20% of breast tissue, the volume replacement (VR) technique was suggested to prevent deformity.[5,6]
Robotic surgery, which has the advantage of a 3-dimensional imaging system and flexibility of robotic arms and instruments, promoted a new revolution in surgical fields.[7–12] Robotic nipple sparing mastectomy (R-NSM)[13–15] and robotic latissimus dorsi flap harvest (RLDFH)[7,8,16–18] were successful examples demonstrating how robotic surgery could be applied in the field of breast cancer operations.
The clinical application of robotic surgery in BCS or VR with RLDFH has been rarely reported. In this study, we report the preliminary experience and clinical outcome of robotic assisted quadrantectomy (RAQ) and immediate partial breast reconstruction (IPBR) with RLDFH.
2. Case summary
A 28-year old female found one palpable mass over her left breast in the spring of 2017 and visited our breast clinic. Sonography revealed one hypoechoic tumor, larger than 4.7 cm by 2.8 cm, located at 2 o’clock/4 cm over the left breast mass, and malignancy was suspected. She received a core needle biopsy. The pathology showed left breast invasive carcinoma, estrogen receptor: negative (<1%), progesterone receptor: negative (<1%), and HER-2/neu: negative, and triple negative breast cancer (TNBC) was diagnosed. Breast magnetic resonance imaging (MRI) was performed, and left breast cancer about 8.5 cm by 6 cm with lymphadenopathy was the impression (Fig. 1A, B). Liver echo and whole body bone scans showed no distant metastasis. Under the impression of left breast cancer, clinical T3N1M0, stage IIIA, neoadjuvant chemotherapy was advised. Four cycles of epirubicin (90 mg/m2) plus cyclophosphamide (600 mg/m2) followed by 4 cycles of docetaxel (75 mg/m2) were performed. Follow-up breast MRI showed a partial response with mild tumor shrinkages of the prior invasive carcinoma; the tumor size decreased to approximately 4.5 cm (Fig. 1C, D). During pre-operative consultation, the patient expressed a strong desire for breast conservation. Left upper outer quadrantectomy was suggested to excise residual cancer, and VR with latissimus dorsi flap (LDF) harvest was suggested to prevent post-operative deformity. After discussion with the patient, single axillary incision RAQ combined with IPBR with RLDFH was planned.
Figure 1.
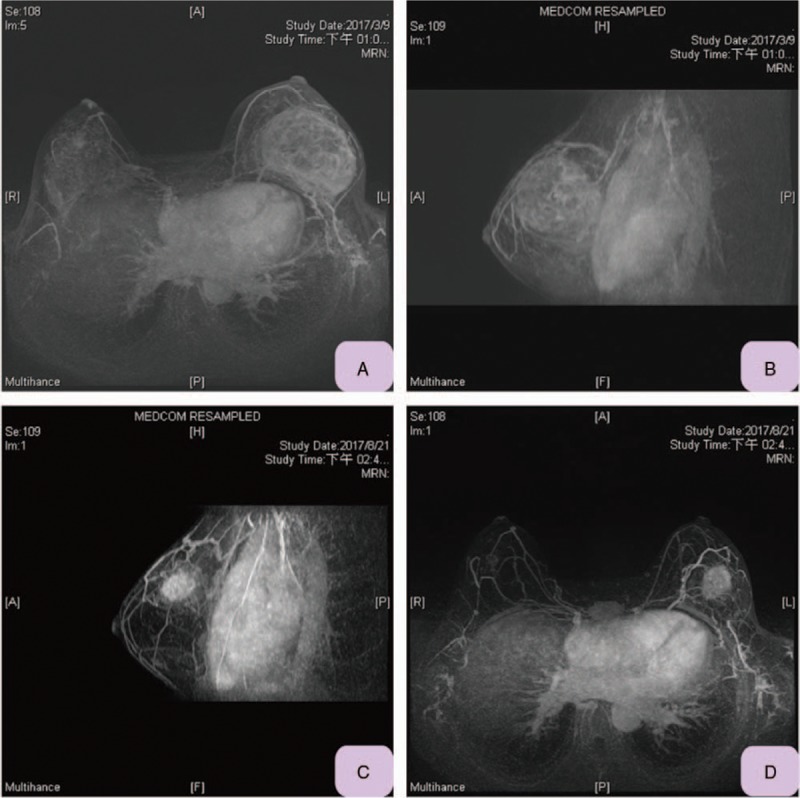
Magnetic resonance imaging (MRI) of current reported case. A, Pre-neoadjuvant chemotherapy MRI (transverse view) showed left breast cancer occupying large area of breast, size about 8.5 × 6 cm2. B, Pre-neoadjuvant chemotherapy MRI (sagittal view) showed left breast cancer occupying left upper half of breast. C, Post neoadjuvant chemotherapy (sagittal view) showed left breast cancer size had decreased to 4.5 cm. D, Post neoadjuvant chemotherapy (transverse view) showed left breast cancer size decreased and confined to upper outer quadrant. MRI = magnetic resonance imaging.
RAQ and IPBR with RLDFH were performed on September 19, 2017. A sentinel lymph node biopsy (SLNB) was done first, and a frozen biopsy showed negative for malignancy (0/3). Then RAQ was performed. An intra-operative resection margin frozen biopsy showed negative for malignancy. It took 82 minutes to complete the upper outer quadrantectomy using the da Vinci Si surgical platform (Fig. 2C), and the partial mastectomy specimen's weight was 203 gm (Fig. 2F). Then IPBR with RLDFH (Fig. 3) was performed; it took 97 minutes. The whole operation was smooth, and overall blood loss was 40 mL.
Figure 2.
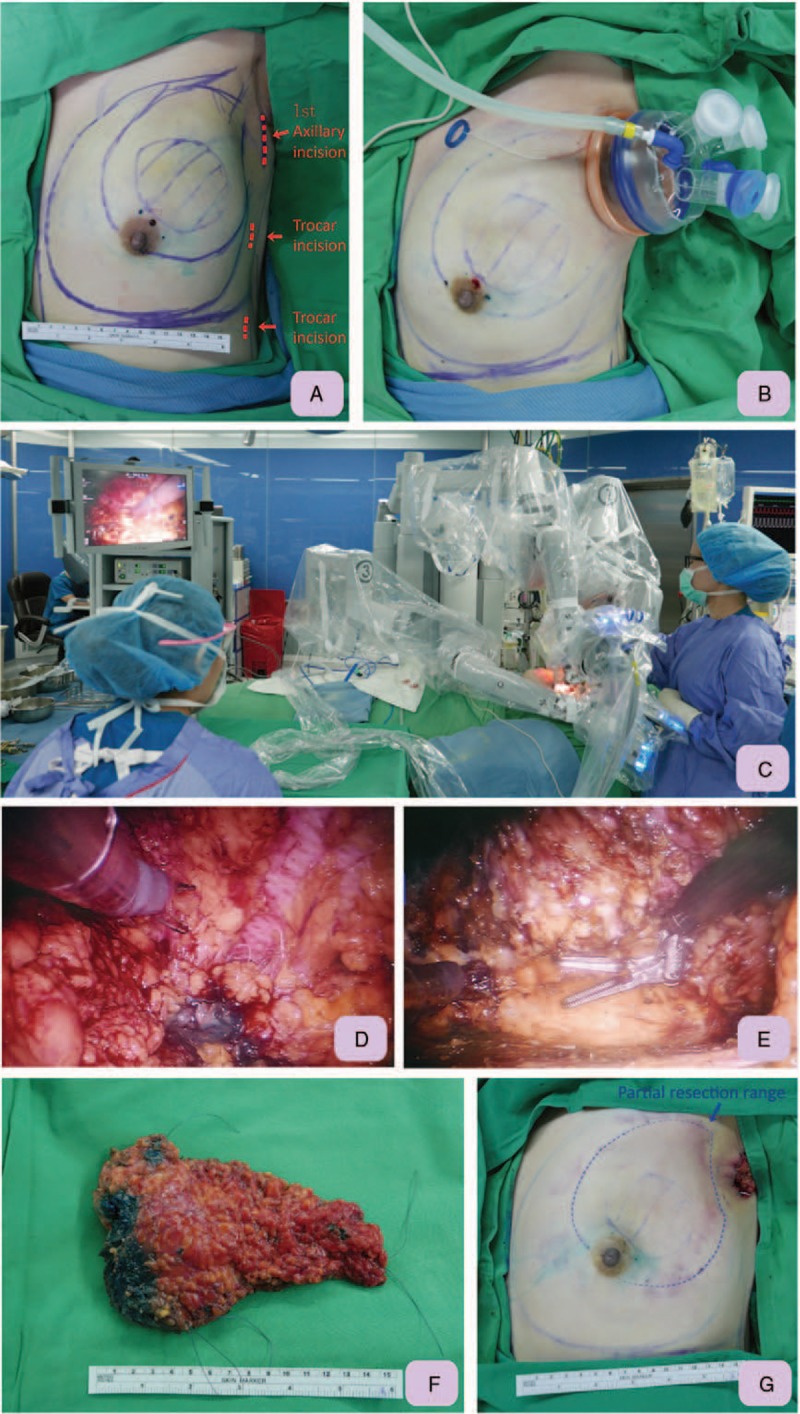
Demonstration of technique tips for RAQ. A, Pre-operative and intra-operative sonography was used to mark the location of the tumor and resection margin. Axillary incision and two additional trocar sites for RAQ and robotic latissimus dorsi flap harvest were marked with red dotted lines and arrows. B, After creation of the working space, a single port (Glove Port, Nelis, Gyeonggi-do, Korea) was inserted over the operating axilla. It was then inflated with carbon dioxide with air pressure kept at 8 mm Hg to create space for partial mastectomy. C, Picture shows the setting of RAQ, which was performed with a da Vinci Si (Intuitive Surgical, Sunnyvale, CA) robotic platform with the operating surgeon controlling at the console. D, Anterior skin flap dissecting was performed by dissection between skin flaps and breast glandular tissue with monopolar scissors. The parenchyma resection was performed with monopolar scissors along the inked resection margin, which was created by methylene blue containing jelly. E, After peripheral dissection and parenchyma resection, the left upper outer quadrantectomy was completed. F, The entire resected quadrantectomy breast specimen, which was intact and marked with silk threads for pathologic orientation, was removed through the axillary wound. G, Immediately post RAQ, the wound was small and hidden in the axilla region. However, a defect over the left upper outer quadrant was obvious due to a large portion of breast being removed. RAQ = robotic assisted quadrantectomy.
Figure 3.
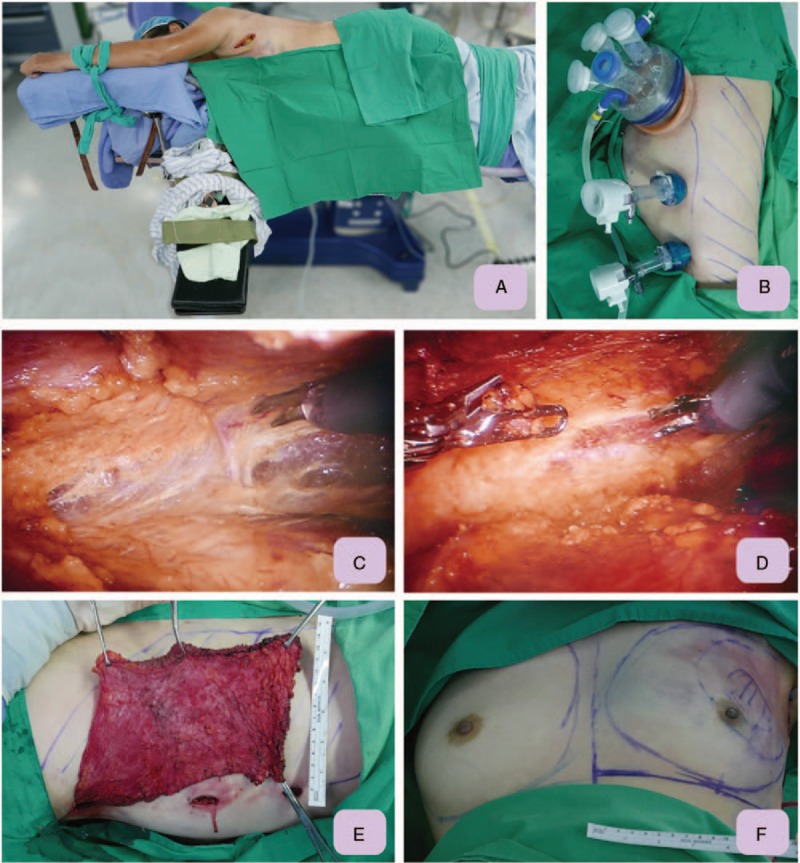
Demonstration of technique tips of IPBR with RLDFH. A, From the axillary wound, the thoracodorsal pedicle was identified and marked with a vessel loop. Then the wound was packed, and the patient was shifted to decubitus position. B, Two 12 mm balloon trocars (Kii Balloon blunt tip system, Applied Medical, Rancho Santa Margarita, CA) were used as port site for monopolar scissor and prograsp forceps. After all the 3 ports were set, the video camera was shifted to the middle port, and the monopolar scissors were shifted to the inferior port. The prograsp forceps were shifted to the inferior opening of the axillary accessory port. C, Dissection was suggested to begin along the undersurface of the muscle. After the undersurface of the muscle is dissected to the borders, the prograsp forceps is used to direct the anterior edge of the muscle toward the chest wall, and dissection proceeds over the superficial surface of the muscle. D, Once dissection is complete along both the deep and superficial surfaces, monopolar scissors are used to disinsert the muscle from the inferoposterior border. As the muscle is released, it is gathered toward the axilla to maintain tension and visualization along the posterior border. E, The pedicled latissimus dorsi flap, which was harvested by robotic means, was ready for transfer to post mastectomy subcutaneous space. The wound was small over the axilla, and 2 1 cm trocar wounds could be used as drain exit sites. F, Front view of immediate post-breast reconstruction outcome. The flap was fixed internally in the resected defect to ensure full coverage of the post partial mastectomy defect. IPBR = immediate partial breast reconstruction, RLDFH = robotic latissimus dorsi flap harvest.
The final pathology of the left resected breast specimen showed carcinoma with medullary features: Grade III, ypT2 (4.2 cm)N0 (sn0/3)M0, stage IIA, TNBC, and 40% Ki-67. The resection margin was free of tumors. Post operation recovery was well. Three JP drains were removed sequentially at an outpatient clinic after draining less than 30 mL/day per tube. The post-operative recovery was event-free except for seroma formation over the back, which was relieved after repeated aspiration at an outpatient clinic. The patient was satisfied with the post-operative scar and aesthetic outcome of RAQ and IBR with RLDFH (Fig. 4). No disease recurrence or discomfort was observed during 5 months of post-operative follow-up.
Figure 4.
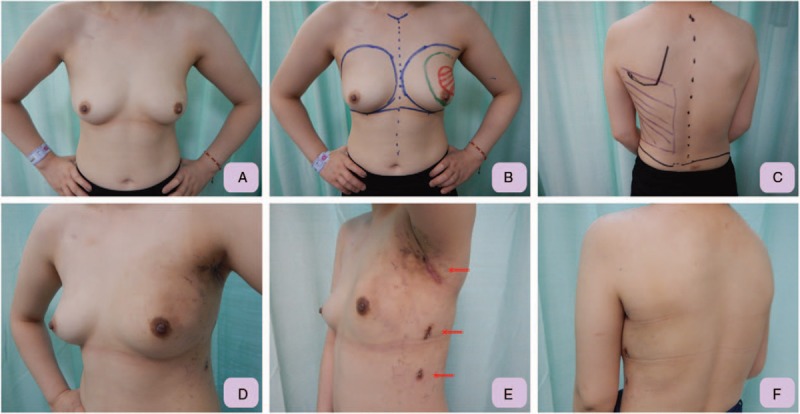
Pre- and post-operative pictures for patient who received RAQ and IPBR with RLDFH. A, Pre-operative front view of the 28 years old female with large left breast cancer post neoadjuvant chemotherapy indicated for left upper outer quadrantectomy. B, The location and presumed resection margin of left breast cancer was marked pre-operatively under the guidance of sonography. C, The borders of the LD muscle are marked: the anterior border was marked during LD muscle contraction, the superior border is marked over the tip of the scapula, and the posterior border is defined about 3 to 4 cm lateral to the spine. D, Left lateral-front view, which was taken 3 weeks post operation, showed that after IPBR with RLDFH, the left breast was in good shape and in symmetry with the right breast. E, Post-operative lateral view showed that the wound was small and well hidden in the inconspicuous axilla region. Two other 1 cm trocar wounds, which were used as drain exit sites, were also small and inconspicuous. F, Post-operative posterior view showed no incision scar was left over the back. IPBR = immediate partial breast reconstruction, LD = latissimus dorsi, RAQ = robotic assisted quadrantectomy, RLDFH = robotic latissimus dorsi flap harvest.
3. Patients and operative methods
The March 2017 to January 2018 medical records of the patient who underwent RAQ and IPBR with RLDFH for breast cancer were searched from our robotic breast surgery database at Changhua Christian Hospital (CCH), a tertiary medical center in central Taiwan. Patient-reported cosmetic outcome was also obtained. This study was approved by the Institutional Review Board of the Changhua Christian Hospital (CCH IRB No. 170806). Written informed consent to the use of clinical records was obtained from each participant. This current report includes photos of one patient, and this patient agreed and signed the consent for publication of her pictures.
4. Technique of RAQ
The patient was placed in the supine position with operative arm abducted about 90°, and her shoulders were elevated to 30 degrees with draping. An approximately 4 cm oblique axillary incision was made over the extra-mammary region (Fig. 2A), and a sentinel lymph node biopsy was performed first. Intra-operative sonography was used to mark the location of the tumor, and the resection margin was marked with methylene blue containing jelly.[19–22] After creation of the working space, a single port (Glove Port, Nelis, Gyeonggi-do, Korea) was inserted; it was inflated with carbon dioxide (CO2) with air pressure kept at 8 mm Hg to create space for partial mastectomy[13,23] (Fig. 2B). Then a robotic side cart (da Vinci, Intuitive Surgical, Sunnyvale, CA) was positioned posterior to the patient with the 2 robotic arms and the endoscope extending over the patient in proximity to the port. A 30 degree 12-mm-diameter camera (Intuitive Surgical, Denzlingen, Germany) was placed in the upper port to prevent collisions with other instruments. Dissection was carried out with a pair of 8 mm monopolar scissors (Intuitive Surgical, Sunnyvale, CA) placed on the right robotic arm. Traction and counter-traction, along with maintaining exposure and stretching out the tissue was carried out with 8 mm prograsp forceps (Intuitive Surgical) fitted on the left robotic arm. The locations of the scissors and forceps in the robotic arms could be adjusted during the operation to complete the dissection and mastectomy. Then the operation was shifted to a da Vinci robotic platform with the operating surgeon controlling the console (Fig. 2C).
The dissection started from the superficial skin flap. Then peripheral and posterior dissections were carried out by pulling breast tissue to create a sufficient working space. Posterior dissection was performed by detaching breast tissue from the pectoralis major muscle. Once the inked resection margin was reached, the parenchyma resection was performed with the monopolar scissors (Fig. 2D, E). After completion of the quadrantectomy, the resected breast specimen was removed through the axillary wound (Fig. 2F, G).
5. IPBR with RLDFH
The techniques for RLDFH have been reported,[7,8,17,18] and here we describe the procedures following RAQ. From the axillary wound, the thoracodorsal pedicle was identified and marked with a vessel loop. The wound was packed, and the patient was shifted to decubitus position (Fig. 3A). After working space was created from the axillary wound, the single port was re-inserted and gas re-inflated to 12 mmHg for re-docking the robotic arms. The prograsp forceps were used to lift the subcutaneous soft tissue, and the monopolar scissors were used to cut and dissect plane. Two additional trocar ports, for 12 mm balloon trocars (Kii Balloon blunt tip system, Applied Medical, CA), were inserted (Fig. 3B). The video camera was shifted to the middle port while monopolar scissor was shifted to the inferior port. The prograsp forceps were shifted to the axillary accessory port.
Dissection was suggested to begin along the undersurface of the muscle. Once dissection was complete along both the deep and superficial surfaces, the monopolar scissors were used to disinsert the muscle from the inferoposterior border (Fig. 3C). Once the muscle had been liberated beyond the tip of the scapula, it was easily accessible through the axillary incision (Fig. 3D). Then the LD muscle could be pulled out from the axilla, and attaching tissue could be dissected easily under direct vision (Fig. 3E). Drains were placed through the two lower trocar ports. A pedicled LDF was transferred into the mastectomy space for breast reconstruction. Then the position was changed to supine position again. The flap was fixed in a different quadrant to ensure full coverage of the post mastectomy defect (Fig. 3F). One drain was left beneath the LDF and exited from the axillary wound.
6. Discussion
The LDF has been widely used in breast reconstruction procedures.[7,24] A long scar on the back, which is usually 15 to 25 cm in length, can be avoided by endoscopic assisted LDF harvest (EALDFH)[25,26] or RLDFH[7,8,18,27] when the skin island is not needed. RLDFH has gradually replaced EALDFH because it overcomes some of the limitations and technical difficulties of EALDFH. In our preliminary experience, it took about 267 minutes to complete our first RLDFH. Our second and third RLDFH operation times were 97 minutes and 90 minutes, respectively.[28] This quick drop in operation time was related to the 3-dimensional vision, flexibility of instruments, and friendly operating system of the da Vinci surgical platform.
In small-to-medium-breast-size breast women with breast cancer who desire breast conservation, VR with autologous flap is in some circumstances a rational alternative to mastectomy.[6] The LDF is one of the most reliable flaps for VR and is often indicated when the resected volume is greater than 150 gm.[6] In this case report, the resected breast weight was 203 gm, which from our estimation was approximately 45% of the breast's volume. That led us to suggest that the patient receive VR with autologous LDF for partial breast reconstruction. The post-operative cosmetic result (Fig. 4) was quite good, and the patient was satisfied with the breast shape and wound result. The most frequent encountered complications of conventional LDF harvest were seroma formation, and wound related complications, like wound dehiscence or long apparent scar over the back.[7,24] RLDFH compared with conventional LDF harvest greatly reduce the wound related complications, however, seroma formation remained observed as sequelae and repeated aspiration still needed.
In the current study, we reported one patient with breast cancer who had undergone RAQ and IPBR with RLDFH; we believe this is the first such case reported in the literature. The main advantage of this approach is that BCS and VR with autologous LDF could be performed in the same operation with only one small and inconspicuous axillary wound. Disadvantages include the long operation time and complexity of the procedures when combining both RAQ and RLDFH. These disadvantages have less of an effect as the operation team becomes more familiar with the procedures. We found that RAQ and IPBR with RLDFH could be beneficial for small-to-medium- breast-size women with upper outer quadrant breast cancer who desire breast conservation surgery and are indicated for VR with autologous LDF.
Acknowledgment
The authors would like to thank Yun-Ting Chang and Shun-Ing Tsai for their assistance in this study.
Author contributions
Conceptualization: Hung-Wen Lai, Shih-Lung Lin.
Data curation: Shou-Tung Chen, Ya-Ling Lin, Shu-Hsin Pai.
Funding acquisition: Shih-Lung Lin.
Investigation: Ya-Ling Lin, Hwa-Koon Wu.
Project administration: Shu-Hsin Pai.
Resources: Shou-Tung Chen.
Supervision: Shou-Tung Chen, Shou-Jen Kuo.
Validation: Dar-Ren Chen.
Writing – original draft: Hung-Wen Lai.
Writing – review & editing: Hung-Wen Lai, Dar-Ren Chen.
Footnotes
Abbreviations: BCS = breast conserving surgery, EALDFH = endoscopic assisted latissimus dorsi flap harvest, IPBR = immediate partial breast reconstruction, LD = latissimus dorsi, LDF = latissimus dorsi flap, MRI = magnetic resonance imaging, RAQ = robotic assisted quadrantectomy, RLDFH = robotic latissimus dorsi flap harvest, R-NSM = robotic nipple sparing mastectomy, SLNB = sentinel lymph node biopsy, VR = volume replacement.
S-TC and H-WL contributed equally in this article as first co-authors.
The authors declare no conflicts of interest. This study was partially funded by the Ministry of Science and Technology of Taiwan, and the number of this funding is 104-2314-B-371-006-MY3. This study was also sponsored by research funding provided by the Changhua Christian Hospital: 104-CCH-ICO-006 and 106-CCH-IRP-011.
None of the authors have conflicts of interest or financial ties to disclose.
References
- [1].Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233–41. [DOI] [PubMed] [Google Scholar]
- [2].Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227–32. [DOI] [PubMed] [Google Scholar]
- [3].Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol 2005;6:145–57. [DOI] [PubMed] [Google Scholar]
- [4].Holmes DR, Schooler W, Smith R. Oncoplastic approaches to breast conservation. Int J Breast Cancer 2011;2011:303879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375–91. [DOI] [PubMed] [Google Scholar]
- [6].Yang JD, Kim MC, Lee JW, et al. Usefulness of oncoplastic volume replacement techniques after breast conserving surgery in small to moderate-sized breasts. Arch Plast Surg 2012;39:489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Selber JC, Baumann DP, Holsinger CF. Robotic harvest of the latissimus dorsi muscle: laboratory and clinical experience. J Reconstr Microsurg 2012;28:457–64. [DOI] [PubMed] [Google Scholar]
- [8].Selber JC, Baumann DP, Holsinger FC. Robotic latissimus dorsi muscle harvest: a case series. Plast Reconstr Surg 2012;129:1305–12. [DOI] [PubMed] [Google Scholar]
- [9].Dal Moro F. Robotic surgery and functional outcomes: a lesson from urology. Surg Laparosc Endosc Percutan Tech 2014;24:392–3. [DOI] [PubMed] [Google Scholar]
- [10].Abramovici L, Cartier C, Pierre G, et al. Robot-assisted transaxillary thyroidectomy: surgical technique. Eur Ann Otorhinolaryngol Head Neck Dis 2015;132:153–6. [DOI] [PubMed] [Google Scholar]
- [11].Alkatout I, Mettler L, Maass N, et al. Robotic surgery in gynecology. J Turk Ger Gynecol Assoc V 17 2016;224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Louie BE. A decade of robotics in lung cancer surgery. J Thorac Dis 2016;8:E1748–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Toesca A, Peradze N, Galimberti V, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique. Ann Surg 2017;266:e28–30. [DOI] [PubMed] [Google Scholar]
- [14].Toesca A, Peradze N, Manconi A, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: feasibility and safety study. Breast 2017;31:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sarfati B, Honart JF, Leymarie N, et al. Robotic da Vinci Xi-assisted nipple-sparing mastectomy: first clinical report. Breast J 2018;24:373–6. [DOI] [PubMed] [Google Scholar]
- [16].Struk S, Leymarie N, Honart JF, et al. Robotic nipple-sparing mastectomy with immediate reconstruction by robotically harvested latissimus dorsi muscle in a single position: Cadaveric study. J Plast Reconstr Aesthet Surg 2018;71:764–6. [DOI] [PubMed] [Google Scholar]
- [17].Selber JC. Can i make robotic surgery make sense in my practice? Plast Reconstr Surg 2017;139:781e–92e. [DOI] [PubMed] [Google Scholar]
- [18].Clemens MW, Kronowitz S, Selber JC. Robotic-assisted latissimus dorsi harvest in delayed-immediate breast reconstruction. Semin Plast Surg 2014;28:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lai HW, Lin HY, Chen SL, et al. Endoscopy-assisted surgery for the management of benign breast tumors: technique, learning curve, and patient-reported outcome from preliminary 323 procedures. World J Surg Oncol 2017;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lai HW, Chen ST, Chen DR, et al. Current trends in and indications for endoscopy-assisted breast surgery for breast cancer: results from a six-year study conducted by the Taiwan Endoscopic Breast Surgery Cooperative Group. PLoS One 2016;11:e0150310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lai HW, Wu HS, Chuang KL, et al. Endoscopy-assisted total mastectomy followed by immediate pedicled transverse rectus abdominis musculocutaneous (TRAM) flap reconstruction: preliminary results of 48 patients. Surg Innov 2015;22:382–9. [DOI] [PubMed] [Google Scholar]
- [22].Hung CS, Chang SW, Liao LM, et al. The learning curve of endoscopic total mastectomy in Taiwan: a multi-center study. PLoS One 2017;12:e0178251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tukenmez M, Ozden BC, Agcaoglu O, et al. Videoendoscopic single-port nipple-sparing mastectomy and immediate reconstruction. J Laparoendosc Adv Surg Tech A 2014;24:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Serletti JM, Fosnot J, Nelson JA, et al. Breast reconstruction after breast cancer. Plast Reconstr Surg 2011;127:124e–35e. [DOI] [PubMed] [Google Scholar]
- [25].Missana MC, Pomel C. Endoscopic latissimus dorsi flap harvesting. Am J Surg 2007;194:164–9. [DOI] [PubMed] [Google Scholar]
- [26].Pomel C, Missana MC, Atallah D, et al. Endoscopic muscular latissimus dorsi flap harvesting for immediate breast reconstruction after skin sparing mastectomy. Eur J Surg Oncol 2003;29:127–31. [DOI] [PubMed] [Google Scholar]
- [27].Chung JH, You HJ, Kim HS, et al. A novel technique for robot assisted latissimus dorsi flap harvest. J Plast Reconstr Aesthet Surg 2015;68:966–72. [DOI] [PubMed] [Google Scholar]
- [28].Lai HW, Lin SL, Chen ST, et al. Robotic Nipple Sparing Mastectomy and immediate Breast Reconstruction with Robotic Latissimus Dorsi Flap Harvest – Technique and preliminary results. J Plast Reconstr Aesthet Surg 2018;in press. [DOI] [PubMed] [Google Scholar]


